Abstract
The oleaginous red yeast Rhodotorula glutinis produces carotenoid pigments, especially torularhodin and β-carotene, in significant amounts. We have analyzed in detail carotenoid and lipid biosynthesis in a torularhodin-producing strain of R. glutinis cultivated at different carbon:nitrogen (C/N) ratios (20:1, 50:1, 70:1, and 100:1). When the strain was cultivated in media with low C/N ratios (20:1 and 50:1), glucose was completely utilized and carotenoid formation was stimulated. Maximum pigment production reached 12.9 mg/L of medium and 2.3 mg/g of biomass at the C/N ratio of 20:1. It was noted that β-carotene synthesis was prominent when glucose was present in the medium. However, glucose exhaustion in the media at C/N ratios of 20:1 and 50:1 was closely accompanied by the predominant formation of torularhodin. The growth of R. glutinis in media with C/N ratios of 70:1 and 100:1 favored lipid accumulation in the cells but carotenoid biosynthesis was reduced. In addition, glucose consumption was linked to a rapid decrease in oleic acid levels in the total intracellular lipids. The kinetic analysis clearly indicated a correlation between oleic acid levels in total lipids and torularhodin accumulation in the cells. The results may suggest that acetyl-CoA formed from oleic acid degradation is metabolized through the mevalonate/isoprenoid/carotenoid pathways directly to torularhodin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Carotenoids are a class of ubiquitous terpenoid pigments whose molecular backbone consists of a polyene chain of alternating single and double bonds. This structure is based on a conjugated system, which is responsible for the delocalization of π electrons and confers several features to the carotenoids, such as chemical reactivity, absorption of light, and pigmentation and coloration (Britton 1995). Carotenoids also play an important role in human health as they serve as antioxidants. One of the most efficient carotenoid antioxidants is torularhodin (Thr), which is involved in the scavenging of singlet oxygen and peroxyl radicals. Thr has higher antioxidant activity than β-carotene owing to its longer polyene chain with a carboxylic polar group (Sakaki et al. 2001).
The industrial production of carotenoids via extraction from plant tissues and chemical synthesis is not economically attractive due to the low yield and high production costs, as well as limitations in the occurrence of the natural sources. Therefore, new alternative sources are being examined, such as pigment-producing microorganisms (Mata-Gómez et al. 2014). Microbial synthesis offers a promising, cost-effective alternative based on the combination of fermentation technology and cultivation. Microbial biotechnology can therefore meet the high demand for valuable natural compounds, which includes not only carotenoid pigments used in food and feed additives but also lipids for the use in biofuel production. The wild-type and mutant forms of several carotenogenic microbes, such as yeast, fungi, bacteria, and algae, have been explored (Cutzu et al. 2013; Mata-Gómez et al. 2014). Urediniomycetes yeast, including the anamorphic Rhodotorula sp. and Sporobolomyces sp. and their teleomorphic analogs Rhodosporidium sp. and Sporidiobolus sp., predominantly synthesize three carotenoid pigments, namely, β-carotene, torulene, and Thr (Wang et al. 2015). In addition, some Rhodotorula strains are characterized by their great capacity to produce Thr, i.e., up to 80% of total carotenoid content (Maldonade et al. 2008). Numerous external fermentation factors, including environmental and physical conditions, influence the cultivation of Rhodotorula glutinis, which is commonly used for the production of both lipids and carotenoid pigments (Yen and Zhang 2011). Thus, the aim of many investigations has been to optimize cultivation conditions, including physical and nutritional factors (e.g., lighting, aeration, temperature, initial pH of cultivation medium, the concentration of a carbon and nitrogen source; Somashekar and Joseph 2000; Aksu and Eren 2007; Yen and Zhang 2011; El-Banna et al. 2012; Braunwald et al. 2013; Zhang et al. 2014). The capability to generate an immediate stress response is important in microorganisms, which thrive in a continuously fluctuating environment. Suboptimal cultivation conditions generate stress in microorganisms, which can induce biochemical reactions involving enhanced enzyme activity and carotenoid accumulation (Salar et al. 2013). The use of specific yeast strains to produced carotenoids is beneficial in industrial systems owing to complete substrate utilization coupled with considerable production of biomass enriched with pigments (Vieira et al. 2013).
The red yeasts are able to grow in a cultivation medium over a wide range of pH (2.5–9.5) and temperature (5–28 °C). However, one of the crucial factors for cell growth and metabolite production is an appropriate carbon and nitrogen ratio (C/N ratio) (Latha et al. 2005; Saenge et al. 2011a). The core process of metabolite formation consists of metabolic incorporation of the carbon source into acetyl-CoA followed by its subsequent utilization. In this process, fatty acids are first accumulated, mainly in the form of triacylglycerols, in cells during the early stationary growth phase, following which carotenoids are produced in the late stationary growth phase (Somashekar and Joseph 2000). These authors found that carotenoid accumulation is enhanced when glucose has been entirely consumed from the production medium with a concurrent utilization of intracellular fatty acids. It must be emphasized that the detailed kinetic study of R. glutinis grown on media with various C/N ratios and the study of metabolic activities of the strain under these conditions have not yet been performed. Therefore, the main novelty of the research reported here was confirmation of the correlation between glucose consumption, lipogenesis, and carotenoid formation by determining the kinetic parameters.
Materials and methods
Microorganism and cultivation conditions
For this study we chose the red yeast R. glutinis JMT 21978, an effective carotenoid producer. R. glutinis was maintained on 7% malt agar in petri dishes at 24 °C for 4 days under continual yellow light. Cultures were re-inoculated into 30 ml of YPG media containing 2% glucose, 1% peptone, and 1% yeast extract, re-incubated, and then transferred to 100-mL Erlenmeyer flasks containing 20 mL of production media (0.5% yeast extract, and 2, 5, 7, or 10% glucose). These glucose/yeast extract ratios in the medium represent C/N ratios of 20:1, 50:1, 70:1 and 100:1, respectively. The C/N ratios were calculated according to the known amount of carbon and nitrogen in glucose and yeast extract (BD Biosciences, San Jose, CA). The yeasts were grown in production media for 8 days, with the exception the lowest C/N ratio of 20:1 in which the yeasts were grown for 6 days. All cultures were grown in the KS 4000 ic control incubator shaker with cooling coil (180 rpm; IKA®-Werke GmbH & Co. KG, Staufen, Germany) at 28 °C under constant yellow light. All experiments were performed in four parallel cultivations.
Isolation of total lipids and carotenoids
Cells of R. glutinis were harvested by centrifugation and washed with a 0.9% NaCl solution and distilled water. Biomass was lyophilized and subsequently homogenized by mortar with sea sand. Carotenoids together with lipids were extracted twice with a chloroform/methanol (2:1) mixture for 2 h at laboratory temperature with occasional stirring (Folch et al. 1957). After extraction the mixture was filtered to remove cells, and the extracts were mixed with distilled water (1.2-fold of total extract volume). The mixture was stirred vigorously for 1 min and centrifuged to effect phase separation. The chloroform layer containing the lipids and pigments was filtered through anhydrous Na2SO4 and evaporated under vacuum. The lipid extract with carotenoid pigments was re-suspended in 1 mL of a hexane/chloroform mixture (9:1, v/v) and subsequently analyzed.
High-performance liquid chromatography analysis of carotenoid pigments
Carotenoids were analyzed by high-performance liquid chromatography (HPLC). Briefly, the carotenoid extract (10 μL) was injected into the column (LiChrospherR 100 RP-18; Merck KGaA, Darmstadt, Germany) and carotenoid content and composition analyzed by HPLC (HP 1100; Agilent Technologies, Santa Clara, CA) equipped with a diode array detector. The solvents used were acetonitrile:water:formic acid (86:10:4 v/v/v; solvent A) and ethyl acetate:formic acid (96:4 v/v; solvent B). The solvent flow rate was 1 mL/ min with a gradient of 100% A at 0 min, 100% B at 20 min, and 100% A at 30 min. Carotenoid pigments were identified using known standards. β-carotene, γ-carotene, and torulene were purchased from Sigma (St. Louis, MO), and Thr was purchased from Novachem (Heidelberg West, Australia). HPLC data were evaluated using ChemStation B.01.03 (Agilent Technologies).
Fatty acid isolation and analysis
Biomass (10–15 mg) was mixed and incubated (50 °C, 3 h) with 1 mL of dichlormetane containing 0.1 mg of internal standard (C17:0) and 2 mL of methanolic HCl solution to form fatty acid methyl esters (FAMEs) (Certik and Shimizu 2000). After incubation 1 mL of distilled water and 1 mL of hexane were added simultaneously and stirred vigorously. The mixture was centrifuged (3000 g, 5 min), and the hexane phase with pre-extracted FAMEs was analyzed by gas chromatography (GC-6890 N; Agilent Technologies). FAME solutions were automatically injected into the DB-23 column (50% cyanopropyl methyl polysiloxane, length 60 m, diameter 0.25 mm, film thickness 0.25 μm) and separated under the following conditions: carrier gas, hydrogen; inlet, temperature 230 °C, hydrogen flow 2.7 mL/min, split 1:10; flame ionization, 260 °C, hydrogen flow 40 mL/min, air flow 450 ml/min; temperature gradient, 130 °C for 1 min, 130–170 °C at 6.5 °C/min, 170 °C for 0 min, 170–220 °C at 3.75 °C/min, 220 °C for 5 min, 220–240 °C for 30 °C/min, 240 °C for 3.5 min). FAMEs were evaluated by retention times of internal standard (Sigma) and quantified using ChemStation B0103 (Agilent Technologies).
Measurement of residual sugars
Residual sugars in the production media were measured spectrometrically by a method using 3,5-dinitrosalicylic acid at 540 nm (Miller 1959).
Determination of kinetic parameters
The kinetic analysis considered the formation of biomass [total dry cell weight (DCW)], total fatty acid content (TFA), and total pigment content (TP) in yeast cells during each day of cultivation. Lipid-free cell weight (ffCW) was used in the other calculations. Several kinetic parameters were determined according to Certik and Shimizu (2000). The basic parameter productivity (P) represents the final production of DCW, TFA, and TP during cultivation process (mg/L−1, g/L−1). The relative productivity (Pr) represents the accumulation of TFA and TP in DCW (%, mg/g). The rate of product synthesis (ν) is the average speed of total production of DCW, TFA and TP (g/L/day; mg/L/day). Specific product synthesis (q) is the total accumulation of TP and TFA in DCW during the entire cultivation of R. glutinis was cultivated (%/day; mg/g/day). Finally, the yield of product (Y) represents the entire amount of DCW, TFA, and TP formation from the total consumed substrate (g/100 g Sc; %).
Results and discussion
Cell growth and glucose utilization
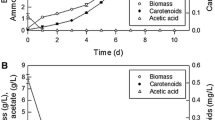
The growth of R. glutinis JMT 21978 was studied in a medium with varying C/N ratios (20:1, 50:1, 70:1 and 100:1, respectively). The C/N ratio of 70:1 was optimal for cell growth yield (14.5 g DCW/L) (Fig.1 a, b). Glucose as an easily digestible carbon source was completely consumed after 72 h of cultivation at a C/N ratio of 20:1, with a maximum cell growth of 6.3 g DCW/L, and after 144 h at a C/N ratio 50:1 with a biomass yield of 11.2 g/L. The C/N ratio of 100:1 prolonged the lag phase of cell growth and thus resulted in a lower cell yield (11.9 g/L) than that at the C/N ratio of 70:1.
Production of total carotenoid pigments
The analysis of carotenoid production delineated the mechanism by which C/N ratio affected the pigment content in the yeast cells (Fig 2a, b). During the first 48 h of cultivation, the rate of carotenoid accumulation was similar at all C/N ratios and was independent of the C/N ratio. However, when glucose was consumed at the C/N ratio of 20:1, there was a rapid accumulation of total pigments in biomass, accounting for the maximum amount of 2.3 mg carotenoids/g of cells after 122 h of cultivation. A similar rapid enhancement of pigment biosynthesis was also triggered at the C/N ratio of 50:1 after 144 h when the glucose in the medium was exhausted. Under this condition, pigment accumulation in the yeast cells reached 1.6 mg/g cells. Aksu and Eren (2007) obtained similar results and observed rapid pigment production after glucose depletion, at the end of stationary phase, with minimal carotenoid content during the lag phase and a progressive increase in the production of carotenoids during the logarithmic growth phase. Although pigment accumulation in cells grown in a medium with C/N ratio 20:1 was 1.4-fold higher than that at C/N ratio 50:1, the total maximum pigment yield of 18 mg/L was reached only upon yeast cell cultivation in the medium with a C/N ratio of 50:1 due to the doubling of cell growth under this condition. However, a significantly lower accumulation of total pigments in the yeast was observed during growth on the media with C/N 70:1 and C/N 100:1. Under these latter conditions, the amount of carotenoids in the cells reached maximum values of 1 and 0.8 mg/g for C/N 70:1 and 100:1, respectively, corresponding to total pigment yields of 12.1 and 8 mg/L, respectively. Thus, the C/N ratios above 50:1 caused a gradual decline in pigment production due to continual lipid formation and the lack of substrate acetyl-CoA for the carotenoid pathway (Somashekar and Joseph 2000).
Metabolite formation in R. glutinis JMT 21978 at different C/N ratios (20:1, 50:1, 70:1, and 100:1, respectively). a Total pigment (TP) yield obtained from red yeast cells, b total pigment accumulation in terms of dry cell weight (DCW), c yield of total lipids (TL) synthesized during cultivation, d total lipid accumulation terms of DCW
The effect of C/N ratio on carotenoid formation also depends on the microbial strain. Libkind et al. (2004) reported that changes in the C/N ratio has no effect on carotenogenesis in R. mucilaginosa. In contrast, the glucose-based increase of the C/N ratio from 70:1 to 120:1 positively affected carotenoid synthesis in R. glutinis (Braunwald et al. 2013). Variations in C/N ratio by employing different concentrations of carbon and nitrogen sources and the nature of either carbon or nitrogen source is yet another parameter that might change carotenoid biosynthesis. Park et al. (2005) observed that both low and high concentrations of ammonium reduced pigment production in R. glutinis. These authors cultivated R. glutinis in a urea-based medium and varied the C/N ratio from 4:1 to 50:1. A C/N ratio of 27:1 resulted in a high carotenoid content of 0.4 mg/g cells and the production of 5.41 mg/L. The effect of different C/N ratios (2.5:1, 5:1, 10:1, 15:1, and 20:1) on carotenoid synthesis by R. glutinis was also studied by El-Banna et al. (2012). These authors found that a C/N ratio of 5:1 was optimal for a good cellular carotenoid production (0.4 mg/g), with a total pigment yield reaching a maximum value of 2.4 mg/L at C/N 10:1.
Biosynthesis of individual carotenoid pigments
The pigments biosynthesized by R. glutinis include four main carotenoids: β-carotene, γ-carotene, torulene, and Thr. The alterations in these pigment profiles produced by these yeast cells grown at the four C/N ratios are shown in Figs. 3 and 4. The results of these experiments clearly show that as long as glucose is not completely utilized, β-carotene is the predominant molecule synthesized, especially during the early phase of cultivation. However, glucose exhaustion in the medium at C/N ratios of 20:1 and 50:1) was closely accompanied by a shift in product synthesis, with Thr becoming the predominant metabolic product. However, the high levels of Thr accumulation in the cells and its total yield at C/N ratio 20:1 was achieved at 120 h of cultivation (1.2 mg/g and 6.6 mg/L, respectively), whereas the growth of the yeast on the medium with a C/N ratio of 50:1 resulted in the maximum Thr levels after 192 h of cultivation (1.1 mg/g and 11.7 mg/L, respectively). Thus, the final total yield of Thr in yeast cells grown in medium with a C/N ratio of 50:1 was 1.8-fold higher than that at C/N ratio 20:1. Carotenoid production during the cultivation of the yeast in media with a C/N 70:1 and 100:1 was significantly reduced. Under all conditions, β-carotene was the main pigment until 42 h of cultivation (42–50%); however, during the stationary growth phase, an accumulation of Thr (30–36%) and a low content of β-carotene (11–18%) were noted. The levels of γ-carotene and torulene (intermediates of carotenoid biosynthesis in this strain) in the total pigments varied from 20 to 30%. On the basis of these observations, it could be claimed that glucose exhaustion at a low C/N ratio and at the late growth phase are favorable conditions for the rapid accumulation of torularhodin in the membranes and the storage lipids of R. glutinis JMT 21978 cells.
The alterations in the pigment profile and in the content of individual carotenoids of yeasts cultivated at various C/N ratios were studied by El-Banna et al. (2012). These authors found that R. glutinis grown on a medium with a low C/N ratio and low glucose concentration synthesized torulene and Thr as the main carotenoids. A higher C/N ratio with an increased glucose level in the medium negatively affected Thr production, with β-carotene then being detected as the major pigment at these conditions. In contrast, Cutzu et al. (2013) reported that the maximum production of β-carotene occurred when R. glutinis was grown in a medium with a low C/N ratio containing a high concentration of both carbon (glycerol, 80 g/L) and nitrogen. Han et al. (2016) observed that the metabolism of carotenoids in Sporidiobolus pararoseus was switched to torulene (up to 58% of total pigments) when the strain was cultivated through fed-batch fermentation with a constant feeding of glucose.
Lipid production
As mentioned, R. glutinis is an oleaginous yeast that accumulates a significant amount of oils in the form of lipid droplets. Lipid overproduction was achieved by cultivating this microorganism on a medium rich in the carbon source and only a limited amount of nitrogen source (Ratledge 2002; Papanikolaou et al. 2004; Meng et al. 2009; Sunja et al. 2011; Ageitos et al. 2011).
Our results clearly show that elevation of the C/N ratio in the medium (from 20:1 to 70:1) was accompanied by an increased lipid accumulation in the yeast cells of up to nearly 32% (Fig. 2c, d). In addition, the maximum total lipid yield of 4.6 g/L was reached at the C/N ratio of 70:1; this yield was 4.5-fold higher than that at the C/N ratio of 20:1. It should be noted that the rate of lipid production was continuously enhanced until glucose was present in the medium (C/N 20:1, 50:1, and 70:1). The positive effect of higher C/N ratios on lipid formation by R. glutinis has also been described by Somashekar and Joseph (2000) and Braunwald et al. (2013). An enhanced lipid synthesis was also observed for R. glutinis grown on a medium with a C/N ratio of 85:1 and supplemented with glycerol (Saenge et al. 2011a). Our experiments also revealed that a high C/N ratio of 100:1 reduced the capability of the yeast to synthesize lipids, probably due to the inhibition of growth by a high concentration of glucose in the medium (longer lag phase of cells was also observed). On the other hand, the rapid stimulation of lipogenesis in R. glacialis was achieved when the yeast was cultivated on a medium with a very high initial glucose concentration and a high C/N ratio (160:1) (Amaretti et al. 2010).
In our study, both the overall and daily rates of glucose conversion to total lipid were the same for C/N ratios of 20:1 and 70:1. When both glucose and the nitrogen source were present in the medium, the daily rate of intracellular lipid formation was in the range of 5–6.5 g lipids/100 g glucose utilized. When the nitrogen source was exhausted, the daily rate of lipid biosynthesis from glucose rapidly increased. In contrast, when glucose was completely consumed and the nitrogen source was still present in the medium, the intracellularly accumulated lipids were catabolized by the yeast cells. These results suggest that complete consumption of glucose in the medium evidently triggered the utilization of storage lipids as the secondary energy source in R. glutinis.
Fatty acid composition
The typical fatty acids of R. glutinis are palmitic acid (C16:0, PA), stearic acid (C18:0, SA), oleic acid (C18:1–9c, OA), linoleic acid (C18:2–9c,12c, LA), and α-linolenic (C18:3–9,12,15c, ALA) acid, of which oleic acid is the predominant fatty acid. Long-chain saturated fatty acids, such as C20:0, 22:0, and 24:0, were formed at a low level via stearic acid elongation reactions. Myristic (C14:0) and palmitoleic (C16:1–9c) acids were also detected in small quantities in the yeast. Fatty acids in the form of triacylglycerols are the main storage of lipids in R. glutinis and serve as an energy source, whereas the fatty acids bound to phospholipids are important constituents of membrane lipids (Davoli et al. 2004; Rani et al. 2013).
The fatty acid profile of intracellular lipids of R. glutinis JMT 21978 was affected by both the C/N ratio of the medium and cultivation time (Fig. 5). Of the intracellular lipids, PA, OA, LA, and ALA were generally the dominant fatty acids during the initial stage of yeast growth at C/N ratios of 20:1 and 50:1 (Fig. 5a, b). When cultivated at C/N ratios of 70:1 and 100:1 (Fig. 5c, d) the yeast synthesized PA, OA, myristic acid (C14:0), palmitoleic acid (C16:1), and long-chain saturated fatty acids. Interestingly, substantial amounts of C14:0 and C16:1 were produced only on the first day of the culture, possibly as a response to a high initial concentration of glucose in the medium. Subsequent growth of the strain was accompanied by a rapid accumulation of OA (mainly incorporated into triacylglycerols), which reached up to 42% of the total fatty acid content until glucose was completely consumed. A significantly high percentage of OA (40–50%) in the total lipid content of R. glutinis grown under sufficient amounts of glucose has also been described by other authors (Saenge et al. 2011b; Wiebe et al. 2012; Schneider et al. 2013). However, when glucose was exhausted from the medium, the levels of OA in the intracellular lipids gradually dropped with a concomitant increase in stearic acid. As mentioned, triacylglycerols are the main storage lipids in R. glutinis while fatty acids serve as an energy source. It would appear that OA is primarily utilized via the β-oxidation reaction after glucose exhaustion and thus it might provide the acetyl-CoA blocks needed for further metabolism by the yeast under these conditions.
Correlation between lipid and carotenoid production
Kinetic analysis is an effective strategy to obtain a better understanding of a substrate flux to metabolite production and to monitor metabolite biosynthesis during the entire cultivation process. The calculated kinetic parameters sketched the metabolic flow of carbon from glucose to lipids and pigments as well as established a correlation between the metabolism of stored fatty acids and the carotenoid pigments after glucose exhaustion. The rates (average velocity) of lipid (νTL) and total pigment (νTP) synthesis by the yeast grown at the C/N ratios of 20:1 and 70:1 were constant until all of glucose was consumed (Fig. 6a, b). Surprisingly, the yields of lipid (YTL) and total pigment (YTP) produced from the total consumption of the glucose in the medium was also similar at both C/N ratios 20:1 and 70:1 until the glucose was actually depleted (Fig. 6c, d). The rates and yields of metabolite biosynthesis by the strain cultivated at C/N ratio 100:1 were significantly reduced compared with those at the lower C/N ratios due to a prolonged lag phase of cell growth at C/N ratio 100:1. However, when glucose was exhausted from the medium (72 h for C/N 20:1 and 144 h for C/N 50:1, respectively), the rate of lipid synthesis and yield rapidly decreased, while the rate and yield of total pigment biosynthesis markedly increased. This rapid accumulation of total pigments after all the glucose in the medium had been consumed was clearly triggered by the biosynthesis of Thr (Fig. 6e, f). This result was also confirmed by the average velocity of Thr synthesis (νThr) and the specific rate of Thr accumulation in cells (qThr), which were found to be 1.6 and 0.27 mg Thr/g cell/day, respectively, at the C/N ratio of 20:1 at 96 h.
Kinetic analysis of metabolite production by R. glutinis JMT 21978 at different C/N ratios (20:1, 50:1, 70:1, and 100:1). Analysis of average speed of total lipid (TL) (a) and total pigment (TP) (b) synthesis (ν) during cultivation and study of glucose conversion (Y) to TL (c) and TP (d) content related to 100 g of glucose (Glc). e, f Kinetic analysis of torularhodin (Thr) production: average speed of Thr formation (ν) (e) and total Thr accumulation in biomass (q) (f)
It is intriguing to know that which carbon source was used for the synthesis of total pigments, and especially for the synthesis of Thr, when the glucose in the medium was completely utilized. In order to address this issue, all measured data were comparatively analyzed, which revealed that glucose consumption was accompanied by a rapid decrease in OA levels in the total intracellular lipid content (Fig. 7a) as well as a rapid reduction of its accumulation in the cells (Fig. 7b). OA was mainly present in triacylglycerols as the main storage lipid structure. Triacylglycerol content in the storage lipids decreased after glucose exhaustion due to the preferential utilization of OA from this lipid structure. Under these same conditions, an increased biosynthesis of Thr was observed. In addition, a correlation between OA levels in the total lipids and Thr accumulation in the cells was clearly established, especially in the exponential growth phase (Fig. 7c). On basis of these observations, the following conclusions can be made: (1) glucose is the main carbon source and primarily utilized and used for yeast growth and lipid accumulation in the cells, (2) glucose consumption is accompanied by a significant reduction in the growth rate and lipid biosynthesis, (3) storage lipids are utilized as the secondary energy source, (4) OA enters the β-oxidation pathway and forms acetyl-CoA for pigment production, (5) because the yeast strain prefers to biosynthesize Thr, acetyl-CoA formed from OA degradation rapidly enters the mevalonate/isoprenoid/carotenoid pathways to synthesize Thr.
Detailed study of OA formation and conversion to Thr synthesis by R. glutinis JMT 21978 in media with different C/N ratios (20:1, 50:1, 70:1, and 100:1). a Percentage amount of OA (C18:1) in accumulated fatty acids (TFA) produced during the cultivation process, b OA (C18:1) accumulation in terms of DCW, c correlation of accumulated Thr in DCW to C18:1 percentage amount related to TFA content. Box Early exponential growth phase, ellipse late exponential and stationary growth phase
It can be inferred from these results that the genes encoding γ-carotene desaturase and hydroxylase/ketolase, which transform γ-carotene to torulene and torulene to Thr, respectively, must be active under these conditions. Therefore, further experiments need to be carried out focusing on biochemical and molecular analyses delineating the molecular mechanism of Thr biosynthesis and its biotechnological overproduction.
Conclusion
The kinetic parameters related to different C/N ratios influencing R. glutinis JMT 21978 growth and affecting metabolite production were analyzed. This study presents an insight into lipid and carotenoid synthesis by R. glutinis JMT 21978 and their interconnections of these processes. For the first time, glucose exhaustion was shown to trigger the rapid accumulation of Thr at the expense of OA from storage lipids. The monitoring of several kinetic parameters of metabolite production may serve as an effective strategy to choose among the optimal conditions for high lipid or carotenoid production.
References
Ageitos JM, Vallejo JA, Veiga-Crespo P, Villa TG (2011) Oily yeasts as oleaginous cell factories. Appl Microbiol Biotechnol 90:1219–1227. doi:10.1007/s00253-011-3200-z
Aksu Z, Eren AT (2007) Production of Carotenoids by the isolated yeast of Rhodotorula glutinis. Bioch Eng J 35:107–113. doi:10.1016/j.bej.2007.01.004
Amaretti A, Raimondi S, Sala M, Roncaglia L, De Lucia M, Leonardi A, Rossi M (2010) Single cell oils of the cold-adapted oleaginous yeast Rhodotorula glacialis DBVPG 4785. Microb Cell Factories 9:1–6. doi:10.1186/1475-2859-9-73
Braunwald T, Schwemmlein L, Graeff-Hönninger S, French WT, Hernandez R, Holmes WE, Claupein W (2013) Effect of different C/N ratios on Carotenoid and lipid production by Rhodotorula glutinis. Appl Microbiol Biotechnol 97:6581–6588. doi:10.1007/s00253-013-5005-8
Britton G (1995) Structure and properties of carotenoids in relation to function. FASEB J 15:1551–1558
Cetik M, Shimizu S (2000) Kinetic analysis of oil biosynthesis by an Arachidonic acid-producing fungus, Mortierella alpina 1S-4. Appl Microbiol Biotechnol 54:224–230. doi:10.1007/s002530000361
Cutzu A, Clemente A, Reis A, Nobre B, Mannazzu I, Roseiro J, da Silva TL (2013) Assessment of β-carotene content, cell physiology and morphology of the yellow yeast Rhodotorula glutinis mutant 400A15 using flow cytometry. J Ind Microbiol Biotechnol 40:865–875. doi:10.1007/s10295-013-1278-2
Davoli P, Mierau V, Weber RWS (2004) Carotenoids and fatty acids in red yeasts Sporobolomyces roseus and Rhodotorula glutinis. Appl Biochem Micro 40:392–397. doi:10.1023/b:abim.0000033917.57177.f2
El-Banna AA, El-Raztek AMA, El-Mahdy AR (2012) Some factors affecting the production of carotenoids by Rhodotorula glutinis var. glutinis. FNS 3:64–71. doi:10.4236/fns.2012.3101
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Han M, Xu Z, Du C, Qian H, Zhang W (2016) Effects of nitrogen on the lipid and carotenoid accumulation of oleaginous yeast Sporidiobolus pararoseus. Bioprocess Biosyst Eng 39:1425–1433. doi:10.1007/s00449-016-1620-y
Latha BV, Jeevaratnam K, Murali HS, Manja KS (2005) Influence of growth factors on carotenoid pigmentation of Rhodotorula glutinis DFR-DPY from natural sources. IJBT 4:353–357
Libkind D, Brizzio S, Van Broock M (2004) Rhodotorula mucilaginosa, a carotenoid producing yeast strain from a Patagonian high altitude Lake. Folia Microbiol 49:19–25. doi:10.1007/BF02931640
Maldonade IR, Rodriguez-Amaya DB, Scamaparini ARP (2008) Carotenoids of yeasts isolated from the Brazilian ecosystem. Food Chem 107:145–150. doi:10.1016/j.foodchem.2007.07.075
Mata-Gómez LC, Montañez JC, Méndez-Zavala A, Aguilar CN (2014) Biotechnological production of carotenoids by yeasts: an overview. Microb Cell Factories 13:12. doi:10.1186/1475-2859-13-12
Meng X, Yang J, Xu X, Zhang L, Nie Q, Xian M (2009) Biodiesel production from oleaginous microorganisms. Renew Energ 34:1–5. doi:10.1016/j.renene.2008.04.014
Miller GL (1959) Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. doi:10.1021/ac60147a030
Papanikolaou S, Komaitis M, Aggelis G (2004) Single cell oil (SCO) production by Mortierella isabellina grown on high-sugar content media. Bioresour Technol 95:287–291. doi:10.1016/j.biortech.2004.02.016
Park PK, Cho DH, Kim EY, Chu KH (2005) Optimization of carotenoid production by Rhodotorula glutinis using statistical experimental design. World J Microbiol Biotechnol 21:429–434. doi:10.1007/s11274-004-1891-3
Rani SH, Saha S, Rajasekharan R (2013) A soluble diacylglycerol acyltransferase is involved in triacylglycerol biosynthesis in the oleaginous yeast Rhodotorula glutinis. Microbiology 159:155–166. doi:10.1099/mic.0.063156-0
Ratledge C (2002) Regulation of lipid accumulation in oleaginous microorganisms. Biochem Soc Trans 30:1047–1050. doi:10.1042/bst0301047
Saenge C, Cheirsilp B, Suksaroge TT, Bourtoom T (2011a) Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem 46:210–218. doi:10.1016/j.procbio.2010.08.009
Saenge C, Cheirsilp B, Suksaroge TT, Bourtoom T (2011b) Efficient concomitant production of lipids and carotenoids by oleaginous red yeast Rhodotorula glutinis cultured in palm oil mill effluent and application of lipids for biodiesel production. Biotechnol Bioproc E 16:23–33. doi:10.1007/s12257-010-0083-2
Sakaki H, Nakanishi T, Tada A, Miki W, Komemushi S (2001) Activation of torularhodin production by Rhodotorula glutinis using weak white light irradiation. J Biosci Bioeng 92:294–297. doi:10.1016/S1389-1723(01)80265-6
Salar RK, Certik M, Brezova V, Brlejova M, Hanusova V, Breierová E (2013) Stress influenced increase in phenolic content and radical Scavening capacity of Rhodotorula glutinis CCY 20-2-26. 3. Biotech 3:53–60. doi:10.1007/s13205-012-0069-1
Schneider T, Graeff-Hönninger S, French WT, Hernandez R, Merkt N, Claupein W, Hetrick M, Pham P (2013) Lipid and carotenoid production by oleaginous red yeast Rhodotorula glutinis cultivated on brewery effluents. Energ J 61:34–43. doi:10.1016/j.energy.2012.12.026
Somashekar D, Joseph R (2000) Inverse relationship between carotenoid and lipid formation in Rhodotorula gracilis according to the C/N ratio of the growth medium. World J Microb Biot 16:491–493. doi:10.1023/A:1008917612616
Sunja C, Lee D, Luong TT, Park S, Oh Y, Lee T (2011) Effects of carbon and nitrogen sources on fatty acid contents and composition in the green microalga, Chlorella sp. 227. J Microb Biot 21:1073–1080. doi:10.4014/jmb.1103.03038
Vieira ÉD, Andrietta MGS, Andrietta SR (2013) Yeast biomass production: a new approach in glucose-limited feeding strategy. Braz J Microbiol 44:551–558. doi:10.1590/S1517-83822013000200035
Wang QM, Yurkov AM, Göker M, Lumbsch HT, Leavitt SD, Groenewald M, Theelen B, Liu XZ, Boekhout T, Bai FY (2015) Phylogenetic classification of yeasts and related taxa within Pucciniomycotina. Stud Mycol 81:149–189. doi:10.1016/j.simyco.2015.12.002
Wiebe MG, Koivuranta K, Penttilä M, Ruohonen L (2012) Lipid production in batch and fed-batch cultures of Rhodosporidium toruloides from 5 and 6 carbon carbohydrates. BMC Biotechnol 12:1–10. doi:10.1186/1472-6750-12-26
Yen HW, Zhang Z (2011) Enhancement of cell growth rate by light irradiation in the cultivation of Rhodotorula glutinis. Bioresour Technol 102:9279–9281. doi:10.1016/j.biortech.2011.06.062
Zhang Z, Zhan X, Tan T (2014) Lipid and carotenoid production by Rhodotorula glutinis under irradiation/high-temperature and dark/low-temperature cultivation. Bioresour Technol 157:149–153. doi:10.1016/j.biortech.2014.01.039
Acknowledgments
The authors acknowledge the financial support from grants VEGA 1/0574/15 and APVV-0662-11.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tkáčová, J., Čaplová, J., Klempová, T. et al. Correlation between lipid and carotenoid synthesis in torularhodin-producing Rhodotorula glutinis . Ann Microbiol 67, 541–551 (2017). https://doi.org/10.1007/s13213-017-1284-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-017-1284-0