Abstract
This research was conducted to evaluate the impact of co-fermentation with Saccharomyces cerevisiae and Williopsis saturnus var. mrakii on the volatile profile of cider. Cider co-fermentation was carried out by co-inoculating S. cerevisiae and W. saturnus var. mrakii at a ratio of 1:100. Changes in yeast cell population, total soluble solid content (degrees Brix [°Bx]), and pH were monitored. Volatiles were analysed using headspace solid-phase microextraction/gas chromatography–mass spectrometry/flame ionization detector (HS-SPME/GC-MS-FID). A diverse group of volatiles, including fatty acids, alcohols, aldehydes, esters and ketones, were identified, among which alcohols and esters were the predominant compounds. Although most of these compounds showed similar dynamics of change, the final concentrations of some volatiles differed significantly between single-culture and co-culture fermentations. Volatiles that were indigenous to apple juice decreased during fermentation, while production of isoamyl acetate, 2-phenylethyl acetate, ethyl acetate, ethyl decanoate, ethyl dodecanoate and ethyl octanoate varied significantly among the monocultures and co-culture. Co-fermentation by S. cerevisiae and W. saturnus resulted in a more complex volatile profile, which could impact on the aromatic characteristics of cider, thus representing a novel way to modulate flavour.
Similar content being viewed by others
Introduction
Cider is an alcoholic beverage fermented from apple juice typically using Saccharomyces cerevisiae yeasts, although cider fermentation can occur spontaneously due to the presence of indigenous yeasts derived from the apple fruits or the environment. Industrially, starter cultures are used to carry out cider fermentation for better process and quality control, just like wine. The selected yeasts, commonly strains of S. cerevisiae, are preferred because of their reliability and their ability to induce a fermentation bouquet and to complete fermentation (Heard 1999). However, industrially produced ciders tend to lack flavor differentiation, complexity and character.
In recent years, inclusion of selected non-Saccharomyces yeasts as part of mixed starters together with S. cerevisiae in order to improve wine flavour has been suggested as a way of maximising the benefits of spontaneous fermentation without risk of spoilage (Ciani 1997; Jolly et al. 2003; Ciani and Comitini 2011; Domizio et al. 2011). The contributions of non-Saccharomyces yeasts include the enhancement of wine flavour due to the increased production of glycerol, esters and higher alcohols (Romano et al. 1997; Egli et al. 1998; Granchi et al. 2002; Fleet 2003).
Growth of non-Saccharomyces yeasts is generally limited to the first few days in spontaneous mixed-culture fermentation; they then die off gradually, and strains of S. cerevisiae become dominant until the completion of fermentation (Constanti et al. 1998). Several studies have shown that some non-Saccharomyces yeasts may survive longer than initially thought (Heard and Fleet 1985; Laplace et al. 1998; Zohre and Erten 2002), which would be dependent on the physiological characteristics of yeast species and strains.
The indigenous yeast profile of traditional cider fermentation is similar to that of spontaneously fermented wine, with non-Saccharomyces yeasts appearing initially and Saccharomyces yeasts taking over at a later stage (Laplace et al. 1998; Morrissey et al. 2004; Suarez et al. 2007; Pando et al. 2010). Cider fermentation with monocultures of non-Saccharomyces yeasts and mixed cultures has received little research attention relative to wine. Bilbao et al. (1997) studied cider fermentation with single and mixed cultures of Kloeckera apiculata and S. cerevisiae while Xu et al. (2006) investigated cider fermentation with pure and co-cultures of Hanseniaspora valbyensis and S. cerevisiae. In both reports, inhibition of non-Saccharomyces by Saccharomyces was observed, but volatile evolution during fermentation was not revealed.
Strains of Williopsis saturnus are high producers of fruity acetate esters and have been well characterized in mixed-culture fermentation of grape, mango, longan and papaya wines (Lee et al. 2010; Trinh et al. 2011; Li et al. 2012; Tanguler 2012). Previously, we assessed volatile changes during cider fermentation with three Williopsis yeasts (W. saturnus var. subsufficiens NCYC 2728, W. saturnus var. saturnus NCYC 22 and W. saturnus var. mrakii NCYC 500; Aung et al. 2014). Therefore, the present research was conducted to evaluate volatile evolution during cider co-fermentation with S. cerevisiae and W. saturnus var. mrakii.
Materials and methods
Yeasts and media
Williopsis saturnus var. mrakii NCYC 500 and S. cerevisiae MERIT.ferm were obtained from the National Collection of Yeast Cultures (Norwich, UK) and Chr. Hansen A/S (Hørsholm, Denmark), respectively, and were used in cider co-fermentation. The yeasts were activated in nutrient broth containing 2 % (w/v) glucose, 0.25 % (w/v) yeast extract, 0.25 % (w/v) bacteriological peptone and 0.25 % (w/v) malt extract (pH 5.0). Cultures were incubated statically at 25 °C for 48 h, then dispensed into 1-ml sterile tubes and stored at −80 °C.
Cider co-fermentation
Commercial apple juice was purchased from a local supermarket in Singapore (Marigold® 100 % apple juice; Malaysia Dairy Industries Pte Ltd.). The juice contained 12 % total carbohydrates and 10.4 % sugar, with no added sugar, preservatives or flavouring, according to the manufacturer.
A total of 220 ml of sterile apple juice was fermented in sterile 250-ml conical flasks after inoculation with 2 % (v/v) of 107–108 CFU ml−1 of respective yeast culture pre-grown in the same sterile apple juice (statically at 25 °C for 2 days). Replicate fermentation for single and co-cultures was carried out for 14 days at 20 °C. Fermentation trials included a single culture of S. cerevisiae MERIT.ferm, a single culture of W. saturnus var. mrakii NCYC500, and a co-culture of Saccharomyces and non-Saccharomyces at a ratio of 1:100 (MERIT.ferm:NCYC500). This ratio was chosen to minimise the inhibition of the Williopsis yeast by the Saccharomyces (Lee et al. 2013).
Determination of degrees Brix (°Bx) and pH using a refractometer (ATAGO CO., LTD, Tokyo, Japan) and a pH meter (Metrohm AG, Herisau, Switzerland) was performed for each sample. Yeasts were enumerated using the spread plate method on potato dextrose agar (PDA). The two yeasts from the co-culture were differentiated by their colony appearance (the colony of strain NCYC 500 appeared dull, wrinkled and rough, while the colony of MERIT.ferm was creamy, shiny and smooth).
Analysis of volatiles
Volatiles were analysed according to a procedure that has been described elsewhere (Aung et al. 2014), by means of headspace (HS) solid-phase microextraction (SPME) using a Carboxen/polydimethylsiloxane (PDMS) fibre (85 μm; Supelco, Inc., Bellefonte, PA, USA), which was coupled with an Agilent 7890A gas chromatograph (GC)-5975 Series mass spectrometer (MS) and flame ionisation detector (FID) (Agilent Technologies Inc., Santa Clara, CA, USA). Five millilitres of samples (pre-adjusted to pH 2.5 with 1 M HCl) were tightly capped into a 20-ml glass vial, sealed with a Teflon septum, and extracted using HS-SPME at 60 °C for 30 min (stirred at 250 rpm). The fibre was then inserted into the injection port of the GC set at 250 °C for 3 min to thermally desorb analytes. A capillary column (DB-FFAP; Agilent Technologies Inc.) 60 m in length × 0.25 mm internal diameter, coated with 0.25-μm-thick polyethylene glycol film (modified with nitroterephthalic acid), was used to separate volatiles. The GC oven was programmed at 50 °C for 5 min, then increased to 230 °C at 5 °C min−1, and kept at this temperature for 20 min. The carrier gas was helium, with a pressure of 19.37 psi and a total flow rate of 42.7 ml min−1. The mass spectra of unknown volatiles were compared with those in the Wiley database (Agilent Technologies Inc.), and identification was confirmed with respective linear retention indices. GC/MSD ChemStation software G1701EA (Agilent Technologies Inc.) was employed for data acquisition. Analyses were carried out in duplicate. The data shown represent the mean values from replicate fermentations.
Statistical treatment
Analysis of variance (ANOVA; Microsoft Office Excel 2003) was applied to the day-14 data to determine the differences in volatiles produced by single and mixed cultures. Results were considered significantly different if the associated P value was below 0.05 at a 95 % confidence level. The mean values and standard deviations were obtained from the data acquired with two independent fermentations of each treatment. Standard deviations expressed in this context indicate system errors (two replicate fermentations and duplicate analyses).
Results
Growth of yeasts and changes in total soluble solids and pH
Figure 1 shows the growth dynamics of S. cerevisiae MERIT.ferm and W. saturnus var. mrakii NCYC 500 inoculated at a ratio of 1:100 (MERIT.ferm:NCYC 500) in respective monoculture and co-culture cider fermentation. The cell count of W. saturnus var. mrakii NCYC 500 increased by about 1 log, reaching a maximum by day 3 in both mono- and co-cultures. Its cell count in the co-culture then declined slightly from day 3, and remained consistently lower than that in the monoculture, but still remained above 106 CFU ml−1 through the end of the experimental period on day 14. The cell population of S. cerevisiae MERIT.ferm increased by approximately 3 log, reaching a maximum by day 3 in the monoculture and by day 6 in the co-culture, and remaining above 107 CFU ml−1 throughout the experiment in both cultures. Although the growth of strain MERIT.ferm was delayed in the co-culture, upon reaching a maximum count, its cell population was similar to that in the monoculture.
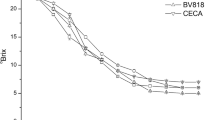
Figure 2 illustrates the dynamic changes in sugars (°Bx) and pH in the single cultures and co-culture during cider fermentation. The single culture of strain MERIT.ferm had the fastest rate of sugar consumption, with a final °Bx of 4.23. The single culture of strain NCYC 500 had the slowest rate of sugar consumption, with a final °Bx of 8.49, and the co-culture had an intermediate rate, with a final °Bx of 3.28. The profiles of pH changes for the two single cultures and the co-culture were similar, with pH values ranging between 3.5 and 3.6, as shown in Fig. 2, with the exception of a progressive decrease in pH from day 9 onwards for the single culture of strain NCYC 500.
Volatile evolution during fermentation of mono- and co-cultures and volatiles in final cider product
The dynamics of selected volatiles during mono- and co-culture fermentation of apple juice are presented in Figs. 3, 4, 5, 6 and 7. The relative amounts of major volatiles in the final cider product are shown in Table 1, and include two acids, nine alcohols, three aldehydes, ten esters and two ketones.
Figure 3 illustrates the evolution of acetic, hexanoic and octanoic acids in mono- and co-cultures. Acetic acid increased consistently in all cultures, with the co-culture containing the lowest final level. Hexanoic acid increased initially and then decreased in all fermentations, with similar final trace amounts. Octanoic acid first increased and then decreased in the co-culture, which differed from the two single cultures in which octanoic acid increased initially, then stabilized in the MERIT.ferm monoculture, although this acid was produced in small amounts in the NCYC 500 monoculture throughout fermentation.
The kinetic changes of alcohols were mostly similar in all cultures with the final concentrations of alcohols being varied with the alcohol and yeast culture (Figs. 4 and 5). The initially present hexanol was produced and then was catabolised with similar final trace amounts in all cultures. cis-3-Hexenol that was indigenous to the apple juice was continuously degraded in the MERIT.ferm single culture and co-culture, but it was formed initially, followed by a continuous reduction in the NCYC 500 monoculture with the highest residual content. Ethanol, isoamyl alcohol, 2-phenylethanol and eugenol increased steadily in all yeast cultures (Figs. 4 and 5). Furfuryl alcohol initially rose sharply followed by a rapid decrease in the MERIT.ferm monoculture and the co-culture; it was generated slowly at very low levels in the NCYC 500 single culture. Ethanol, isoamyl alcohol and 2-phenethyl alcohol were produced mostly by the MERIT.ferm single culture, followed by the co-culture and the NCYC 500 single culture, with final levels differing significantly.
Most of the esters present in the apple juice, including hexyl acetate, ethyl butanoate, cis-3-hexenyl acetate, trans-2-hexenyl acetate and menthyl acetate, were reduced the most by the single MERIT.ferm during cider fermentation, followed by the co-culture and single NCYC 500, with the exception of trans-2-hexenyl acetate, which initially increased and then decreased in the co-culture and the NCYC 500 single culture (Figs. 6 and 7; Table 1). Synthesis of isoamyl acetate and 2-phenylethyl acetate was greatest with single NCYC 500, followed by the co-culture, and least by single MERIT.ferm (Fig. 6).
Ethyl acetate, ethyl octanoate and ethyl decanoate increased in all fermentations; the latter two ethyl esters declined slightly after reaching a maximum in the single MERIT.ferm and co-culture (Fig. 7). The co-culture produced an intermediate amount of ethyl acetate, between the two single cultures; it also synthesized the highest concentrations of ethyl octanoate and ethyl decanoate. The NCYC 500 monoculture generated the highest amount of ethyl acetate but the lowest amount of ethyl octanoate and ethyl decanoate.
The final levels of aldehydes and ketones in the cider varied by culture type, with the co-culture tending to be similar to the MERIT.ferm monoculture but differing from the NCYC 500 monoculture, with exceptions such as β-damascenone (Table 1).
Discussion
This study investigated yeast and volatile evolution during cider fermentation with a pure strain of S. cerevisiae (MERIT.ferm), a pure strain of W. saturnus var. mrakii (NCYC 500) and a co-culture of MERIT.ferm and NCYC 500 at a ratio of 1:100. This ratio was chosen in order to avoid inhibition of the Williopsis yeast by the Saccharomyces (Lee et al. 2013). Even at this ratio, however, lower cell populations of W. saturnus var. mrakii and delayed growth of S. cerevisiae were observed in the co-culture (Fig. 1), indicating mutual inhibition under the current conditions. This finding is in accordance with reports that the interaction between Saccharomyces and non-Saccharomyces and their persistence during growth and fermentation are inoculum ratio-dependent (Domizio et al. 2011; Lee et al. 2013).
The ecological evolution of yeasts in spontaneous fermentation of wine and cider has been well characterized, and both share common features, with non-Saccharomyces yeasts initially dominant and then later overtaken over by Saccharomyces, presumably due to inhibition of the former by the latter as result of accumulation of high ethanol levels (Laplace et al. 1998; Morrissey et al. 2004; Suarez et al. 2007; Pando et al. 2010). In induced cider co-fermentation with non-Saccharomyces and Saccharomyces, inhibition of non-Saccharomyces by Saccharomyces has also been reported, including Kloeckera apiculata by S. cerevisiae and Hanseniaspora valbyensis by S. cerevisiae (Bilbao et al. 1997; Xu et al. 2006).
However, inhibition of Saccharomyces by non-Saccharomyces has not been noted in cider fermentation, as can be observed in Fig. 1 (retardation of growth of S. cerevisiae MERIT.ferm by W. saturnus var. mrakii NCYC 500), which may be attributable to mycocin (killer toxin) production by W. saturnus (Liu and Tsao 2009, 2010). On the other hand, multiple factors may contribute to the inhibition of non-Saccharomyces by Saccharomyces, including nutrient depletion, production of toxic substances, exhaustion of oxygen and cell-to-cell contact-mediated mechanisms (Holm Hansen et al. 2001; Nissen et al. 2003; Nissen and Arneborg 2003; Pérez-Nevado et al. 2006; Albergaria et al. 2010).
It is worth noting that the rate of sugar utilization by the co-culture was much faster than that of the monoculture of strain NCYC 500, but clearly slower than that of the monoculture of strain MERIT.ferm, which corresponds to the trend of ethanol production in the respective fermentations; this pattern of sugar consumption and ethanol formation may be ascribed to the dominance of W. saturnus var. mrakii NCYC 500 during the early stage of co-fermentation (Figs. 1, 2 and 4). Williopsis yeasts are known to have a weaker capacity for sugar fermentation and to produce low levels of ethanol (Erten and Campbell 2001; Lee et al. 2010; Trinh et al. 2011; Aung et al. 2014; Li et al. 2014).
Non-Saccharomyces wine yeasts play key roles in the synthesis of high concentrations of fermentation metabolites of oenological importance, particularly with the largest contribution of esters that enhance the characteristic fruity wine fermentation bouquet (Romano et al. 2003; Ciani and Comitini 2011). However, the behaviour of non-Saccharomyces in co-fermentation would differ from that in pure cultures as a result of the interactions discussed above. In fact, volatile evolution in cider co-fermentation of S. cerevisiae and W. saturnus var. mrakii resembled or differed from that in respective single strain fermentation depending on the volatile compound (Figs. 3–7).
Acetic acid is an undesirable volatile compound in alcoholic beverages, contributing a vinegary off-flavour. In this work, the co-culture of S. cerevisiae and W. saturnus var. mrakii produced a lower amount of acetic acid compared to the respective monocultures, but no statistically significant differences were exhibited in the final amounts among the three fermentations. Octanoic acid was synthesized in a greater amount by the co-culture than the single NCYC 500 culture, although the co-fermentation resulted in a statistically similar amount of octanoic acid to that of the MERIT.ferm monoculture (Table 1). Strain MERIT.ferm was likely the key contributor to octanoic acid production in the co-fermentation.
The production of higher alcohols by S. cerevisiae is generally greater than that formed by pure cultures of non-Saccharomyces and in mixed cultures (Ciani 1997; Rojas et al. 2003; Romano et al. 2003). Indeed, the major higher alcohol content (isoamyl alcohol and 2-phenylethanol) was greatest in the cider fermented by S. cerevisiae, followed by cider co-fermentation and then cider fermented with W. saturnus var. mrakii (Table 1). However, in the co-culture during cider co-fermentation, the concentration of each alcohol was affected differently, as has been reported by other researchers (Viana et al. 2009). It is noteworthy that eugenol (absent in apple juice) increased in all fermentations, especially in the co-fermentation and single fermentation of W. saturnus var. mrakii (Fig. 5). Eugenol imparts a clove and spice-like aroma, and may be released from its glucoside precursor via the action of glucosidase (Pando et al. 2012), suggesting that W. saturnus var. mrakii might have higher glucosidase activity than S. cerevisiae. On the other hand, alcohols are precursors to esters, and the increased alcohol level in cider co-fermentation would be expected to raise the concentration of respective esters relative to the single fermentation of W. saturnus var. mrakii.
Low molecular weight esters, especially acetate and ethyl esters, are known to be crucial to the pleasant fruity aroma of alcoholic beverages such as cider. Non-Saccharomyces such as Williopsis and Saccharomyces such as S. cerevisiae yeasts are regarded as the principal producers of acetate and ethyl esters, respectively (Romano et al. 1997; Egli et al. 1998; Fleet 2003; Lee et al. 2010; Li et al. 2012; Tanguler 2012). Indeed, acetate ester production was significantly enhanced in the co-fermented cider compared to the S. cerevisiae-fermented cider; conversely, ethyl ester formation in the co-fermented cider was on par with that in the S. cerevisiae-fermented cider but was much higher than that in the W. saturnus var. mrakii-fermented cider (Table 1, Fig. 6 and 7).
In this study, sensory evaluation was not conducted, and this should be considered in any follow-up research. Furthermore, only volatiles that could be identified with confidence by the GC-MS system are presented in Table 1. There may well be aroma-active volatiles that were present at levels below the detection limits, in addition to volatiles that could not be identified with confidence, both of which could exert a sensory impact and should be further characterized with other appropriate methods such as solvent-assisted flavour extraction.
HS-SPME is commonly used today in volatile analysis, despite its inherent limitation in producing results prone to variation. Nonetheless, peak area differences between the two single-strain fermentations with W. saturnus var. mrakii NCYC 500 (Aung et al. 2014 and the current study) were relatively small for most volatiles, considering larger standard deviations in some cases. The main exception was acetic acid, which may be the result of batch-to-batch biological variation. For example, acetic acid can sometimes be activated to generate acetyl-CoA, which may enter the tricarboxylic acid (TCA) cycle or may be converted into various acetate esters (Ugliano and Henschke 2009; Cherry et al. 2012). This could well happen, given the comparatively aerobic nature of Williopsis yeasts (Erten and Campbell 2001). Indeed, the lower amount of acetic acid correlated with the higher level of acetate esters detected in our previous single fermentation with strain NCYC 500 (Aung et al. 2014) as compared with the current study.
In conclusion, incorporation of a strain of Williopsis saturnus into the conventional cider starter culture S. cerevisiae at the correct ratio may be a novel approach for modulation of cider flavour by modifying the profile of volatile compounds such as acetate esters, ethyl esters and higher alcohols.
References
Albergaria H, Francisco D, Gori K, Arneborg N, Gírio F (2010) Saccharomyces cerevisiae CCMI 885 secretes peptides that inhibit the growth of some non-Saccharomyces wine-related strains. Appl Microbiol Biotechnol 86:965–972
Aung MT, Lee PR, Yu B, Liu S-Q (2014) Cider fermentation with three Williopsis saturnus yeast strains and volatile changes. Ann Microbiol. doi:10.1007/s13213-014-0935-7
Bilbao A, Irastorza A, Duenas M, Fernandez K (1997) The effect of temperature on the growth of strains of Kloeckera apiculata and Saccharomyces cerevisiae in apple juice fermentation. Lett Appl Microbiol 24:37–39
Cherry JM, Hong EL, Amundsen C, Balakrishnan R, Binkley G, Chan ET, Wong ED (2012) Saccharomyces genome database: the genomics resource of budding yeast. Nucleic Acids Res 40:700–705
Ciani M (1997) Role, enological properties and potential use of non-Saccharomyces wine yeasts. Recent Res Dev Microbiol 1:317–331
Ciani M, Comitini F (2011) Non-Saccharomyces wine yeasts have a promising role in biotechnological approaches to winemaking. Ann Microbiol 61:25–32
Constanti M, Reguant C, Poblet M, Zamora F, Mas A, Guillamon JM (1998) Molecular analysis of yeast population dynamics: effect of sulphur dioxide and inoculum on must fermentation. Int J Food Microbiol 41:169–175
Domizio P, Romani C, Comitini F, Gobbi M, Lencioni L, Mannazzu I, Ciani M (2011) Potential spoilage non-Saccharomyces yeasts in mixed cultures with Saccharomyces cerevisiae. Ann Microbiol 61:137–144
Egli CM, Edinger WD, Mitrakul CM, Henick-Kling T (1998) Dynamics of indigenous and inoculated yeast populations and their effect on the sensory character of Riesling and Chardonnay wines. J Appl Microbiol 85:779–789
Erten H, Campbell I (2001) The production of low-alcohol wines by aerobic yeasts. J Inst Brew 107:207–215
Fleet GH (2003) Yeast interactions and wine flavor. Int J Food Microbiol 86:11–22
Granchi L, Ganucci D, Messini A, Vincenzini M (2002) Oenological properties of Hanseniaspora osmophila and Kloeckera cortices from wines produced by spontaneous fermentations of normal and dried grapes. FEMS Yeast Res 2:403–407
Heard GM (1999) Novel yeasts in winemaking-looking to the future. Food Aust 51:347–352
Heard GM, Fleet GH (1985) Growth of natural yeast flora during the fermentation of inoculated wines. Appl Environ Microbiol 50:727–728
Holm Hansen E, Nissen P, Sommer P, Nielsen J, Arneborg N (2001) The effect of oxygen on the survival of non-Saccharomyces yeasts during mixed culture fermentations of grape juice with Saccharomyces cerevisiae. J Appl Microbiol 91:541–547
Jolly NP, Augustyn PPH, Pretorius IS (2003) The effect of non-Saccharomyces yeasts on fermentation and wine quality. S Afr J Enol Vitic 24:55–62
Laplace J-M, Apery S, Frere J, Auffray Y (1998) Incidence of indigenous microbial flora from utensils and surrounding air in traditional French cider making. J Inst Brew 104:7–74
Lee P-R, Ong Y-L, Yu B, Curran P, Liu S-Q (2010) Profile of volatile compounds during papaya juice fermentation by a mixed culture of Saccharomyces cerevisiae and Williopsis saturnus. Food Microbiol 27:253–261
Lee P-R, Kho SHC, Yu B, Curran P, Liu S-Q (2013) Yeast ratio is a critical factor for sequential fermentation of papaya wine by Williopsis saturnus and Saccharomyces cerevisiae. Microb Biotechnol 6:385–393
Li X, Chan LJ, Yu B, Curran P, Liu S-Q (2012) Fermentation of three varieties of mango juices with a mixture of Saccharomyces cerevisiae and Williopsis saturnus var. mrakii. Int J Food Microbiol 158:28–35
Li X, Chan LJ, Yu B, Curran P, Liu S-Q (2014) Influence of Saccharomyces cerevisiae and Williopsis saturnus var. mrakii on mango wine characteristics. Acta Aliment 43:473–481
Liu S-Q, Tsao M (2009) Inhibition of spoilage yeasts in cheese by killer yeast Williopsis saturnus var. saturnus. Int J Food Microbiol 131:280–282
Liu S-Q, Tsao M (2010) Biocontrol of spoilage yeasts and moulds by Williopsis saturnus var. saturnus in yoghurt. Nutr Food Sci 40:166–175
Morrissey WF, Davenport B, Querol A, Dobson ADW (2004) The role of indigenous yeasts in traditional Irish cider fermentations. J Appl Microbiol 97:647–655
Nissen P, Arneborg N (2003) Characterization of early deaths of non-Saccharomyces yeasts in mixed cultures with Saccharomyces cerevisiae. Arch Microbiol 180:257–263
Nissen P, Nielsen D, Arneborg N (2003) Viable Saccharomyces cerevisiae cells at high concentration cause early growth arrest of non-Saccharomyces yeasts in mixed cultures by a cell-cell contact-mediated mechanism. Yeast 20:331–341
Pando R, Querol A, Suarez B (2010) Genetic and phenotypic diversity of autochthonous cider yeasts in a cellar from Asturias. Food Microbiol 27:503–508
Pando R, Lastra A, Suarez B (2012) Screening of enzymatic activities in non-Saccharomyces cider yeasts. J Food Biochem 36:683–689
Pérez-Nevado F, Albergaria H, Hogg T, Girio F (2006) Cellular death of two non-Saccharomyces wine-related yeasts during mixed fermentations with Saccharomyces cerevisiae. Int J Food Microbiol 108:336–345
Rojas V, Gil JV, Pinaga F, Manzanares P (2003) Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int J Food Microbiol 86:181–188
Romano P, Suzz G, Comi G, Zironi R, Maifreni M (1997) Glycerol and other fermentation products of apiculate wine yeasts. J Appl Microbiol 82:615–618
Romano P, Fiore C, Paraggio M, Caruso M, Capece A (2003) Function of yeast species and strains in wine flavor. Int J Food Microbiol 86:169–180
Suarez B, Pando R, Fernandez N, Querol A, Madrera R (2007) Yeast species associated with the spontaneous fermentation of cider. Food Microbiol 24:25–31
Tanguler H (2012) Evaluation of Williopsis saturnus inoculum level on fermentation and flavor compounds of white wines made from Emir (Vitis vinifera L.) grown in Anatolia. Food Biotechnol 26:351–368
Trinh T-T-T, Woon WY, Yu B, Curran P, Liu S-Q (2011) Growth and fermentation kinetics of mixed cultures of Saccharomyces cerevisiae var. bayanus and Williopsis saturnus var. saturnus at different ratios in longan juice. Int J Food Sci Technol 46:130–137
Ugliano M, Henschke PA (2009) Yeasts and wine flavour. In: Moreno-Arribas MV, Polo MC (eds) Wine chemistry and biochemistry. Springer, New York, pp 313–392
Viana F, Gil JV, Valles S, Manzanares P (2009) Increasing the levels of 2-phenylethyl acetate in wine through the use of a mixed culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int J Food Microbiol 135:68–74
Xu Y, Zhao GA, Wang LP (2006) Controlled formation of volatile components in cider making using a combination of Saccharomyces cerevisiae and Hasenispora valbyensis yeast species. J Ind Microbiol Biotechnol 33:192–196
Zohre DE, Erten H (2002) The influence of Kloeckera apiculata and Candida pulcherrima yeasts on wine fermentation. Process Biochem 38:310–324
Acknowledgments
This work was supported in part by an Academic Research Fund (AcRF) grant from the Ministry of Education, Singapore (WBS No. R-143-000-368-133).
Conflict of interest
The authors declare no conflict.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, SQ., Aung, M.T., Lee, PR. et al. Yeast and volatile evolution in cider co-fermentation with Saccharomyces cerevisiae and Williopsis saturnus . Ann Microbiol 66, 307–315 (2016). https://doi.org/10.1007/s13213-015-1110-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-015-1110-5