Abstract
Quercetin is one of the most abundant dietary flavonoids and one of the most studied antioxidants. In this work, the effect of quercetin on stress resistance of exponentially growing Saccharomyces cerevisiae cells was estimated. Pre-treatment with low concentrations (10 μM and 100 μM) of quercetin enhanced yeast viability under exposure to hydrogen peroxide, copper ions (Cu2+), and heat shock. The stress-protective effects of quercetin were not connected with involvement of oxidative stress regulator Yap1p, or with induction of endogenous antioxidant defense. However, pre-treatment of wild type and ∆yap1 strains with quercetin prevented protein oxidation and antioxidant enzyme inactivation in yeast cells under H2O2-induced oxidative stress. Cycloheximide—an inhibitor of mRNA translation—or a deficiency in the transcriptional regulators Msn2p and Msn4p abolished the protective effects of low concentrations of quercetin against H2O2 toxicity. It was shown in vitro that quercetin can scavenge H2O2 and produce low amounts of H2O2 in a pH-dependent manner. The antioxidant-related and antioxidant-independent mechanisms of quercetin effects are discussed.
Similar content being viewed by others
Introduction
Production of reactive oxygen species (ROS) is a part of normal aerobic cellular metabolism. These species, such as superoxide anion radical (O2 •−), hydrogen peroxide (H2O2) and hydroxyl radical (HO•), are potentially dangerous due to their high reactivity and capability to interact with virtually all cellular components, including proteins, lipids, nucleic acids, and carbohydrates. To protect themselves against ROS-induced damage, cells possess effective defense mechanisms, including high molecular mass antioxidants, such as the enzymes superoxide dismutase (SOD), catalase, and several types of peroxidases, as well as low molecular mass antioxidants such as ascorbate, tocopherol and glutathione. Under certain circumstances, the balance between generation and elimination of ROS by the antioxidant defense system is disturbed, leading to an increase in ROS levels causing so-called “oxidative stress” (Lushchak 2011, 2014). This imbalance is associated with aging and numerous diseases, including cancer, cardiovascular and neurodegenerative diseases (Halliwell and Gutteridge 1989; Lushchak 2011, 2014). The interest in natural antioxidants preventing ROS-induced oxidative damage in living organisms has increased steadily during the last few decades (Prior 2003; Landete 2013).
Plants are potential sources of natural antioxidants, particularly phenolic compounds, such as phenolic acids, flavonoids, and tannins (Dai and Mumper 2010). Flavonoids are among the most studied phytochemicals found in plant foods and including a large number of different molecules with various biological activities (Prior 2003; Dai and Mumper 2010; Landete 2013). They have chemical structures favoring protection against ROS via chelation of metal ions, direct or indirect interaction with ROS, and inhibition of ROS-producing enzymes (Andrade et al. 2005; Dai and Mumper 2010). Recently, flavonoids were found to exert not only antioxidant, but also prooxidant properties and to increase cellular antioxidant capacity via activation of redox-sensitive transcriptional factors such as Yap1 in yeast and Nrf2 in animals (Maeta et al. 2007; Lushchak 2011; Bayliak et al. 2014; Kim et al. 2014).
The flavonol quercetin (3,3′,4′,5,7-pentahydroxyflavone) is one of the most abundant dietary flavonoids being found ubiquitously in onions, shallots, apples, berries, grapes, capers, cruciferous vegetables, tea, and red wine (Scalbert and Williamson 2000). The antioxidant, anti-inflammatory, neuroprotective, and anticarcinogenic effects of quercetin are well documented (Prior 2003; Boots et al. 2008; Dajas 2012; Russo et al. 2012). Quercetin has been studied extensively in many biological models, such as the nematode Caenorhabditis elegans (Pietsch et al. 2011), mammalian cell cultures (Suematsu et al. 2011), mice (Alam et al. 2014) and rats (Özyurt et al. 2014), and also to some extent in humans (Guo et al. 2014). Some beneficial effects of quercetin were reported for the yeast Saccharomyces cerevisiae. For instance, it was found that a short-term pre-treatment with quercetin increased life span and oxidative stress resistance (Belinha et al. 2007). As an antioxidant, quercetin also prevented translocation of the yeast transcription factor Yap1p into the nucleus under oxidative stress conditions (Bednarska et al. 2008). It was also reported that quercetin might act as a modulator of cellular signaling pathways related to carbohydrate metabolism and cell integrity to exert its protective effects against ROS in S. cerevisiae (Vilaça et al. 2012). Taking these facts into account, this work aimed to study the protective effects of quercetin against different stressors and their potential relationship with oxidative stress markers in S. cerevisiae cells. In addition, we investigated whether the biological effects of quercetin were associated with up-regulation of antioxidant enzymes, and studied the involvement of the transcriptional factors Msn2/Msn4p and Yap1p in this up-regulation.
Materials and methods
Strains and growth conditions
The following S. cerevisiae strains were used in this study: YPH250 (MATa trp1-Δ1 his3-Δ200 lys2-801 leu2-Δ1 ade2-101 ura3-52) and its isogenic mutant Δyap1 (as YPH250 but yap1-∆1::HIS3) kindly provided by Dr. Y. Inoue (Kyoto University, Kyoto, Japan); W303-1A (MATa leu2-3,112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15) and its isogenic mutant Δmsn2Δmsn4 (as W303-1A but msn2::HIS3 msn4::TRP1) kindly provided by Dr K. Kuchler (Vienna University, Vienna, Austria). Cells were grown at 28 °C with shaking at 175 rpm in liquid medium containing 1 % yeast extract, 2 % peptone, 2 % glucose (YPD). The initial cell concentration in the growth medium was about 0.3 × 106 cells/mL.
Stress treatment
Exponential-phase cells were harvested after cultivation for 15 h and resuspended in fresh medium (pH 6.0) containing 0.5 % glucose (Maeta et al. 2007). The resulting cell suspension was divided into three aliquots, which were then treated with 10 μM and 100 μM quercetin [from 100 mM stock solution of quercetin diluted in dimethylformamide (DMF)] or with DMF (volume identical to the volume of quercetin solution added) for 2 h. To distinguish the effects of quercetin that depend on gene expression, yeast cells were pre-treated with 10 μM quercetin in the presence of cycloheximide (CH)—an inhibitor of eukaryotic translation—which was added to a final concentration of 100 μg/mL (Gerashchenko and Gladyshev 2014). The cells were then harvested, washed and re-suspended in equal volume of 50 mM potassium phosphate buffer (pH 7.0). Aliquots of the experimental cultures were exposed for 1 h to the following stressors: 10 mM H2O2, 2 mM CuSO4, or heat shock (41 °C). Control cells (with DMF) were incubated under the same conditions but without stressors. Cell survival after stress exposure was monitored by counting of dead cells stained by methylene blue (Smart et al. 1999) or colony-forming units (CFU) on YPD agar plates.
Assay of metabolic activity
Yeast suspensions containing 3 × 108 cells were harvested by centrifugation at 3000 g for 5 min and washed twice with distilled water as described above. The yeast pellets were resuspended in 1 mL 50 mM potassium phosphate buffer (pH 7.0) and mixed with 0.35 mL 0.5 % 2,3,5-triphenyl tetrazolium chloride. Metabolically active cells are capable of reducing the dye to water-insoluble red formazan, which was extracted from the cells with ethanol:acetone mixture (2:1); the absorbance of this solution was measured at 485 nm (Conconi et al. 2000). The metabolic activity was expressed as arbitrary units of optical density at 485 nm per 108 cells.
Preparation of cell-free extracts, assay of enzyme activities, protein carbonyls, total antioxidant capacity, and protein concentration
The parameters were measured spectrophotometrically using Spekol 211 (Zeiss, Jena, Germany) and SF-46 (LOMO, St. Petersburg, Russia). The preparation of cell extracts, measurement of SOD and catalase activities were conducted as described earlier (Lushchak et al. 2005).
The content of carbonyl groups in proteins (CP) was measured by determining the amount of 2,4-dinitrophenylhydrazone formed upon the reaction with 2,4-dinitrophenylhydrazine (Lushchak et al. 2005). Carbonyl content was calculated from the absorbance maximum of 2,4-dinitrophenylhydrazone at 370 nm with molar extinction coefficient of 22 000 M−1 cm−1. The values are expressed as nanomoles of CP per milligram protein.
The total antioxidant capacity (TAC) of supernatants was measured by 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) assay (Erel 2004). The rate of decrease in ABTS•+ absorbance at wavelength 414 nm in the presence of supernatant was monitored using a Labsystems Multiskan MCC/340 (Labsystems, Nelsirrki, Finland). Solutions of Trolox in a concentration range from 0.015 to 0.180 mM were used as standards for the calibration curve.
Protein concentration was determined by Bradford (1976) based on binding of Coomassie Brilliant Blue G-250 dye with protein. Bovine serum albumin was used as a standard.
Assay of low-molecular mass thiols and disulfides
For determination of low-molecular mass thiols (L-SH), aliquots of the cell-free extracts were mixed with trichloroacetic acid to a final concentration of 10 %, centrifuged (16,000 g, 5 min) to remove pellet protein and the resulting supernatants were used for assay. Low-molecular mass thiol-containing compounds were measured in cellular extracts by the light absorption of thiol conjugates with 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) at 412 nm (Ellman 1959). The concentration of reduced thiol groups was measured after incubation of 60 μL supernatant in solution containing 50 μM DTNB in 100 mM potassium phosphate buffer (pH 8.0) for 30 min at room temperature. To measure total thiol content, disulfide bonds in samples were reduced as follows: aliquots of the supernatant were incubated for 30 min with 50 μM DTNB in 100 mM Tris-HCl buffer (pH 10.5) and total low molecular mass thiol groups, reduced thiols and disulfides (L-S2) were determined (Robyt et al. 1971). Corresponding blank samples (without supernatant) were used in all cases. Thiol levels were expressed as nanomoles of SH-groups per milligram of protein. The levels of L-S2 were calculated by subtracting the L-SH concentration from total thiol concentration. The thiol-disulfide ratio was calculated by dividing the L-SH level by the L-S2 level.
Assay of H2O2-scavenging and H2O2-producing activities of quercetin
To determine the H2O2-scavenging activity of quercetin, a mixture containing 10 mM H2O2, 10 μM or 100 μM quercetin, and 50 mM potassium phosphate buffer (pH 6.0) was incubated for 30 min at 28 °C whereas quercetin was omitted in the control sample. After incubation, the samples were diluted 100-fold and 200 μL diluted sample was mixed with 1.8 mL FOX reagent (250 μL FeSO4, 25 mM H2SO4, 100 μL xylenol orange and 100 mM sorbitol) according to the previously described FOX method (Nourooz-Zadeh et al. 1994). The reaction mixture was then vortexed and incubated at room temperature for 30 min. The absorbance of the ferric-xylenol orange complex was measured at 580 nm. Solutions of H2O2 of known concentration were used as standards. The percentage of hydrogen peroxide scavenged by quercetin was calculated as follows: % Scavenged [H2O2] = [(A0 − A1)/A0] × 100, where A0 was the absorbance of the control and A1 was the absorbance in the presence of quercetin.
To estimate quercetin H2O2-generating activity, solutions of 10 μM or 100 μM quercetin in 50 mM potassium phosphate buffer at pH 6.0 and 7.5 were incubated for 30 min at 28 °C. After incubation, the concentration of H2O2 in samples was determined by the FOX method as described above.
Statistical analysis
Experimental data are expressed as mean of four to six independent experiments ± the standard error of the mean (SEM), and statistical analysis used Student’s t-test.
Results and discussion
Quercetin increases yeast viability under stress exposure
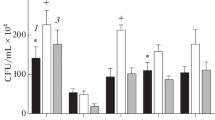
Quercetin is one of the most common flavonoids in the diet. Its antioxidant and anti-inflammatory properties have been studied extensively in animals and in vitro (Prior 2003; Boots et al. 2008; Dajas 2012; Russo et al. 2012). In this work, the unicellular yeast S. cerevisiae was used as a eukaryotic model organism to investigate the stress-protective effects of quercetin at the cellular level. We examined the effects of quercetin at different concentrations on resistance of exponentially growing S. cerevisiae YPH250 cells to several stressors, including hydrogen peroxide, metal ions and heat. Yeast cells were pre-incubated with quercetin for 2 h and then exposed to 10 mM H2O2, 2 mM Cu2+, and heat shock (41 °C). The number of live cells in yeast cultures incubated without quercetin but with solvent (DMF) only, was about 80 ± 5 % while this number in untreated cell suspension (without DMF) was 93 ± 5 %. Therefore, DMF had a certain toxicity for yeast cells. However, only 28 %, 44 % and 26 % live cells remained in cultures pre-treated with DMF and then exposed to 10 mM H2O2, heat shock and 2 mM Cu2+, respectively (Fig. 1). Pre-treatment with quercetin increased cell survival under lethal stress exposure, but the effective concentration of quercetin differed for particular stressors. Pre-incubation with 10 μM and 100 μM quercetin enhanced the viability of the cells by 22 % under H2O2 treatment. Pre-treatment with 10 μM quercetin had little protective effect on cells treated with 2 mM Cu2+ or heat shock. However, 30 % and 20 % more cells survived after heat shock and 2 mM Cu2+ treatment, respectively, when pre-treated with 100 μM quercetin. The protective effect against Cu2+ can be associated partly with the chelating activity of quercetin (Dai and Mumper 2010). Although it is highly possible that other defense mechanisms are also involved. The concentration of Cu2+ used in the current work was quite high and could not be chelated by quercetin at the 10-fold lower concentration. In general, the results suggest that quercetin induces cross-adaptation to different stressors in the yeast S. cerevisiae in a dose-dependent manner.
Effect of different stressors on viability of Saccharomyces cerevisiae YPH250 cells after pre-incubation with quercetin. Cells growing exponentially in YPD medium were pre-incubated with quercetin or dimethylformamide (DMF; control for quercetin treatment) for 2 h, and were then harvested and resuspended in 50 mM potassium phosphate buffer (pH 7.0) and exposed to different stressors for 1 h at 28 °C. The total number of cells in each yeast suspension was set as 100 %. * Significantly different from respective values of cultures without quercetin (with DMF); # significantly different from respective values of control cultures (without stressors) with P < 0.05. Results shown are mean ± SEM (n = 5–6)
Msn2/Msn4 but not Yap1 proteins may mediate the stress-protective effects of quercetin
The ability of quercetin to increase oxidative stress resistance in yeast was demonstrated earlier. Particularly, it was shown that quercetin can act as an antioxidant in yeast (Belinha et al. 2007) or can influence some signaling pathways (Vilaça et al. 2012). Transcription regulators Yap1p and Msn2/4p play a pivotal role in the yeast adaptive response to oxidative stress (Lushchak 2010, 2011). However, these transcription factors were found to play little role in the adaptogenic effects of quercetin (Vilaça et al. 2012). At the same time, yeast strains with different genetic backgrounds may differ in stress resistance and adaptive capabilities (Abrat et al. 2007; Semchyshyn 2014; Homza et al. 2014). Taking this into account, we used yeast strains deficient in genes MSN2/4 and YAP1 to establish role of the proteins they encode in the stress-protective effects of quercetin. The current study used strain ∆msn2∆msn4—a derivative of wild-type S. cerevisiae strain W303-1A. Since wild type strains may differ in stress resistance (Bayliak et al. 2006, 2014; Abrat et al. 2007), we first examined survival under stress exposure in the parental W303-1A strain. Resistance of W303-1A to stressors investigated was similar to that of strain YPH250 (Fig. 2). Hydrogen peroxide decreased survival of the control (with DMF) and the sample pre-incubated with 10 μM quercetin W303-1A cells by 72 % and 53 %, respectively. Thus, quercetin at a concentration of 10 μM enhanced the resistance of W303-1A cells to 10 mM H2O2. The ∆msn2∆msn4 mutant was more susceptible to hydrogen peroxide than the parental W303-1A strain. At the same time, pre-incubation of ∆msn2∆msn4 cells with 10 μM quercetin did not affect yeast resistance to H2O2. The ∆yap1 strain is an isogenic derivative strain of YPH250, for which we observed the positive effects of quercetin described above. The sensitivity of Δyap1 mutant to H2O2 was 2.5-fold higher than that of the parental strain (Fig. 2), which could be expected because Yap1p regulates yeast resistance to hydrogen peroxide (Jamieson 1998; Lushchak 2010). Pre-incubation with 10 μM quercetin increased the resistance of ∆yap1 cells to H2O2 similarly to the parental strain. Hence, unlike transcription factors Msn2/4p, Yap1p is not involved in the protective effects of quercetin to H2O2. Similar results were obtained by other authors (Bednarska et al. 2008; Vilaça et al. 2012).
Effect of hydrogen peroxide on the survival of exponentially growing S. cerevisiae YPH250 (wt) and ∆yap 1, W303-1A (wt) and ∆msn2∆ msn4 cells after pre-incubation with quercetin. Cells growing exponentially in YPD medium were pre-incubated with quercetin or DMF for 2 h, and were then harvested and resuspended in 50 mM potassium phosphate buffer (pH 7.0) and exposed to 10 mM H2O2 for 1 h at 28 °C, n = 4–5. The percent of colony forming units (CFUs) was calculated as the ratio of the number of cells forming colonies to the total number of cells plated. * Significantly different from respective control values (without quercetin); # significantly different from respective control values of parental strain (wt) with P < 0.05. Results shown are mean ± SEM (n = 4–5)
Quercetin does not induce endogenous antioxidant defense, but prevents protein oxidation under stress exposure
Stressors used in this study affect cells in different ways but all may increase ROS production directly or indirectly and subsequently lead to oxidative modification of biomolecules (Lushchak 2011). Protection against stress conferred by quercetin at low concentrations could be associated with activation of antioxidant defense and a decrease in uncontrolled oxidation. Therefore, in the next part of our study, we measured oxidative stress indices and total metabolic activity in yeast cells.
Total metabolic activity was 1.5- and 2.0-fold higher in YPH250 cells pre-incubated with 10 μM and 100 μM quercetin, respectively, compared to control cells (Fig. 3a). At the same time, pre-incubation with quercetin did not influence the metabolic activity of ∆yap1 cells. Metabolic activity was measured by reduction of the colorless tetrazolium salt (ТТС), yielding red formazan. The reaction occurs in the mitochondria where TTC is reduced at different sites along the electron-transport chain (Conconi et al. 2000). In fact, metabolic activity reflects the intensity of redox processes in cells. It can be assumed, therefore, that in wild type cells pretreated with quercetin, redox processes proceed more actively than in cells under control treatment (with DMF). Intensity of redox processes is connected closely with ROS production and antioxidant system capacity in cells (Lushchak 2011). Surprisingly, we found a 15 % decrease in total antioxidant capacity (TAC) measured by a reduction of ABTS•+-radical in supernatants from YPH250 cells pre-treated with quercetin (Fig. 3b). Quercetin exposure did not affect TAC in ∆yap1 cells, although this parameter was 6-fold lower than in the parental strain. Treatment with 10 μM quercetin decreased TAC by 12 % in the ∆msn2∆msn4 mutant (4.26 ± 0.12 versus 3.77 ± 0.17 μM Trolox equivalents in control cells and cells pre-treated with quercetin, respectively) as observed in the wild-type strain. Similar results were obtained regarding levels of low molecular mass thiols (L-SH). Thiols are important reductants that can interact with ROS directly or mediate their elimination in coupled reactions. The cysteine-containing tripeptide glutathione is the most important endogenous antioxidant among L-SHs (Lushchak 2012). Pre-treatment with 10 μM quercetin did not affect the level of L-SH in YPH250 and ∆yap1 cells, but decreased levels in YPH250 cells treated with 100 μM quercetin (Fig. 3c). The ratio between reduced and oxidized levels of thiols (L-SH/L-S2) was similar in YPH250 and ∆yap1 cells, at 5.8 ± 1.0 and 5.1 ± 1.3, respectively, in control conditions. This did not change upon exposure to 10 μM quercetin in ∆yap1 cells and was increased by 55 % in YPH250 cells compared to untreated cells (Fig. 3d). Pre-incubation with quercetin did not influence either the activities of catalase and SOD, or the level of carbonyl proteins in YPH250 cells, only that catalase activity was ~25 % lower in cells incubated with 100 μM quercetin in comparison with the control (Fig. 4b). Thus, quercetin at low concentrations does not increase the antioxidant potential of yeast cells and, moreover, it may attenuate endogenous antioxidant defense in certain cases. In addition, the deficiency in Yap1p diminished the antioxidant and metabolic capacity of yeast cells but quercetin still displayed a stress-protective effect on these mutant cells. Next, we analyzed the effects of oxidative and heat stresses on the oxidative stress parameters in YPH250 cells pre-treated with quercetin. In the case of H2O2 exposure, SOD activity did not differ in the control and in cells pre-treated with 10 μM quercetin, but was lower in cells pre-treated with 100 μM quercetin (Fig. 4a). Heat stress increased SOD activity by 1.7, 1.6 and 2.1 times in the control and in cells pre-treated with 10 μM and 100 μM quercetin, respectively. Increase in SOD activity could result from elevated production of ROS, namely superoxide anion-radical, as was observed earlier in S. cerevisiae subjected to heat shock (Kim et al. 2006). Wild-type YPH250 cells that were not incubated with quercetin showed 65 % and 72 % lower catalase activity after exposure to H2O2 and heat shock, respectively (Fig. 4b). This may have been due to the oxidative inactivation of this enzyme as was already shown in our previous works (Bayliak et al. 2006; Lushchak 2006; Semchyshyn and Lozinska 2012). Pre-treatment with quercetin at both concentrations used prevented a decrease in catalase activity under H2O2-stress and heat shock.
(a) Metabolic activity, (b) total antioxidant capacity, (c) levels of low molecular mass thiols (L-SH) and (d) ratio of reduced/oxidized thiols (L-SH/L-S2) in exponentially growing S. cerevisiae cells after pre-incubation with quercetin for 2 h. * Significantly different from respective control values (without quercetin) with P < 0.05. #Significantly different from respective values of YPH250 strain (wt) with P < 0.05. Results are mean ± SEM (n = 4)
Effect of exposure to 10 mM H2O2 and heat shock (41 °C) for 1 h on (a) superoxide dismutase (SOD) activity, (b) catalase activity and (c) level of carbonyl proteins in exponentially growing S. cerevisiae YPH250 cells after pre-incubation with quercetin in the presence or the absence of cycloheximide (CH, 100 μg/mL) for 2 h. * Significantly different from respective values in control cultures (without quercetin); # significantly different from respective values of untreated cells (without stressors); δ significantly different from respective values in group “10 mM H2O2 + CH” with P < 0.05. Results are mean ± SEM (n = 4–6)
The content of carbonyl groups in proteins was taken as a marker of ROS-induced protein oxidation. Oxidation of several amino acid residues—cysteine, histidine and some others—by ROS results in the formation of additional carbonyl groups in proteins. Carbonyl groups are used commonly to evaluate the intensity of ROS-induced modifications in proteins (Lushchak 2006). Upon exposure to hydrogen peroxide and heat shock, the content of carbonyl groups in proteins was 2.8-fold and 2.0-fold higher, respectively, in the control (Fig. 4c). However, pre-treatment with 10 and 100 μM quercetin abolished the increase in CP level induced by the stressors.
Taken together, our results suggest that pre-treatment with quercetin increased stress resistance of yeast cells via prevention of oxidation of proteins, including antioxidant enzymes. Quercetin does not stimulate, but may rather inhibit, the first line of antioxidant defense such as catalase. This might indicate that quercetin may act in yeast via a mechanism that does not require activation of endogenous antioxidant defense. To test this assumption, we investigated the ability of quercetin to scavenge H2O2 in vitro. As seen in Table 1, after 60 min of incubation with 10 μM and 100 μM quercetin, the concentration of 10 mM H2O2 decreased by 10 % and 70 %, respectively. Thus, the effects of 100 μM quercetin can be accounted for by its antioxidant activity. That 10 μM quercetin exhibits only antioxidant activity is doubtful. The latter is confirmed by our experiments with cycloheximide—an inhibitor of protein synthesis. In this case, yeast cells pre-treated with 10 μM quercetin in the presence of cycloheximide showed an increase in the level of oxidized proteins (Fig. 4c) and a decrease in catalase activity (Fig. 4b) under H2O2-exposure at a level similar to that of YPH250 cells that were not incubated with quercetin. This disappearance of the protective effects of quercetin after cycloximide treatment suggests that quercetin may act via certain signaling pathways requiring protein synthesis. Our results regarding the survival of different yeast mutants after stress (Fig. 2) indicate the involvement of the transcriptional regulators Msn2/Msn4p but not Yap1 in the stress-protective effects of quercetin. To verify this, we measured the level of oxidized proteins and the activity of catalase in ∆yap1 and ∆msn2∆msn4 cells pre-treated with 10 μM quercetin and subsequently exposed to 10 mM H2O2. The stress response of the ∆yap1 mutant was similar to that of the parental YPH250 strain (Table 2). Treatment with 10 mM H2O2 led to a 1.4-fold decrease in catalase activity and a 1.6-fold increase in CP level in control ∆yap1 cells. Pre-treatment with 10 μM quercetin prevented these H2O2-induced changes in ∆yap1 cells. This confirmed our suggestion that Yap1 does not mediate the stress-protective effect of quercetin. At the same time, we did not observe the stress-protective action of quercetin in the ∆msn2∆msn4 mutant (Table 2). Hydrogen peroxide caused a decrease in catalase activity and an increase in the level of CP in ∆msn2∆msn4 cells under control conditions and after pre-treatment with quercetin. It is worth noting that catalase activity demonstrated a steeper decline upon H2O2 exposure in ∆msn2∆msn4 cells as compared with ∆yap1 cells. This fact can be explained by the influence of the genetic background, which differed in the strains investigated, and it also suggests that proteins Msn2 and Msn4 may play a more important protective role against exposure to high H2O2 concentrations as it was shown earlier (Hasan et al. 2002). In general, the absence of the protective effects of quercetin in strain ∆msn2∆msn4 suggests that Msn2/Msn4 proteins are necessary for the stress-protective action of quercetin in yeast. Quercetin may act by activating Msn2/Msn4 proteins via its pro-oxidant properties. We measured the production of H2O2 by quercetin in 50 mM potassium phosphate buffer at pH 6.0 and pH 7.5 during 30 min (Table 1): 100 μM quercetin generated 0.89 ± 0.48 and 31.7 ± 2.5 μM H2O2 during this time at pH 6.0 and pH 7.5, respectively. Thus, quercetin can produce very low amounts of H2O2 in culture medium (pH 6.0), but this effect is increased after penetration of quercetin into cells, where the pH is close to neutral. Hydrogen peroxide at low concentrations can induce adaptive responses via different transcriptional regulators, such as Msn2p/Msn4p (Hasan et al. 2002). The fact that the transcriptional regulator Yap1p, a main regulator of the response to H2O2 in yeast species, was not involved can be explained by assuming that quercetin maintains this protein in reduced form, preventing its translocation into the nucleus (Bednarska et al. 2008).
In summary, the results presented here suggest that quercetin increases stress resistance in the yeast S. cerevisiae via both antioxidant-related and antioxidant-independent mechanisms. Quercetin at high concentrations can act as an antioxidant, providing partial protection to proteins against ROS-induced modification under heat shock and oxidative stress exposure. Simultaneously, the stress-protective action of quercetin at low concentrations is connected with the involvement of the transcription factors Msn2/Msn4p, but not Yap1p. Taken together, these findings suggest that stress resistance conferred by quercetin can be accounted for by changes in gene expression that does not include major components of antioxidant defense.
References
Abrat OB, Semchyshyn HM, Lushchak VI (2007) Acid stress increases the activity of superoxide dismutase and catalase in the yeast Saccharomyces cerevisiae. Ukr Biokhim Zh 79(2):17–23
Alam MM, Meerza D, Naseem I (2014) Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci 109(1):8–14
Andrade RG Jr, Dalvi LT, Silva JM Jr, Lopes GK, Alonso A, Hermes-Lima M (2005) The antioxidant effect of tannic acid on the in vitro copper-mediated formation of free radicals. Arch Biochem Biophys 437(1):1–9
Bayliak M, Semchyshyn H, Lushchak V (2006) Effect of hydrogen peroxide on antioxidant enzyme activities in Saccharomyces cerevisiae is strain-specific. Biochemistry (Mosc) 71:1013–1020
Bayliak MM, Burdyliuk NI, Izers’ka LI, Lushchak VI (2014) Concentration-dependent effects of Rhodiola rosea on long-term survival and stress resistance of yeast Saccharomyces cerevisiae: the involvement of Yap 1 and Msn2/4 regulatory proteins. Dose Response 11(1):93–109
Bednarska S, Leroy P, Zagulski M, Bartosz G (2008) Efficacy of antioxidants in the yeast Saccharomyces cerevisiae correlates with their effects on protein thiols. Biochimie 90(10):1476–1485
Belinha I, Amorim MA, Rodrigues P, De Freitas V, Moradas-Ferreira P, Mateus N, Costa V (2007) Quercetin increases oxidative stress resistance and longevity in Saccharomyces cerevisiae. J Agric Food Chem 55:2446–2451
Boots AW, Haenen GR, Bast A (2008) Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 585(2-3):325–337
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72:248–254
Conconi A, Jager-Vottero P, Zhang X, Beard BC, Smerdon MJ (2000) Mitotic viability and metabolic competence in UV-irradiated yeast cells. Mutat Res 459:55–64
Dai J, Mumper RJ (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15(10):7313–7352
Dajas F (2012) Life or death: neuroprotective and anticancer effects of quercetin. J Ethnopharmacol 143(2):383–396
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Erel O (2004) A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 37:277–285
Gerashchenko MV, Gladyshev VN (2014) Translation inhibitors cause abnormalities in ribosome profiling experiments. Nucleic Acids Res 42(17):e134. doi:10.1093/nar/gku671
Guo Y, Mah E, Bruno RS (2014) Quercetin bioavailability is associated with inadequate plasma vitamin C status and greater plasma endotoxin in adult. Nutrition 30(11-12):1279–1286
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine, 2nd edn. Oxford University Press, London
Hasan R, Leroy C, Isnard AD, Labarre J, Boy-Marcotte E, Toledano MB (2002) The control of the yeast H2O2 response by the Msn2/4 transcription factors. Mol Microbiol 45(1):233–241
Homza BV, Vasyl'kovs'ka RA, Semchyshyn HM (2014) Defects in TOR regulatory complexes retard aging and carbonyl/oxidative stress development in yeast Saccharomyces cerevisiae. Ukr Biokhim Zh 86(1):85–92
Jamieson DJ (1998) Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast 14:1511–1527
Kim IS, Moon HY, Yun HS, Jin I (2006) Heat shock causes oxidative stress and induces a variety of cell rescue proteins in Saccharomyces cerevisiae KNU5377. J Microbiol 44:492–501
Kim HS, Quon MJ, Kim JA (2014) New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol 2:187–195
Landete JM (2013) Dietary intake of natural antioxidants: vitamins and polyphenols. Crit Rev Food Sci Nutr 53(7):706–721
Lushchak VI (2006) Budding yeast Saccharomyces cerevisiae as a model to study oxidative modification of proteins in eukaryotes. Acta Biochim Pol 53:679–684
Lushchak VI (2010) Oxidative stress in yeast. Biochemistry (Mosc) 75:281–296
Lushchak VI (2011) Adaptive response to oxidative stress: bacteria, fungi, plants and animals. Comp Biochem Physiol C. Toxicol Pharmacol 153:175–190
Lushchak VI (2012) Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids 2012: Article ID 736837. doi:10.1155/2012/736837
Lushchak VI (2014) Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 224:164–175
Lushchak V, Semchyshyn H, Mandryk S, Lushchak O (2005) Possible role of superoxide dismutases in the yeast Saccharomyces cerevisiae under respiratory conditions. Arch Biochem Biophys 441:35–40
Maeta K, Nomura W, Takatsume Y, Izawa S, Inoue Y (2007) Green tea polyphenols function as prooxidants to activate oxidative-stress-responsive transcription factors in yeasts. Appl Environ Microbiol 73:572–580
Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolff SP (1994) Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal Biochem 220:403–409
Özyurt H, Çevik Ö, Özgen Z, Özden AS, Çadırcı S, Elmas MA, Ercan F, Gören MZ, Şener G (2014) Quercetin protects radiation-induced DNA damage and apoptosis in kidney and bladder tissues of rats. Free Radic Res 48(10):1247–1255
Pietsch K, Saul N, Chakrabarti S, Stürzenbaum SR, Menzel R, Steinberg CE (2011) Hormetins, antioxidants and prooxidants: defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extension in C. elegans. Biogerontology 12(4):329–347
Prior RL (2003) Fruits and vegetables in the prevention of cellular oxidative damage. Am J Clin Nutr 78(3 Suppl):570S–578S
Robyt JF, Ackerman RJ, Chittenden CG (1971) Reaction of protein disulfide groups with Ellman's reagent: a case study of the number of sulfhydryl and disulfide groups in Aspergillus oryzae -amylase, papain, and lysozyme. Arch Biochem Biophys 147(1):262–269
Russo M, Spagnuolo C, Tedesco I, Bilotto S, Russo GL (2012) The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochem Pharmacol 83(1):6–15
Scalbert A, Williamson G (2000) Dietary intake and bioavailability of polyphenols. J Nutr 130:2073S–2085S
Semchyshyn HM (2014) Hormetic concentrations of hydrogen peroxide but not ethanol induce cross-adaptation to different stresses in budding yeast. Int J Microbiol 2014: Article ID 485792. doi:10.1155/2014/485792
Semchyshyn HM, Lozinska LM (2012) Fructose protects baker's yeast against peroxide stress: potential role of catalase and superoxide dismutase. FEMS Yeast Res 12:761–773
Smart K, Chambers K, Lambert I, Jenkins C (1999) Use of methylene violet staining procedures to determine yeast viability and vitality. J Am Soc Brew Chem 57:18–23
Suematsu N, Hosoda M, Fujimori K (2011) Protective effects of quercetin against hydrogen peroxide-induced apoptosis in human neuronal SH-SY5Y cells. Neurosci Lett 504(3):223–227
Vilaça R, Mendes V, Mendes MV, Carreto L, Amorim MA, de Freitas V, Moradas-Ferreira P, Mateus N, Costa V (2012) Quercetin protects Saccharomyces cerevisiae against oxidative stress by inducing trehalose biosynthesis and the cell wall integrity pathway. PLoS One 7(9):e45494
Acknowledgments
We are grateful to Drs. Y. Inoue and K. Kuchler for the providing S. cerevisiae strains, our students I. Pavlykivskyy, M. Lylyk and K. Voroshylo for their technical assistance, and Dr. D. Gospodaryov for English editing of the manuscript. The work was supported partially by a Ministry of Education and Science of Ukraine grant (#0106U002245) to V.I.L.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bayliak, M.M., Burdylyuk, N.I. & Lushchak, V.I. Quercetin increases stress resistance in the yeast Saccharomyces cerevisiae not only as an antioxidant. Ann Microbiol 66, 569–576 (2016). https://doi.org/10.1007/s13213-015-1136-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-015-1136-8