Abstract
Cancer is a major health problem worldwide, which is responsible for more than 10 million deaths annually. Cancer treatment has traditionally been based on chemotherapy and surgery; however, owing to cytotoxicity, drug resistance, and non-specificity, cancer immunotherapy, which involves using the patient’s own immune system in treatment, has recently gained prominence as a new cancer treatment strategy. Cancer immunotherapy includes strategies such as adoptive T-cell therapy, immune checkpoint blockade, and cancer vaccines, all of which have shown significant anticancer effects. To improve the therapeutic effectiveness and safety and lower the side effects of these strategies, nano- and micro-technologies are being applied to advance the technology. Several studies have reported the use of liposomes (i.e., lipid nanoparticles) in the context of cancer treatment. Liposomes, which are excellent carriers with biocompatibility, amphiphilicity, and drug protection, can be used for passive and active targeting to enhance the effectiveness of cancer immunotherapy. In this review, we summarize cancer immunotherapy and discusses the strategies and benefits of using various liposomes in cancer immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cancer is characterized by uncontrolled abnormal cell growth, usually caused by mutations in genes involved in cell proliferation, death, metabolism, and DNA [1]. Cancer cells may also invade and metastasize to surrounding tissues, leading to death [2] The risk of developing cancer between the ages of 0 and 74 years is 20.2%, and the incidence is expected to increase in the coming decades [3,4,5,6]. According to the World Health Organization (WHO), cancer is the second leading cause of death worldwide, with 10 million deaths per year as of 2020; however, it is likely to become the leading cause of death by 2060 given population growth and aging [3, 7]. Therefore, cancer treatment is a major public health concern worldwide. To date, several advances have been made in cancer treatment; however, existing therapies have serious side effects. Therefore, considerable research has been conducted on therapies that can mitigate side effects or achieve higher therapeutic efficacy. For decades, surgery, chemotherapy, and radiotherapy have been primarily used for cancer treatment. More recently, following the development of targeted and hormonal therapies, immunotherapy, which modulates the activity of the patient’s own immune cells, has been actively introduced [8]. Anticancer treatments are generally divided into four types [9]. First, chemotherapy is a systemic treatment that uses cytotoxic anticancer drugs to kill rapidly dividing and proliferating cells by blocking cell division or damaging DNA [10, 11] second, hormonal therapy involves stopping or slowing cancer growth using drugs that interfere with the transmission of growth signals through hormone receptors on cancer cells [12] third, targeted therapy involves the use of small-molecule drugs or monoclonal antibodies that specifically target proteins involved in the growth signaling pathways of cancer cells without affecting the surrounding environment [13, 14]; and fourth, immunotherapy is gaining prominence as an innovative treatment that boosts the patient’s immune system to attack and eliminate cancer cells [15,16,17].
The most commonly used cytotoxic anticancer drugs may not selectively act on cancer cells, resulting in the nonspecific destruction of rapidly dividing normal cells such as hematopoietic stem cells in the bone marrow, mucosal cells, and hair root cells [18, 19]. Additionally, the clinical efficacy of anticancer drugs is significantly limited by antitumor drug resistance, which may result in a decrease in or disappearance of the therapeutic effect [20,21,22]. Therefore, side effects, such as cytotoxicity, low specificity, and drug resistance, pose considerable challenges in cancer treatment using chemotherapy.
The goal of cancer immunotherapy is to induce an anticancer response that enhances or restores the ability of the immune system to detect and destroy cancer cells by overcoming the mechanisms by which cancer evades and suppresses the immune response [23,24,25]. Recently, cancer immunotherapy has emerged as a promising strategy, with the introduction of strategies such as chimeric antigen receptor (CAR)-T cell therapy, immune checkpoint blockade therapy, and cancer vaccines [26, 27]. CAR-T cell therapy induces a strong antitumor response by genetically modifying the patient’s own T cells to express specific CARs, so that the T cells can recognize and eliminate cells expressing specific target antigens [28, 29]. CAR-T cells have shown significant therapeutic benefits in B cell leukemia, and several CAR-T cell therapies have received food and drug administration (FDA) approval [30]. Immune checkpoint blockade reverses tumor immune resistance by blocking immune checkpoints that suppress immune responses, such as cytotoxic T lymphocyte-associated protein-4 (CTLA-4), programed cell death ligand-1 (PD-L1), and programed cell death 1 (PD-1) to produce antitumor effects [31,32,33]. Currently, CTLA-4 inhibitors (e.g., ipilimumab), PD-L1 inhibitors (e.g., atezolimumab, avelumab, and durvalumab), and PD-1 inhibitors (e.g., nivolumab, pembrolizumab, and cemiplimab), which block immune checkpoints, have been FDA approved [34]. Cancer vaccines are another promising type of cancer immunotherapy that uses tumor antigens to stimulate antitumor immunity and kill tumor cells. Cancer vaccines are delivered in various forms, including peptides, nucleic acids, and antigens [35, 36].
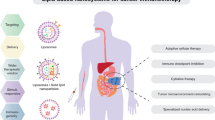
The efficacy of anticancer drugs varies from patient to patient, and several obstacles hinder the delivery of the injected drug to the lesion. To overcome these obstacles, various nanotechnologies are being used in the field of anticancer therapy; treatment using nanoparticles reduces side effects by specifically delivering drugs to target sites and controlling their release and increases therapeutic efficacy by protecting drugs and increasing their half-life [7, 41, 42]. Therefore, various approaches using nano-sized particles have been investigated as strategies for successfully delivering drugs to the tumor microenvironment and have shown improved safety and therapeutic efficacy [43, 44]. Nanocarriers used in cancer immunotherapy include micelles, dendrimers, carbon nanotubes, and gold nanoparticles, among which liposomes are one of the most promising and useful innovations for delivering drugs and other molecules (Fig. 1) [19]. Liposomes have become an integral component of the field of nanomedicine since they were first discovered in the 1960s by Bangham et al. [45]. They consist of a bilayer surrounding an aqueous interior compartment that can contain various hydrophilic and lipophilic drugs, enabling efficient multidrug delivery [46, 47]. Spherical lipid-based vesicles are simple self-assembling systems, which can prevent drug degradation, reduce drug half-life, and control drug release [48]. Moreover, the biofilm-like membrane structure of liposomes renders them safe, biocompatible, and biodegradable. Additionally, the long circulation time and enhanced permeability and retention (EPR) effect obtained at 100–200 nm allow preferential leakage into tumor tissues, which can enhance the anticancer effect and reduce systemic exposure to drugs [49, 50]. Furthermore, the fluid lipid surface of liposomes allows the attachment of targeting ligands, enabling targeting to the desired site, which can increase the specificity of liposomes and their therapeutic efficacy [51, 52].
Strategies for using liposomes in cancer immunotherapy. a Chimeric antigen receptor (CAR)-T cell therapy, a representative strategy used in adoptive cell therapy, involves the collection of the patient’s T cells through leukapheresis and the generation of CAR-T cells through more efficient and safer gene transfer through liposomes. Upon reinjection into the patient, the manufactured CAR-T cells recognize and attack cancer cells [37, 38]. b Immune checkpoint blockade therapy blocks immune checkpoints, a group of proteins that suppress immune responses and are used by cancer cells to evade the body’s immune response. Modifying the liposome surface with an antibody that targets an immune checkpoint effectively blocks the immune checkpoint, preventing immune escape and reactivating T cells, further increasing the anticancer response [39]. c Cancer vaccines use tumor-associated antigens, nucleic acids, and peptides to increase the activity of T cells and destroy cancer cells. When cancer vaccines are delivered using liposomes, antigen-presenting cells can effectively absorb them, activating immune responses using cytotoxic T cells and enhancing anticancer effects [40]
Liposomes were the first nanodrug carriers to be successfully used in clinical applications; the first product, which received clinical approval in 1990, was amphotericin B (Ambisome®), which was used for fungal infections [49]. The first FDA-approved liposomal product for cancer therapy was doxorubicin HCl liposomal injection (Caelyx® in Europe and Doxil® in the USA) in 1995 for AIDS-associated Kaposi’s sarcoma [53]. Several other liposomal formulations have received FDA approval for cancer therapy, and research on cancer therapy using liposomes remains ongoing (Table 1).
In this review, we discuss the limitations of chemotherapy, propose liposome use and cancer immunotherapy as strategies to overcome these limitations, and describe the major strategies, mechanisms, and research trends in cancer immunotherapy. Furthermore, we discuss the strategies and benefits of liposome use in adoptive cell therapy, immune checkpoint blockade, and cancer vaccines to increase the efficacy of cancer immunotherapy.
2 Liposomal Application to Deliver Chemotherapeutics
Conventional chemotherapy has been highly successful against cancers with complex metastases. However, certain chemotherapeutic drugs can activate multiple signaling pathways in the tumor microenvironment and increase the secretion of inflammatory mediators [56, 57]. Additionally, they are generally unable to kill all cancer cells, leading to the possibility of cancer recurrence within a short period [58]. Therefore, to address these issues, researchers are actively working on developing drug delivery vehicles that target only the desired sites. To reduce the side effects of anticancer drugs, carriers are used to transport the drug to the desired site of therapeutic action. Liposomes are the most recently studied carriers, and liposomal nanoparticles have been used to develop cancer therapeutics [48]. Liposomes continue to be developed as delivery systems for improving cancer chemotherapy and reducing side effects. In particular, the encapsulation of drugs can provide benefits such as preventing degradation in the bloodstream, enhancing drug solubility, improving drug stability, targeted drug delivery, reducing toxic side effects, and improving pharmacokinetic properties [59].
2.1 Conventional Chemotherapeutics and Their Limitations
Advances in cancer treatment have been made worldwide over the past few decades. In the past, surgical therapy was used to treat cancer; however, micrometastases have been treated using adjuvant chemotherapy, in which drugs are employed along with surgery and radiotherapy [60]. Recent advances have resulted in more efficient and less burdensome chemotherapy, targeted drug therapy, gene therapy, and immunotherapy [8]. The clinical use of chemotherapy, the most commonly used modality, has improved through improved dosing regimens, upfront or adjuvant administration, and combination therapy [52]. Chemotherapy, which inhibits cancer cell proliferation by interfering with cell division or DNA and RNA synthesis, is the primary modality for cancer treatment [61].
Chemotherapy is excellent for inhibiting rapid tumor growth [62]. Alkylating agents are the main class of chemotherapeutic drugs that interfere with the formation and linkage of DNA double strands, which is achieved by transferring one alkyl group to the guanidine base of DNA [63]. Cross-linking of nucleic acids and proteins affects DNA structure, causing incorrect base pairing and DNA strand breaks, eventually leading to irreversible aging. It is the oldest class of anticancer drugs in common use, and it plays an important role for treating several types of cancer [64]. Antimetabolites are the next generation of substances that interfere with cellular metabolism by competing with and inhibiting certain metabolites inside the cell. Most of these substances, including 5-fluorouracil, gemcitabine, platinum, and zebularine, have structures similar to those of cellular metabolites and enzyme substrates, and they are usually identified and processed by enzymes [65].
Pyrimidine-derived antimetabolites are chemotherapeutic agents that interfere with DNA synthesis and are cytotoxic to cancer cells; these include cytosine arabinosides, which induce cell death during progression through the S phase of the cell cycle by misincorporation into RNA and DNA or by inhibiting their core [66]. Although the substances used in these drug therapies can cause sufficient death of cancer cells, they can cause many side effects and may even harm healthy cells when administered at high doses; therefore, dosage and duration are important considerations, with drug resistance becoming possible if cancer cells are exposed to drugs for an extended period [64]. These substances also pose problems such as low bioavailability, side effects, and nonspecific targeting; therefore, efforts are underway to develop targeted delivery methods for anticancer drugs.
2.2 Drug Delivery Systems (DDS) for Cancer Therapy
Controlled DDSs have been used to overcome the limitations of chemotherapy. A multi-DDS is used in cancer treatment to accurately deliver anticancer drugs to target tissues or cells, maximize effectiveness, and minimize side effects [67]. Multi-DDSs are used to modify the properties of anticancer drugs by packaging or modifying them in a material or device in a specific manner with the aim to achieve high drug concentrations in cancerous tissues [68]. A controlled DDS is an excellent carrier of chemotherapeutic agents, which serves to guide the agent to the tumor site, increasing the drug concentration in cancer cells, and preventing toxicity to normal cells, as well as protecting the drug from degradation and elimination, facilitating the delivery of proteins and novel therapeutics such as gene therapy and RNA interference [42].
Recently, nanoparticles have been actively studied for use in controlled DDSs (Fig. 2) [69, 70]. Various techniques and strategies have been used to improve drug delivery efficiency and provide high therapeutic efficacy in specific cancer tissues [68]. Nanoparticles have diameters ranging from 10 to 100 nm, and are generally divided into inorganic and organic particles. Among the inorganic class, metallic nanoparticles, such as gold and iron oxide, which exhibit optical and electronic properties, are the most commonly studied and are particularly advantageous for biomedical imaging [71]. Hayashi et al. reported that magnetic nanoparticles can be used to treat cancer cells through drug delivery and hyperthermia by applying high-frequency magnetic fields [72]. Additionally, organic nanoparticle families, including protein- and polysaccharide-based natural particles and synthetic polymeric nanoassemblies such as dendrimers and fluorescent organic nanoparticles, are used to increase biocompatibility and biodegradability. Lipid-based particles, such as micelles and liposomes, are commonly used in preclinical and clinical studies because of their excellent biocompatibility [59, 73]. However, lipid-based nanoparticles have limitations owing to their low loading capacity and relative lack of stability, which leads to drug leakage.
a Conventional chemotherapy mechanism and different types of chemotherapeutic drugs. Reproduced with permission from [63]. Copyright (2023) by Elsevier. b General working mechanism of drug delivery systems (DDSs). A DDS has a stimulus response mechanism that is endowed with a controlled release function. Reproduced with permission from [70] Copyright (2016) by MDPI. c Chemically engineered nanoparticles for cancer therapy. Nanocarriers are classified as inorganic and organic materials. Inorganic nanoparticles are characterized by high stability, low biodegradability, and unique electronic and optical properties. Organic nanoparticles show low stability but good biocompatibility and exhibit various possibilities for drug functionalization on the surface or in the interior space. Reproduced with permission from [71] Copyright (2021) by Springer Nature. d Structures of conventional and functionalized liposomes. Conventional liposomes are composed of a phospholipid, and functionalized liposomes may contain polyethylene glycol (PEG), targeting ligands, and multifunctional molecules on the surface to enable cancer diagnosis and treatment. Reproduced with permission from [48] Copyright (2018) by MDPI
2.3 Liposomal Approaches to Cancer DDS
As explained previously, chemotherapy has limitations such as low bioavailability, high dose requirements, side effects, low therapeutic index, development of multidrug resistance, and nonspecific targeting. Nanoparticles and multiple carriers may be used to deliver chemotherapeutic drugs to cancer cells. Among them, we noted the encapsulation method of drugs using liposomes [48]. Liposomes are phospholipid vesicles consisting of one or more concentric lipid bilayers that enclose individual aqueous spaces. A range of drugs can be encapsulated by these vesicles because of the unique ability of the liposomal system to entrap both lipophilic and hydrophilic compounds. Hydrophobic drugs are inserted between the bilayer membranes, whereas hydrophilic drugs can be entrapped in the aqueous site, which is the center of the liposome [74].
Different types of drug delivery systems use liposomes. Conventional liposomes are composed of a lipid bilayer comprising molecules, including cationic, anionic, or neutral lipids and cholesterol. Thus, hydrophobic compounds can be encapsulated inside the lipid bilayer, while hydrophilic compounds can be encapsulated inside the liposome [47, 75]. PEGylated liposomes are liposomes whose properties and in vivo behavior can be modified by adding PEG, a hydrophilic polymeric coating, to the liposome surface to impart steric stabilization [76]. Ligand-targeted liposomes can be used for specific targeting by attaching ligands to the ends of PEG chains [77]. Liposomes for therapeutic use consist of nanoparticles, targeting elements, imaging components, and therapeutic components. Harris et al. evaluated the efficacy and toxicity of liposome-encapsulated doxorubicin and conventional single-agent doxorubicin for the treatment of metastatic breast cancer and observed lower cardiac toxicity with liposome-encapsulated doxorubicin [78]. As drug carriers, liposomes have excellent properties, including protecting the encapsulated material from physiological degradation, prolonging the half-life of the drug, and controlling the release of drug molecules, with good biocompatibility and stability [50].
3 Liposomal Application for Targeting Immune Cells
Cancer immunotherapy generates or modulates the immune response to cancer. Over the past two decades, various immunotherapies have been developed to improve antitumor responses by modulating stimulatory, inhibitory, or regulatory mechanisms [79, 80]. Adoptive cell therapy (ACT) involves the infusion of autologous or allogeneic T cells into patients with cancer to eliminate cancer cells. Originating in 1966, ACT was based on a study that showed that transplantation of a mixture of white blood cells and tumor cells from patients with cancer resulted in the inhibition of tumor cell growth in more than half of the patients evaluated. This sparked a wave of research on autoimmune therapies, including the transplantation of immune cells [81]. Representative ACTs include tumor-infiltrating lymphocyte (TIL), engineered T cell receptor (TCR), chimeric antigen receptor (CAR) T-cell, and dendritic cell (DC) therapies. ACTs generate a potent immune-mediated antitumor response by reinfusing ex vivo engineered T cells into lymphocyte-depleted patients [82]. Here, we elaborate on the principles of the aforementioned ACT strategies and briefly discuss the recent application of nanomaterials for increasing their therapeutic efficiency. In particular, we discuss advances in immunotherapy by focusing on therapeutic strategies that use liposomes as delivery vehicles.
3.1 Conventional Immune Cell Therapy for Anticancer Treatment
Because ACTs have shown promise as a new treatment for patients with hematologic malignancies, many studies are being conducted to develop cancer treatment strategies. TILs are mononuclear cells that infiltrate the stroma surrounding tumor cells and can be used as a form of ACT. ACT using TILs was initiated at the National Cancer Institute in the late 1980s for the treatment of metastatic melanoma [83]. The principle of TILs as an ACT involves the isolation of naturally infiltrating lymphocytes from the tumor material, before expanding them ex vivo to therapeutic numbers, approximately 1–10 billion cells, in the presence of high-dose interleukin-2 (IL-2). These cells are then infused into patients along with high-dose IL-2, which supports their growth and survival in the tumor microenvironment [84, 85].
Engineered TCRs are composed of α- and β-chain non-covalent bonds that are closely associated with the CD3 complex on the surface of T cells, which plays an important function in response to abnormal or foreign cells in the body. T-cell activation occurs when the TCR recognizes a peptide that is non-covalently bound to a major histocompatibility complex (MHC) on the surface of an antigen-presenting cell (APC) or tumor cell [86]. In vitro and in vivo experiments on TCR T-cells were performed very early, and various studies have demonstrated that they can effectively kill tumor cells in animal models of melanoma [87,88,89].
CAR T-cell therapy, which uses chimeric antigen receptor proteins attached to a patient’s T cells, is one of the most prominent ACT strategies. These receptors consist of single-chain proteins designed to target cancer cell surface antigens [90]. It consists of three domains. an extracellular single-chain fragment variant domain (scFv) that recognizes the antigen, a transmembrane domain that crosses the cell membrane, and an intracellular activation domain of CD3ζ. The antigen-recognition domain contains an exotic component that allows it to bind to a target antigen with high affinity [91]. The endodomains containing CD3ζ or FcεRIγ have three ITAMs, and after antigen recognition, signals are transmitted to T cells by activating receptor clusters and signaling. The patient’s T cells are transduced using viral vectors, such as retrovirus, lentivirus, and adenovirus vectors, to introduce chimeric antigen receptors. After proliferation, they undergo lymphodepletion and are activated by IL-7 and IL-15 clearance. After isolating low CD4/CD8 T cells and inducing T cell growth with IL-2, the CAR is reverse transcribed from RNA to DNA and integrated into the genome, before expanding the cells for therapy. Currently, all approved CAR T-cells are indicated for hematological cancers. CD19, a marker of B cells, is a common target of CAR T-cells [92]. FDA-approved CAR-T therapies to date include the CD19-targeting CAR-T products tisagenlecleucel (Novartis, 2017) for acute lymphoblastic leukemia, axicabtagene ciloleucel (Gilead, 2017) for large B-cell lymphoma, brexucabtagene autoleucel (Gilead, 2020) for mantle cell lymphoma, and lisocabtagene maraleucel (Bristol Myers Squibb, 2021) for relapsed or refractory large B-cell lymphoma. B-cell maturation antigen (BCMA) is another target, and CAR-T cells that target BCMA include Abecma (2021), the first BCMA CAR-T cell treatment from Bristol Myers Squibb and Bluebird Bio, and ciltacabtagene autoleucel (2022) from Legend Biotech and Janssen Pharmaceutical company [93, 94].
Natural killer (NK) cells are another type of cytotoxic innate immune lymphocyte that can kill tumor cells and mediate immune surveillance and clearance of viral infections and tumor-transforming cells [95]. NK cells do not express MHC class I molecules on their cell surface, rendering them potentially available as off-the-shelf cell therapies, with clinical evidence indicating that the adoptive transfer of allogeneic NK cells is safe for patients [96].
3.2 Nanomaterials to Enhance Immune Cell Therapy
Current cancer immunotherapy focuses on T cell-mediated tumor immunity. However, for adoptive T cells, cell migration and infiltration into solid tumors remain challenging. The number of immune cells that penetrate deeply into solid tumors should be increased to efficiently improve the therapeutic potential of ACT [97]. Nanomaterials have emerged as promising tools for enhancing the effectiveness of immune cell therapy and have revolutionized the field of cancer treatment. Researchers have endeavored to use the unique properties of nanoscale materials to improve the precision, targeting, and overall treatment outcomes of immune-based approaches such as CAR-T and TCR therapies. In recent years, the use of nanomaterials in immune cell therapy has focused on the development of nanoparticle-based drug delivery systems. These nanocarriers are characterized by their ability to encapsulate therapeutic agents, such as anticancer drugs or immunomodulators, and precisely deliver them to the tumor site. This not only minimizes nonspecific delivery but also enhances the accumulation of immune cells in the tumor, which can increase the therapeutic effect [98]. Surface modification of nanoparticles can be designed to actively modulate the immune response, enabling controlled interactions with immune cells to influence their activation, proliferation, and tumor infiltration. Nanomaterials can be designed to mimic immune cell components to promote antigen presentation and immune recognition and enhance antitumor responses.
Metal nanoparticles (MNPs) have favorable characteristics in the field of cancer immunotherapy because of the precise control of their size, shape, charge, and surface modifications. MNPs have been used to improve the delivery of TLR-9 adjuvants such as CpGs and synthetic oligodeoxynucleotides that mimic bacterial DNA [99, 100]. For example, the combination of gold nanoparticles (AuNPs) with modified CpG attenuates side effects and stimulates macrophages and DCs, resulting in significant inhibition of tumor growth [101, 102]. Mirkin et al. demonstrated that AuNP-CpG formulated with the OVA antigen significantly increased IgG2a antibody titers and consequently improved T-cell activation compared to unformulated particles, leading to reduced tumor growth and improved survival in a lymphoma model system (Fig. 3c) [99, 103, 104]. Additionally, the unique properties of MNPs can be leveraged in ablative therapies using techniques such as NIR-mediated photothermal therapy (PTT), and MNP-mediated tumor ablation can induce systemic antitumor immunity even without co-delivery of immunotherapeutic agents [105, 106]. Many studies have identified the potential for improving ACT-based cancer immunotherapy using polymeric NPs such as poly(lactic-co-glycolic acid) (PLGA), dendrimers and hydrogels, liposomes and exosomes, and delivery vehicles with porous structures [107,108,109].
a Schematic showing various ACT platforms, including tumor-infiltrating lymphocyte (TIL) therapy, T-cell receptor (TCR) therapy, chimeric antigen receptor (CAR) therapy, and dendritic cell (DC) therapy [119] Copyright (2023) by Wiley. b Nanomaterials that can be used for current in vivo T-cell therapy. Nanomaterials optimized for characteristics such as surface area, physicochemical properties, and encapsulation and release properties can maximize the efficiency of T cell-based cancer immunotherapy [94] Copyright (2021) by Springer Nature. c Enhanced immunomodulatory properties of AuNP-CpG formulated with OVA antigen result in reduced tumor growth and increased survival rate [103] Copyright (2015) by PMC. d Liposome-based immunostimulatory delivery system for DC and T cells. Liposomes can directly target DCs, and immune responses mediated by liposome induction can increase the potency of cytotoxic T lymphocytes (CTLs). Liposomes can activate cytotoxic CTLs to induce a sustained and robust immune response [110] Copyright (2020) by MDPI. e Tumor growth, survival rate, and number of effective memory T cells (TEM) and central memory T cells (TCM) following the delivery of liposomes loaded with CHI and BMS-202 [116] Copyright (2022) by BMC
3.3 Advances in Immune Cell Therapy via Liposomal Delivery
In nanotechnology-based immunotherapy, liposomes exhibit unique features such as improved drug efficacy, reduced toxicity, better physicochemical properties, ability to deliver macromolecular drugs, and ability to bypass tumor-induced resistance mechanisms [110]. Liposome-based immune cell therapy enables targeted drug delivery and enhances the stability of therapeutic agents such as cytokines, immune checkpoint inhibitors, and antigens. Liposomes loaded with tumor-specific antigens enhance antigen presentation by immune cells, which improves the accuracy of the immune response by preparing immune cells to recognize and attack cancer cells more accurately. immunomodulators that modify the tumor microenvironment can also be loaded into liposomes to reprogram immune cells within the tumor, suppress immunosuppressive factors, and promote an environment that is conducive to immune cell function [111,112,113].
Current adjuvants used in cancer immunotherapy include cytokines, CpG oligonucleotides (ODNs), monophosphoryl lipid A (MPLA), and lipopolysaccharide (LPS) derivatives (Fig. 3). Liposomes have the advantages of enhancing the adjuvant effects of internal cargo, reducing systemic distribution, and minimizing side effects. Mannose-modified liposomes co-encapsulated with CpG ODN and melanoma-specific TRP2180-188 peptide have been reported to specifically target DCs and produce a strong synergistic effect on the immune response. In addition to their delivery capabilities, liposomes can act as adjuvants to stimulate immune responses (Table 2). Indeed, cationic liposomes can induce greater immune activation than anionic or neutral liposomes, even without the addition of adjuvants, rendering them promising vehicles for cancer vaccination [114, 115]. Tu et al. studied liposomes co-delivering the epigenetic modulator chidamide (CHI) and the PD-L1 inhibitor BMS-202, and demonstrated that CHI induced ICD in triple-negative breast cancer (TNBC), enhanced cancer immunoreactivity, activated NK cells, promoted antigen presentation, T cell recognition, and DC maturation, and effectively inhibited tumor growth and metastasis [116,117,118].
These studies have shown that loading immunomodulatory factors into liposomes can lead to more effective treatment. Furthermore, the examples demonstrate that immune cell therapy using liposomes is a promising field that requires further research.
4 Liposomal Application for Targeting Immune Checkpoints
With tremendous progress in the field of immunotherapy, nanotechnology-based immunotherapies are increasingly showing the potential to overcome the limitations of conventional therapies such as therapeutic monoclonal antibodies and cancer vaccines. Nanotechnology can improve the efficacy of drugs, reduce unnecessary toxicity, and enhance their physicochemical stability, as well as bypass tumor-derived defense mechanisms to enhance therapeutic efficacy and enable large-molecule drug delivery. Liposomes are one such system that can be used for nanotechnology-based cancer immunotherapy. Drugs developed as liposomes are being evaluated in clinical trials to target cancer and develop vaccines. In addition, liposomal therapies such as Doxil are currently used clinically for cancer treatment [110]. Liposome nanomedicine has shown significant results as a drug delivery platform; therefore, the use of liposomal delivery systems in immune checkpoint-mediated tumor therapy is becoming increasingly promising [39]. Immune checkpoint inhibitors are typically encapsulated inside liposomes or attached to their surface to improve their stability and delivery. For example, PEGylated liposomes as delivery vehicles protect drugs from clearance by the reticuloendothelial system and allow for a longer circulation time in the body. Some liposomes are composed of lipids that are responsive to the tumor microenvironment, such as temperature and pH, and only release cargo in specific areas, thus minimizing systemic toxicity [120]. Here, we discuss immune checkpoint-targeted therapies using liposomes and describe the current status and challenges in the development of existing immune checkpoint inhibitors. As strategies to address these issues, we introduce immunotherapies that use liposomes and provide specific examples of how liposomes can improve treatment effectiveness.
4.1 Immune Checkpoints During Cancer Treatment
Immune checkpoints are a group of proteins involved in various inhibitory and stimulatory pathways that act as homeostatic regulators of the immune system. Immune checkpoints are important immune modulators that maintain self-tolerance, prevent autoimmune responses, and allow the immune system to respond to pathogens under normal physiological conditions [121]. Simultaneously, they limit the immune response by activating negative feedback immune gateways that provide signals to suppress the immune response and protect normal cells from damage caused by an overactive immune response [122]. However, cancer cells evade immunosurveillance and use an immune gateway mechanism to protect themselves through immunosuppression, which involves inactivating immune cells to neutralize T cells. Consequently, they evade the immune response, making it difficult to eliminate the cancer. The mechanism by which cancer evades immunity was first proposed by Thomas and Burnet in the twentieth-century. However, this theory remained unexplored for a long time, until Dunn et al. revealed the presence of stages of elimination, equilibrium, and escape that are driven by interactions between immune cells and tumor cells in the tumor microenvironment [121]. Tumor cells use immune gateways to evade host immune responses. Unlike ideal cancer antigens, which elicit a strong immune response, tumor-associated antigens typically have low immunogenicity or avoid presentation on the surface of APCs. This prevents the activation of cytotoxic T lymphocytes (CTLs), resulting in an inadequate host immune response. Additionally, several inhibitory factors in the tumor microenvironment result in immunosuppressive phenomena, such as the upregulation of immune pathways, production of immunosuppressive cytokines, and recruitment of immunosuppressive cells. Furthermore, chronic inflammation exacerbates tumor progression, attenuates T-cell activity, and consequently causes challenges in eliminating tumor cells, which evade the host immune system [123].
4.2 Conventional Method for Inhibiting Immune Checkpoints
Immune checkpoint inhibitors have emerged as effective and revolutionary third-generation anticancer agents over the past decade. CTLA-4, PD-1, and PD-L1 are the most extensively studied immune checkpoint molecules for cancer immune checkpoint inhibition therapy. Since the initial FDA approval of the anti-CTLA-4 antibody ipilimumab for the treatment of metastatic melanoma in 2011, additional immune checkpoint inhibitors have been approved for tumor treatment, including the anti-PD-1 antibodies nivolumab and pembrolizumab and the anti-PD-L1 antibodies atezolizumab, durvalumab, and avelumab [31]. In addition to the aforementioned classical immune pathways, new immune pathways have recently been studied, including LAG-3, VISTA, TIM-3, B7/H3, and TIGIT. Newly investigated immune pathways that use stimulatory pathways include CD40, OX40, 4-1BB, GITR, and ICOS pathways. Studies have also investigated the value of indoleamine-2,3-dioxygenase (IDO), an immune gateway that targets components of the tumor microenvironment [121]. These immune gateway inhibitors block the binding of immune gateway receptors to their ligands, thereby removing the inhibitory signals that prevent (inhibit or block) T cell activation, thereby inducing T cells to recognize tumor cells and activate an antitumor response.
4.3 Immune Checkpoint Modulation Using Liposomes
Immune gateway inhibitors increase immune responses by blocking certain negative feedback pathways; however, they present limitations because they are administered systemically and are often accompanied by side effects that can damage normal organs and tissues. Therefore, liposomes can be used to minimize toxicity (side effects). In immune gateway inhibition therapy, drug delivery using liposomes enables the protection, effective targeting, and controlled release of the drug by preventing its degradation in the surrounding biological environment (Table 3). This increases the amount of inhibitor reaching the target site, enabling effective immune checkpoint inhibition therapy. It can also limit the amount of the inhibitor at non-target sites, thereby reducing side effects such as toxicity [110, 124]. In cancer therapy, tumor-targeted drug delivery can be enhanced by attaching monoclonal antibodies (mAbs) against tumor-associated antigens on the surface of liposomes [125]. This strategy is discussed by presenting examples of immune checkpoints, such as CTLA-4, PD-1, PD-L1, and CD47, and reviewing trends in liposome research on tumor targeting through surface modification of liposomes and immune checkpoints for cancer immunotherapy (Fig. 4).
a Potential of liposomal delivery systems. T cells activated by APCs eliminate cancer cells through various immune mechanisms. However, cancer cells can evade these immune mechanisms when immune checkpoints are blocked. To prevent this, liposomes modified with immune checkpoint inhibitors (ICBs) can be used. Liposomes can reactivate T cells and induce cancer cell death through localized drug release [39] Copyright (2023) by BMC. b Mechanism of tumor-microenvironment- and pH-responsive liposomes. Both in vitro apoptosis in B16F10 cells and in vivo experimental results in mice demonstrated the highest tumor inhibition effect when liposomes were used as delivery vehicles [133] Copyright (2019) by the American Chemical Society. c Mechanism of liposomes with photothermal therapy and IDO-blocking functionality; these liposomes prominently exhibited not only cytotoxicity in 4T1 cells but also photothermal effects [141] Copyright (2019) by Theranostics
Studies have confirmed the benefits of using liposomes for targeting and blocking immune checkpoints, as well as for drug delivery. The results suggest that immune checkpoint inhibition therapy has potential clinical usefulness and provide insight into its clinical applicability.
4.3.1 Blockade of CTLA-4 by Liposomes
In the early stages of the T cell response, CTLA-4 signaling regulates T cell activity in the lymph nodes [126]. The binding of CD28 on T cells to the APC ligands B7-1 (CD80) and B7-2 (CD86) leads to IL-2 production and T cell proliferation and survival [127]. However, CTLA-4 shows a higher affinity than CD28 and outcompetes CD28 in binding to CD80 and CD86, thereby transmitting inhibitory signals and attenuating T cell activation. Thus, CTLA-4 plays a crucial inhibitory role in early T-cell activation and proliferation [128,129,130].
Nikpoor et al. evaluated the therapeutic efficacy of liposomes containing an anti-CTLA-4 antibody to address immune-related adverse events (irAEs) associated with mAbs. Comparing PEGylated liposomes containing anti-CTLA-4 antibody with non-PEGylated liposomes containing anti-CTLA-4 antibody and free antibody, the authors found that PEGylated liposomes containing CTLA-4 antibody had a prolonged blood half-life, significantly higher tumor accumulation, improved therapeutic response, and a greater impact on the antitumor immune response [128]. Alimohammadi et al. studied the combination therapy of doxorubicin and PEGylated liposomes to reduce the side effects of anti-CTLA-4 and increase its therapeutic effect. For this purpose, a B16 mouse melanoma model was treated with non-liposomal anti-CTLA-4 or liposomal anti-CTLA-4 antibodies. The results revealed that only liposomal anti-CTLA-4 significantly reduced the tumor size relative to that in the control group, while enhanced CD8 + cells and CD8 + /Treg ratios were observed in the tumor-infiltrated lymphocytes of liposomal anti-CTLA-4-treated mice [130]. Yang et al. synthesized anti-CTLA-4 nanobody (Nb)-modified liposomes to enhance the effect of CTLA-4 blocking immunotherapy. Nb36/liposome complexes were synthesized and used as CTLA4–B7 pathway blockers and combined with APCs and tumor fusion vaccines to enhance CD8 + T-cell cytokine secretion and activation. In both in vitro and in vivo experiments, we found that treatment with anti-CTLA-4 Nb liposomes enhanced the anticancer activity of CD8 + T cells, causing them to aggregate at the tumor site with high tumor-targeting ability and increase the apoptosis and inflammatory response of tumor cells. This shows that liposome-mediated CTLA-4 blockade could serve as an innovative strategy to overcome the limitations of conventional immunotherapy and inhibit T cell inactivation at the immune gateway [131].
4.3.2 Blockade of PD-1/PD-L1 by Liposomes
The programed death ligand-1 (PD-L1)/PD-1 pathway plays an important role in tumor immunosuppression [111] PD-1 is a member of the B7/CD28 family of costimulatory receptors and is highly expressed on activated T cells, B cells, DCs, and NK cells, and suppresses T cells later in the immune response, mainly in peripheral tissues. In contrast, PD-L1 is expressed on several types of tumor cells, including urothelial cancers, gastrointestinal cancers, lung cancer, breast cancer, and melanoma, where it regulates T cell activation by binding to PD-1, inhibits T cell proliferation and survival, and suppresses the production of IFN-γ, TNF-α, and IL-2 [126, 127]. Several studies have shown that blocking the interaction between PD-1 and PD-L1 mediates antitumor responses via T cell activation and activated CTLs [132].
Various studies have been conducted to use liposomes to address irAEs associated with anti-PD-1 or anti-PD-L1 antibody or to achieve higher therapeutic efficacy. Liu et al. demonstrated an enhanced antitumor effect by combining a PD-L1 inhibitor with liposomes loaded with low doses of doxorubicin, which effectively disrupted PD-1/PD-L1 interactions and achieved a high tumor inhibition efficiency of 78.7% compared to a free PD-L1 inhibitor [133]. Merino et al. conducted a similar study, in which Dox immunoliposomes functionalized with PD-L1 mAb were developed, and their antitumor efficacy and immune activation were evaluated in a B16 OVA melanoma murine cell line overexpressing PD-L1. Compared to conventional liposomes, these immunoliposomes specifically bound to PD-L1 + cells, showed increased interaction and internalization, and induced complete tumor regression in 20% of mice, as well as increased survival and significantly increased CTLs [134]. Du et al. modified PD-L1 antibodies on the surface of liposomes and loaded them with doxorubicin to target PD-L1 expressed on tumor cells and block the PD-1/PD-L1 interaction, which inhibited tumor growth and metastasis through the reversal of immunosuppression [135]. Hei et al. developed liposomes loaded with catalase that presented anti-PD-L1 on the surface, which showed good therapeutic effects and low systemic toxicity [136] Yang et al. fabricated liposomes containing 10 mol% PD-L1 binding peptides to improve the low response rate of conventional therapies for immune checkpoint blockade. The fabricated particles induced significant PD-L1 degradation compared to anti-PD-L1 antibody, interfered with the immune escape mechanism of tumor cells, and enhanced T cell-mediated antitumor immunity by promoting multivalent binding with PD-L1 [137]. Furthermore, Li et al. demonstrated the therapeutic effect of liposomal nanoparticles containing a polyethyleneimine (PEI)-siRNA-PD-L1 complex at the center and imatinib on the surface in melanoma. Both in vitro and in vivo experiments confirmed that the synthesized particles effectively inhibited PD-L1 protein expression and significantly reduced p-S6k protein expression, thereby blocking the mTOR pathway and inhibiting tumorigenesis [138]. Gu et al. synthesized pH-sensitive PD-L1-targeted docetaxel-carrying liposomes to accurately target tumors. By responding to pH changes, liposomes can accumulate within tumor cells, specifically releasing drugs and activating the immune system. This targeted inhibition of the immune gateway can alleviate immune suppression by tumor cells and enhance the immune response to eliminate the tumor [111].
4.3.3 Blockade of CD47 by Liposomes
Along with PD-L1, CD47 is a cell membrane protein that is highly expressed on the surface of many malignant tumor cells where it acts as a “don’t eat me” signal to prevent the phagocytosis of tumor cells by macrophages, thereby inhibiting the immune response to tumors. Several studies have shown that blocking CD47 promotes macrophage differentiation and T-cell activation [111]. In addition, CD47 blockade improves the effectiveness of other immune checkpoint inhibitory treatments (ICB treatments), such as PD-L1 blockade and radiotherapy. Four anti-CD47 antibodies have shown promise in clinical trials; however, when these CD47 inhibitors are administered by ex vivo injection, they can cause CD47 overexpression on the surface of red blood cells, leading to serious side effects such as anemia and thrombocytopenia. Therefore, such hurdles must be overcome before CD47 blockade immunotherapy can be approved for clinical use. Use of liposomal delivery vehicles may be a key to overcome these issues [139]. Chang et al. recently synthesized imiquimod-encapsulated CD47-targeted liposomes termed coupled Fc-CV1 to imiquimod-loaded liposomes (CILPs). The results of in vitro experiments demonstrated that CILPs showed long-lasting release and specific targeting effects, while in vivo results showed that the particles were effectively taken up by tumor cells and exhibited good tumor treatment efficiency and biosafety because of their dual functions of inhibiting the immune response and innate immune gateway [140].
4.3.4 Blockade of Other Immune Checkpoints by Liposomes
Huang et al. investigated methods for inhibiting cancer metastasis while increasing the effectiveness of breast cancer treatments. IDO is highly expressed in many tumors, where it serves to attenuate T cell proliferation and activation; therefore, Huang et al. used IDO inhibitors to reactivate the host immune system, reverse immunosuppression, and enhance therapeutic effects. To improve the anti-tumor effect, a photosensitizer for photodynamic therapy and cancer immunotherapy and an IDO inhibitor were delivered to the tumor microenvironment via liposomes [141]. CXCR4 is a G-protein-coupled receptor that is highly expressed on both tumor cells and tumor-associated fibroblasts in various cancer types, including TNBC. The interaction between CXCR4 and its ligand, stromal cell-derived factor 1 (CXCL12), affects tumor growth, angiogenesis, and metastasis [142]. Lu et al. developed a CXCR4-targeted liposomal delivery vehicle to enhance the immunotherapeutic effects of plerixafor (AMD3100), a CXCR4 antagonist. The researchers designed AMD3100 to not only to be encapsulated within liposomes but also to be coated on the liposomal surface so that it could act as a target molecule, thus acting as a dual blocker inhibiting CXCR4 activation both inside and outside the cell. Compared to the conventional formulation of plerixafor, liposomal-AMD3100 more effectively reconstituted the immune and tissue environments and improved the pharmacodynamic profile of the drug. Moreover, in a mouse model of TNBC, liposomal-AMD3100 showed a stronger antitumor effect and prolonged survival time than a single treatment [143].
5 Liposomal Application in Cancer Vaccines
Cancer cells neutralize immune responses in various ways, such as by secreting chemical signaling substances that render T cells inactive or prevent them from presenting antigens. To restore the immune response during cancer treatment, research is underway to develop therapeutic cancer vaccines that modulate T-cell activity to destroy cancer cells and enhance their antitumor effects. Cancer vaccines primarily use tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs) to activate the immune system; this can be achieved by directly inducing a T cell response or using DCs to activate T cells [144,145,146]. Despite several efforts to develop cancer vaccines, most are still in the preclinical and clinical research stages [147]. Owing to the large diversity of tumor-associated antigens, many of which are yet to be identified, research on specific antigens is essential, and methods of delivering antigens to induce an efficient immune response need to be further explored.
5.1 Vaccine Application for Cancer Treatment
Cancer vaccines are categorized into cellular, DNA, RNA, virus-based, and peptide vaccines [35, 148]. The earliest cancer vaccines were cell-based vaccines that used cancer cells themselves, such as irradiated cancer cells or cell lysates, to generate a specific immune response through cancer antigens on the surface of cancer cells. Because living cells can suppress immune cells, dead cells are used or modified to increase their immunogenicity. For example, MYC oncogenes suppress cellular immunity, and Myc-suppressed tumor cells have shown great efficacy as whole-cell vaccines by inducing immunogenicity [149]. DC vaccines are another class of cell-based vaccines. DCs act as APCs that induce CTL responses by degrading antigens on the surface of cancer cells and presenting them to T cells for recognition by the immune system. Based on these features, DC vaccines are used to induce immune responses; DCs serve as carriers for antigens from various sources such as tumor lysates, tumor-derived mRNA, tumor-associated antigen (TAA)-based peptides, and whole tumors [150].
Recently, the structures of several cancer antigens have been elucidated and highly specific cancer antigens have been mass produced. Antigenic vaccines, which use the cancer antigen itself as a vaccine to efficiently elicit an immune response, have also been introduced into cancer therapy. Peptide vaccines are polypeptides composed of known or predicted immunogenic tumor-associated antigenic epitopes that can be easily mass-produced and administered with minimal toxicity to generate anticancer immune responses [151]. However, peptide-based vaccines have weak immunogenicity because of the limitations of MHC polymorphism and the small size of the antigen epitopes. Additionally, the simple use of peptides without considering the targeting of activated APCs is likely to result in MHC class I tolerance and the loading of non-professional APCs. Therefore, they are used in combination with immune adjuvants to stimulate T-cell responses and increase antitumor effectiveness [152]. However, when antigens are administered as vaccines, the desired immune response is initially generated, but their efficacy decreases over time. Therefore, to induce a sustained immune response, nucleic acid-based cancer vaccines, such as DNA- or RNA-encoding tumor antigens, have been developed. Nucleic acid vaccines are based on the discovery that the administration of recombinant plasmid DNA to animals results in the expression of a foreign protein encoded by the plasmid [153]. Encoded genetic information can be transferred to the host to induce an immune response through the expression of antigenic proteins. Because mRNA enters the cytoplasm to directly translate and express antigens, mRNA vaccines are immediate and efficient. However, once the plasmid DNA enters the nucleus, a single plasmid DNA can generate multiple copies of mRNA and thus produce more antigens than a single mRNA molecule. Therefore, adjuvants have been used to effectively deliver DNA [154].
5.2 Conventional Cancer Vaccine Approaches Using Nanomaterials
Vaccines can be used to present various antigens and target and treat cancer; however, insufficient concentrations of antigens can lead to T cell tolerance. In particular, tumor lysates can prompt immunomodulatory cytokines such as IL-10 and TGF-β to induce tolerogenic transformation [155]. In addition, peptide vaccines have the disadvantages of low immunogenicity and short immune responses. These issues can be mitigated by synthetic long peptides (SLPs), which are sufficiently long to contain multiple MHC class I and II epitopes; nevertheless, they exhibit low immunogenicity and require adjuvants [156]. Similarly, the use of DNA vaccines remains limited because of their low transduction rates in vivo [157].
Various methods have been developed to prevent antigen degradation and improve vaccine efficacy, among which nanotechnology-based vaccine strategies have recently gained prominence [158,159,160]. Nanocarriers can be designed from various materials, including lipids, polymers, proteins, and inorganic nanoparticles, to encapsulate cancer-specific antigens (Fig. 5). These nanocarriers protect antigens, extend their circulation time in the body, and enhance their uptake by immune cells. Nanoparticles can also be used as adjuvants to enhance antigen presentation to immune cells and activate immune responses, thereby improving the effectiveness of cancer vaccines compared to traditional adjuvants. Gong et al. proposed a proton-driven nanotransformer-based vaccine (NTV) in which an antigenic peptide (AP) was loaded onto a polymer-peptide conjugate-based nanotransformer (NT) that can be used as an adjuvant for cancer vaccines [161]. NTVs can effectively deliver APs into the cytoplasm in an acidic endo/lysosomal environment and simultaneously induce antigen presentation and tumor-specific CD8+ T-cell responses by activating the NLRP3 inflammatory vesicle pathway. Nam et al. found that the combination of a synergistic dual adjuvant-based neoantigen cancer vaccine and spiky gold nanoparticle (SGNP)-based photothermal therapy produced synergistic effects with respect to local tumor ablation and local and systemic immune activation compared to either therapy alone. Combination therapy effectively eradicated both small and large local tumors and showed a strong abscopal effect on pre-established distant tumors with robust systemic anti-tumor immunity [162].
a Proton-driven nanotransformer-based vaccine (NTV). The NTV delivers antigenic peptides into the cytosol by transforming their structure under acidic conditions and inducing robust antitumor immunity [161] Copyright (2020) by Springer Nature. b Unlike free cGAMP, PEG-containing cationic liposomes internalize into the endosomal compartment and facilitate cGAMP release into the cytosol. cGAMP induces type I interferon production by binding to the stimulator of interferon genes (STING) adaptor molecule [158, 163] Copyright (2017) by Wiley. c Liposomal vaccine containing CD169/Siglec-1-binding ganglioside GM3 and non-binding ganglioside GM1. GM3 liposomes stimulate effector T and B cell responses in the presence of adjuvants more effectively than non-targeted GM1. [164] Copyright (2021) by Elsevier B.V
5.3 Liposomal Cancer Vaccines
Among the previously investigated nanocarriers, liposomes can simultaneously load hydrophobic substances within the lipid bilayer and hydrophilic substances within them, enabling the sustained or controlled release of substances. Additionally, the high stability of liposomes protects the substance from degradation, and they can be easily delivered to the desired target through surface modification. Liposome delivery can enhance the immune response by presenting antigens to immune cells for effective uptake by cells such as APCs [50, 165]. The use of naked DNA to induce cell-mediated protective immune responses has been studied for DNA delivery-based vaccines [166]. However, naked DNA exhibits a low transfection rate because of its low penetration into the nucleus. DNA delivery via liposomes may enhance the efficacy of DNA vaccines by promoting APC plasmid uptake. Perrie et al. investigated the effect of liposome composition and surface charge on vaccine efficacy and found that liposome-mediated DNA showed a higher antitumor effect than naked DNA and that the appropriate content of cationic lipids contributed to the promotion of the immune response [167]. Anionic lipids in cells help to release nucleic acids from liposomes by neutralizing the charge of cationic lipid carriers [168, 169].
Koshy et al. constructed a liposomal vaccine to induce innate immune activation through stimulation of the interferon gene (STING) pathway in the tumor microenvironment (TME) [163]. DNA shed by dying tumor cells triggers STING pathway activation in DCs to induce an immune response; however, exogenous delivery of STING agonists such as 2′3'-cyclic GMP-AMP (cGAMP) is more effective than endogenous STING activation [170]. STING agonists applied to the TME induce antitumor effects via innate and adaptive immune responses [171, 172]. However, as these agonists have poor membrane permeability and reduced ability to react with cells, this group presented cGAMP to melanoma tumors using cationic liposomes containing PEG, which bind to anionic cell membranes and effectively achieve cytoplasmic delivery of cGAMP, thereby stimulating the STING pathway and increasing type I interferon production in TMEs.
Additional targeting ligands can be introduced into liposomes to enhance vaccine efficacy (Table 4). For example, CD169 + macrophages present antigens to DCs and have been used as alternative targets for cancer vaccines [164, 173]. Grabowska et al. evaluated the ability of liposomes containing ganglioside GM3, a CD169/Siglec-1-binding ligand, and ganglioside GM1, a non-binding ligand, to target antigens in CD169 + macrophages and induce immune responses using a combination of these two ligands [164]. In vivo, CD169 + macrophages captured control and GM1 liposomes; however, their uptake of GM3 liposomes was more than threefold. Furthermore, when GM3 liposomes were loaded with ovalbumin as an antigen and adjuvant, antigen-specific CD8 + and CD4 + T and B cell responses were observed, confirming that the inclusion of GM3 in liposomes enhanced the immune response and increased the efficacy of the vaccine. Similarly, Zhao et al. constructed a mannose-modified liposomal vaccine to target and activate DCs via mannose receptors. This vaccine not only co-delivered HPV16 E7 peptide and CpG oligodeoxynucleotides (CpG ODNs) but also induced an immune response via DCs, which enhanced the antitumor effect [174].
6 Future Direction and Outlook
As illustrated by the examples presented in this review, nanomedicine has been used in various ways to solve conventional problems in cancer therapy. Previous studies have shown that the physicochemical properties of nanoparticles can be modified to modulate the human immune response. Studies continue to explore the correlation of nanoparticles with immune responses in the body, and analyzing the interactions between nanoparticles and immune cells may provide a basis for expanding the application of nanoparticles in immunotherapy.
Moreover, among the nanoparticles used in anticancer immunotherapy, lipid-based liposomal particles continue to demonstrate excellent stability and drug delivery efficiency, and the use of liposomes has significantly increased the survival rates of patients with cancer in clinical trials. Owing to the superiority of liposomes, recent years have seen a rapid increase in the use of not only liposomes loaded with conventional chemotherapeutic agents but also improved liposomal delivery systems for immunotherapy. To address the current challenges in anticancer therapy, various strategies have been explored to develop and optimize liposomes, such as controlling the physicochemical properties of the liposome surface using various surface modification techniques, binding antibodies and ligands that target specific cells, and mRNA loading for application as anticancer vaccines.
Nevertheless, anticancer immunotherapies using liposomal delivery systems require further improvements with respect to production cost, yield, and process technology. Anticancer therapy with liposomes can be expensive due to high production costs, low yields, and challenging quality control. Drug encapsulation and targeting ligand attachment involve multiple synthetic and formulation steps, which can further increase production costs and reduce yield, making production challenging. Immunotherapies may require individualization or customization for each patient, which can increase costs and complicate the manufacturing process. However, liposomal drug formulations have continued to attract attention due to their potential for efficient drug loading, mass production, improved cost-effectiveness, and increased therapeutic efficacy. Consequently, the FDA has approved products such as Doxil, Myocet, and ONPATTRO over the past few decades. Various attempts have been made in academia and industry to address these issues. Liposome-based immunotherapeutics, similar to liposome-based chemotherapeutics, are expected to be clinically approved and made available to patients soon [116, 175, 176]. Various attempts have been made in academia and industry to address these issues. Similar to liposome-based chemotherapeutics, liposome-based immunotherapeutics will be clinically approved and made available to patients soon.
References
Nia, H.T., Munn, L.L., Jain, R.K.: Physical traits of cancer. Science 370, 6516 (2020)
Fares, J., et al.: Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct. Target. Ther. 5(1), 28 (2020)
Mattiuzzi, C., Lippi, G.: Current cancer epidemiology. J Epidemiol Glob Health 9(4), 217–222 (2019)
Soerjomataram, I., Bray, F.: Planning for tomorrow: global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 18(10), 663–672 (2021)
Zugazagoitia, J., et al.: Current challenges in cancer treatment. Clin. Ther. 38(7), 1551–1566 (2016)
Son, M.H., et al.: Recent advances in electrochemical and optical biosensors for cancer biomarker detection. BioChip J. 17(1), 44–67 (2023)
Gavas, S., Quazi, S., Karpinski, T.M.: Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res. Lett. 16(1), 173 (2021)
Debela, D.T., et al.: New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 9, 20503121211034370 (2021)
Lesterhuis, W.J., Haanen, J.B., Punt, C.J.: Cancer immunotherapy–revisited. Nat. Rev. Drug Discov. 10(8), 591–600 (2011)
Wei, G., et al.: Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics 11(13), 6370–6392 (2021)
Perez-Herrero, E., Fernandez-Medarde, A.: Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 93, 52–79 (2015)
Yip, H.Y.K., Papa, A.: Signaling pathways in cancer: therapeutic targets, combinatorial treatments, and new developments. Cells 10(3), 659 (2021)
Zhong, L., et al.: Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 6(1), 201 (2021)
Vanneman, M., Dranoff, G.: Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 12(4), 237–251 (2012)
Tan, S., Li, D., Zhu, X.: Cancer immunotherapy: pros, cons and beyond. Biomed. Pharmacother. 124, 109821 (2020)
Riley, R.S., et al.: Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 18(3), 175–196 (2019)
Choi, S.Y., et al.: Enhanced antitumor effect of the combination of Bacille Calmette-Guérin and an immune checkpoint inhibitor in bladder cancer-on-a-chip. BioChip J. 83, S610 (2023)
Huda, S., Alam, M.A., Sharma, P.K.: Smart nanocarriers-based drug delivery for cancer therapy: an innovative and developing strategy. J. Drug Deliv. Sci. Technol. 60, 102018 (2020)
Kenchegowda, M., et al.: Smart nanocarriers as an emerging platform for cancer therapy: a review. Molecules 27(1), 146 (2021)
Wang, X., Zhang, H., Chen, X.: Drug resistance and combating drug resistance in cancer. Cancer Drug Resist 2(2), 141–160 (2019)
Kaur, G., et al.: Drug-metabolizing enzymes: role in drug resistance in cancer. Clin. Transl. Oncol. 22(10), 1667–1680 (2020)
Liu, S., et al.: The reversal of chemotherapy-induced multidrug resistance by nanomedicine for cancer therapy. J. Control. Release 335, 1–20 (2021)
Disis, M.L.: Mechanism of action of immunotherapy. Semin. Oncol. 41(Suppl 5), S3-13 (2014)
He, X., Xu, C.: Immune checkpoint signaling and cancer immunotherapy. Cell Res. 30(8), 660–669 (2020)
Gao, S., et al.: Nanotechnology for boosting cancer immunotherapy and remodeling tumor microenvironment: the horizons in cancer treatment. ACS Nano 15(8), 12567–12603 (2021)
Zhang, H., Chen, J.: Current status and future directions of cancer immunotherapy. J. Cancer 9(10), 1773–1781 (2018)
Christofi, T., et al.: Current perspectives in cancer immunotherapy. Cancers (Basel) 11(10), 1472 (2019)
Sterner, R.C., Sterner, R.M.: CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11(4), 69 (2021)
Marofi, F., et al.: CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res. Ther. 12(1), 81 (2021)
Safarzadeh Kozani, P., Safarzadeh Kozani, P., Rahbarizadeh, F.: CAR-T cell therapy in T-cell malignancies: Is success a low-hanging fruit? Stem Cell Res. Ther. 12(1), 527 (2021)
Shiravand, Y., et al.: Immune checkpoint inhibitors in cancer therapy. Curr. Oncol. 29(5), 3044–3060 (2022)
Wei, S.C., Duffy, C.R., Allison, J.P.: Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8(9), 1069–1086 (2018)
Robert, C.: A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 11(1), 3801 (2020)
Marin-Acevedo, J.A., Kimbrough, E.O., Lou, Y.: Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 14(1), 45 (2021)
Liu, J., et al.: Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J. Hematol. Oncol. 15(1), 28 (2022)
Elsheikh, R., Makram, A.M., Huy, N.T.: Therapeutic cancer vaccines and their future implications. Vaccines (Basel) 11(3), 660 (2023)
Rurik, J.G., et al.: CAR T cells produced in vivo to treat cardiac injury. Science 375(6576), 91–96 (2022)
Ye, Z., et al.: In vitro engineering chimeric antigen receptor macrophages and T cells by lipid nanoparticle-mediated mRNA delivery. ACS Biomater. Sci. Eng. 8(2), 722–733 (2022)
Ma, G.L., Lin, W.F.: Immune checkpoint inhibition mediated with liposomal nanomedicine for cancer therapy. Mil. Med. Res. 10(1), 20 (2023)
Mai, Y., et al.: Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell. Immunol. 354, 104143 (2020)
Yao, Y., et al.: Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 7, 193 (2020)
Dang, Y., Guan, J.: Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 1, 10–19 (2020)
Akkin, S., Varan, G., Bilensoy, E.: A review on cancer immunotherapy and applications of nanotechnology to chemoimmunotherapy of different cancers. Molecules 26(11), 3382 (2021)
Debele, T.A., Yeh, C.F., Su, W.P.: Cancer immunotherapy and application of nanoparticles in cancers immunotherapy as the delivery of immunotherapeutic agents and as the immunomodulators. Cancers (Basel) 12(12), 3773 (2020)
Nsairat, H., et al.: Liposomes: structure, composition, types, and clinical applications. Heliyon 8(5), e09394 (2022)
Tenchov, R., et al.: Lipid nanoparticles horizontal line from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 15(11), 16982–17015 (2021)
Sercombe, L., et al.: Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 6, 286 (2015)
Olusanya, T.O.B., et al.: Liposomal drug delivery systems and anticancer drugs. Molecules 23(4), 907 (2018)
Alavi, M., Hamidi, M.: Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 34(1), (2019)
Liu, P., Chen, G., Zhang, J.: A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules 27(4), 1372 (2022)
Liu, Y., Castro Bravo, K.M., Liu, J.: Targeted liposomal drug delivery: a nanoscience and biophysical perspective. Nanoscale Horiz. 6(2), 78–94 (2021)
Alavizadeh, S.H., Soltani, F., Ramezani, M.: Recent advances in immunoliposome-based cancer therapy. Curr. Pharmacol. Rep. 2(3), 129–141 (2016)
Wang, S., et al.: Liposomes for tumor targeted therapy: a review. Int. J. Mol. Sci. 24(3), 2643 (2023)
Beltrán-Gracia, E., et al.: Nanomedicine review: clinical developments in liposomal applications. Cancer Nanotechnol. 10(1), (2019)
Bulbake, U., et al.: Liposomal formulations in clinical use: an updated review. Pharmaceutics 9(2), 12 (2017)
Behranvand, N., et al.: Chemotherapy: a double-edged sword in cancer treatment. Cancer Immunol. Immunother. 71(3), 507–526 (2022)
Schirrmacher, V.: From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 54(2), 407–419 (2019)
Advancing Cancer Therapy: Nat Cancer 2(3), 245–246 (2021)
Senapati, S., et al.: Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 3, 7 (2018)
DeVita, V.T., Jr. and E. Chu, A history of cancer chemotherapy. Cancer Res, 2008. 68(21): p. 8643–53.
Goldstein, M., Kastan, M.B.: The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu. Rev. Med. 66, 129–143 (2015)
Liu, G., et al.: A Review on Drug Delivery System for Tumor Therapy. Front. Pharmacol. 12, 735446 (2021)
Anand, U., et al.: Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis 10(4), 1367–1401 (2023)
Kondo, N., et al.: DNA damage induced by alkylating agents and repair pathways. J Nucleic Acids 2010, 543531 (2010)
Valenzuela, M.M., J.W. Neidigh, and N.R. Wall, Antimetabolite Treatment for Pancreatic Cancer. Chemotherapy (Los Angel), 2014. 3(3).
Parker, W.B.: Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer. Chem. Rev. 109(7), 2880–2893 (2009)
Allen, T.M., Cullis, P.R.: Drug delivery systems: entering the mainstream. Science 303(5665), 1818–1822 (2004)
Wilczewska, A.Z., Niemirowicz, K., Markiewicz, K.H., Car, H.: Nanoparticles as drug delivery systems. Pharmacol. Rep. 64(5), 1020–1037 (2012)
Karimi, M., et al.: Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 45(5), 1457–1501 (2016)
Ding, C., et al.: Recent advances in stimuli-responsive release function drug delivery systems for tumor treatment. Molecules 21(12), 1715 (2016)
Briolay, T., et al.: Delivery of cancer therapies by synthetic and bio-inspired nanovectors. Mol. Cancer 20(1), 55 (2021)
Hayashi, K., et al.: High-frequency, magnetic-field-responsive drug release from magnetic nanoparticle/organic hybrid based on hyperthermic effect. ACS Appl. Mater. Interfaces 2(7), 1903–1911 (2010)
Husseini, G.A.W.G.P.: Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 30(10), 1137–1152 (2008)
Rommasi, F., Esfandiari, N.: Liposomal nanomedicine: applications for drug delivery in cancer therapy. Nanoscale Res. Lett. 16(1), 95 (2021)
Allen, T.M., Cullis, P.R.: Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 65(1), 36–48 (2013)
Chang, H.I., Yeh, M.K.: Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 7, 49–60 (2012)
Hua, S.P.J.C.: Targeted nanoparticles that mimic immune cells in pain control inducing analgesic and anti-inflammatory actions: a potential novel treatment of acute and chronic pain conditions. Pain Physician 16, E199–E216 (2013)
Harris, L., et al.: Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer 94(1), 25–36 (2002)
Khalil, D.N., et al.: The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 13(5), 273–290 (2016)
Burugu, S., Dancsok, A.R., Nielsen, T.O.: Emerging targets in cancer immunotherapy. Semin. Cancer Biol. 52(Pt 2), 39–52 (2018)
Southam, C.M., Brunschwig, A., Levin, A.G., Dizon, Q.S.: Effect of leukocytes on transplant ability of human cancer. Cancer 19(11), 1743–1753 (1966)
Met, Ö., et al.: Principles of adoptive T cell therapy in cancer. Semin. Immunopathol. 41(1), 49–58 (2019)
Baars, J.W., Fonk, J.C., Scheper, R.J., von Blomberg-van, B.M., der Flier, H., von Bril, P., Valk, H.M., Pinedo, J.W.: Treatment with tumour infiltrating lymphocytes and interleukin-2 in patients with metastatic melanoma A pilot study. Clin. Trial 4(4), 289–297 (1992)
Fang, K.K., Lee, J.B., Zhang, L.: Adoptive cell therapy for T-cell malignancies. Cancers (Basel) 15(1), 94 (2022)
Wang, S., et al.: Perspectives of tumor-infiltrating lymphocyte treatment in solid tumors. BMC Med. 19(1), 140 (2021)
Zhang, Y., et al.: TCR engineered T cells for solid tumor immunotherapy. Exp. Hematol. Oncol. 11(1), 38 (2022)
Clay, T.M., Custer, M.C., Sachs, J., Hwu, P., Rosenberg, S.A., Nishimura, M.I.: Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J. Immunol. 163(1), 507–513 (1999)
Frankel, T.L., et al.: Both CD4 and CD8 T cells mediate equally effective in vivo tumor treatment when engineered with a highly avid TCR targeting tyrosinase. J. Immunol. 184(11), 5988–5998 (2010)
Wei, T., et al.: Generation of neoantigen-specific T cells for adoptive cell transfer for treating head and neck squamous cell carcinoma. Oncoimmunology 10(1), 1929726 (2021)
Zhang, C., et al.: Engineering CAR-T cells. Biomark. Res. 5, 22 (2017)
Larson, R.C., Maus, M.V.: Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 21(3), 145–161 (2021)
Dagar, G., et al.: Harnessing the potential of CAR-T cell therapy: progress, challenges, and future directions in hematological and solid tumor treatments. J. Transl. Med. 21(1), 449 (2023)
Byun, J.M., Yoon, S.-S.: Chimeric antigen receptor-T cell therapy. Korean J. Med. 97(4), 229–237 (2022)
Gong, N., et al.: Nanomaterials for T-cell cancer immunotherapy. Nat. Nanotechnol. 16(1), 25–36 (2021)
Di Vito, C., et al.: NK cells to cure cancer. Semin. Immunol. 41, 101272 (2019)
Wang, X., et al.: Glycoengineering of natural killer cells with CD22 ligands for enhanced anticancer immunotherapy. ACS Cent. Sci. 6(3), 382–389 (2020)
Lanitis, E., Irving, M., Coukos, G.: Targeting the tumor vasculature to enhance T cell activity. Curr. Opin. Immunol. 33, 55–63 (2015)
Postow, M.A., Sidlow, R., Hellmann, M.D.: Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378(2), 158–168 (2018)
Evans, E.R., et al.: Metallic nanoparticles for cancer immunotherapy. Mater Today (Kidlington) 21(6), 673–685 (2018)
Bode, C., et al.: CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 10(4), 499–511 (2011)
Lin, A.Y., et al.: Gold nanoparticle delivery of modified CpG stimulates macrophages and inhibits tumor growth for enhanced immunotherapy. PLoS ONE 8(5), e63550 (2013)
Almeida, J.P., Figueroa, E.R., Drezek, R.A.: Gold nanoparticle mediated cancer immunotherapy. Nanomedicine 10(3), 503–514 (2014)
Radovic-Moreno, A.F., et al.: Immunomodulatory spherical nucleic acids. Proc. Natl. Acad. Sci. U. S. A. 112(13), 3892–3897 (2015)
Almeida, J.P.M., et al.: In vivo gold nanoparticle delivery of peptide vaccine induces anti-tumor immune response in prophylactic and therapeutic tumor models. Small 11(12), 1453–1459 (2015)
Frey, B., et al.: Old and new facts about hyperthermia-induced modulations of the immune system. Int. J. Hyperthermia 28(6), 528–542 (2012)
Toraya-Brown, S., et al.: Local hyperthermia treatment of tumors induces CD8(+) T cell-mediated resistance against distal and secondary tumors. Nanomedicine 10(6), 1273–1285 (2014)
Daftarian, P., et al.: Peptide-conjugated PAMAM dendrimer as a universal DNA vaccine platform to target antigen-presenting cells. Cancer Res. 71(24), 7452–7462 (2011)
Simhadri, V.R., et al.: Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS ONE 3(10), e3377 (2008)
Guo, H.C., et al.: Immunization of mice by hollow mesoporous silica nanoparticles as carriers of porcine circovirus type 2 ORF2 protein. Virol J 9, 108 (2012)
Gu, Z., et al.: Liposome-based drug delivery systems in cancer immunotherapy. Pharmaceutics 12(11), 1054 (2020)
Gao, A., et al.: Overview of recent advances in liposomal nanoparticle-based cancer immunotherapy. Acta Pharmacol. Sin. 40(9), 1129–1137 (2019)
Rani, V., Venkatesan, J., Prabhu, A.: Liposomes-a promising strategy for drug delivery in anticancer applications. J. Drug Deliv. Sci. Technol. 76, 103739 (2022)
Moholkar, D.N., et al.: Advances in lipid-based carriers for cancer therapeutics: liposomes, exosomes and hybrid exosomes. Cancer Lett. 565, 216220 (2023)
Bayyurt, B., et al.: Encapsulation of two different TLR ligands into liposomes confer protective immunity and prevent tumor development. J. Control. Release 247, 134–144 (2017)
Barnier Quer, C., et al.: Cationic liposomes as adjuvants for influenza hemagglutinin: more than charge alone. Eur. J. Pharm. Biopharm. 81(2), 294–302 (2012)
Ding, Y., et al.: Application of lipid nanovesicle drug delivery system in cancer immunotherapy. J. Nanobiotechnol. 20(1), 214 (2022)
Liu, B., et al.: Equipping cancer cell membrane vesicles with functional DNA as a targeted vaccine for cancer immunotherapy. Nano Lett. 21(22), 9410–9418 (2021)
Tu, K., et al.: Combination of chidamide-mediated epigenetic modulation with immunotherapy: boosting tumor immunogenicity and response to PD-1/PD-L1 blockade. ACS Appl. Mater. Interfaces 13(33), 39003–39017 (2021)
Du, S., et al.: Adoptive cell therapy for cancer treatment. Exploration 3(4), (2023)
Cremolini, C., et al.: Advanced nanotechnology for enhancing immune checkpoint blockade therapy. Nanomaterials (Basel) 11(3), 661 (2021)
Marin-Acevedo, J.A., et al.: Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 11(1), 39 (2018)
Fujii, T., et al.: Biomarkers of response to immune checkpoint blockade in cancer treatment. Crit. Rev. Oncol. Hematol. 130, 108–120 (2018)
Fobian, S.F., Cheng, Z., Ten Hagen, T.L.M.: Smart lipid-based nanosystems for therapeutic immune induction against cancers: perspectives and outlooks. Pharmaceutics 14(1), 26 (2021)
Boone, C.E., et al.: Combining nanomedicine and immune checkpoint therapy for cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 14(1), e1739 (2022)
Park, J.W., et al.: Immunoliposomes for cancer treatment. Adv. Pharmacol. 40, 399–435 (1997)
Dermani, F.K., et al.: PD-1/PD-L1 immune checkpoint: potential target for cancer therapy. J. Cell. Physiol. 234(2), 1313–1325 (2019)
Buchbinder, E.I., Desai, A.: CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 39(1), 98–106 (2016)
Nikpoor, A.R., et al.: Improved tumor accumulation and therapeutic efficacy of CTLA-4-blocking antibody using liposome-encapsulated antibody: In vitro and in vivo studies. Nanomedicine 13(8), 2671–2682 (2017)
Pandey, P., et al.: Revolutionization in cancer therapeutics via targeting major immune checkpoints PD-1, PD-L1 and CTLA-4. Pharmaceuticals (Basel) 15(3), 335 (2022)
Alimohammadi, R., et al.: Encapsulated checkpoint blocker before chemotherapy: the optimal sequence of anti-CTLA-4 and doxil combination therapy. Int. J. Nanomed. 15, 5279–5288 (2020)
Yang, W., et al.: A novel CTLA-4 blocking strategy based on nanobody enhances the activity of dendritic cell vaccine-stimulated antitumor cytotoxic T lymphocytes. Cell Death Dis. 14(7), 406 (2023)
Parry, R.V., et al.: CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell Biol. 25(21), 9543–9553 (2005)
Liu, Y., et al.: Tumor microenvironmental ph and enzyme dual responsive polymer-liposomes for synergistic treatment of cancer immuno-chemotherapy. Biomacromol 20(2), 882–892 (2019)
Merino, M., et al.: Dual activity of PD-L1 targeted Doxorubicin immunoliposomes promoted an enhanced efficacy of the antitumor immune response in melanoma murine model. J. Nanobiotechnol. 19(1), 102 (2021)
Kim, M., et al.: Aptamer-conjugated nano-liposome for immunogenic chemotherapy with reversal of immunosuppression. J. Control. Release 348, 893–910 (2022)
Hei, Y., et al.: Multifunctional immunoliposomes combining catalase and PD-L1 antibodies overcome tumor hypoxia and enhance immunotherapeutic effects against melanoma. Int. J. Nanomed. 15, 1677–1691 (2020)
Yang, S., et al.: Liposome-mediated PD-L1 multivalent binding promotes the lysosomal degradation of PD-L1 for T cell-mediated antitumor immunity. Biomaterials 290, 121841 (2022)
Li, C., Han, X.: Melanoma cancer immunotherapy using PD-L1 siRNA and imatinib promotes cancer-immunity cycle. Pharm. Res. 37(6), 109 (2020)
Zhou, F., et al.: Tumor microenvironment-activatable prodrug vesicles for nanoenabled cancer chemoimmunotherapy combining immunogenic cell death induction and CD47 blockade. Adv. Mater. 31(14), e1805888 (2019)
Chang, R., et al.: Liposome-based co-immunotherapy with TLR agonist and CD47-SIRPalpha checkpoint blockade for efficient treatment of colon cancer. Molecules 28(7), 3147 (2023)
Huang, Z., et al.: Enhanced cancer therapy through synergetic photodynamic/immune checkpoint blockade mediated by a liposomal conjugate comprised of porphyrin and IDO inhibitor. Theranostics 9(19), 5542–5557 (2019)
Guo, F., et al.: CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene 35(7), 816–826 (2016)
Lu, G., Qiu, Y., Su, X.: Targeting CXCL12-CXCR4 signaling enhances immune checkpoint blockade therapy against triple negative breast cancer. Eur. J. Pharm. Sci. 157, 105606 (2021)
Duong, E., et al.: Type I interferon activates MHC class I-dressed CD11b(+) conventional dendritic cells to promote protective anti-tumor CD8(+) T cell immunity. Immunity 55(2), 308-323e9 (2022)
Farhood, B., Najafi, M., Mortezaee, K.: CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J. Cell. Physiol. 234(6), 8509–8521 (2019)
Miao, L., Zhang, Y., Huang, L.: mRNA vaccine for cancer immunotherapy. Mol. Cancer 20(1), 41 (2021)
Saxena, M., et al.: Therapeutic cancer vaccines. Nat. Rev. Cancer 21(6), 360–378 (2021)
Lin, M.J., et al.: Cancer vaccines: the next immunotherapy frontier. Nat Cancer 3(8), 911–926 (2022)
Wu, X., et al.: MYC oncogene is associated with suppression of tumor immunity and targeting Myc induces tumor cell immunogenicity for therapeutic whole cell vaccination. J. Immunother. Cancer 9(3), e001388 (2021)
Sprooten, J., et al.: Trial watch: dendritic cell vaccination for cancer immunotherapy. Oncoimmunology 8(11), e1638212 (2019)
Schneble, E., et al.: Peptide-based cancer vaccine strategies and clinical results. Methods Mol. Biol. 1403, 797–817 (2016)
Liu, W., et al.: Peptide-based therapeutic cancer vaccine: current trends in clinical application. Cell Prolif. 54(5), e13025 (2021)
Cui, Z.: DNA vaccine. Adv. Genet. 54, 257–289 (2005)
Rezaei, T., et al.: Strategies in DNA vaccine for melanoma cancer. Pigment Cell Melanoma Res. 34(5), 869–891 (2021)
Zhang, X., et al.: Engineered tumor cell-derived vaccines against cancer: The art of combating poison with poison. Bioact. Mater. 22, 491–517 (2023)
Stephens, A.J., Burgess-Brown, N.A., Jiang, S.: Beyond just peptide antigens: the complex world of peptide-based cancer vaccines. Front. Immunol. 12, 696791 (2021)
Cole, G., et al.: DNA vaccination for prostate cancer: key concepts and considerations. Cancer Nanotechnol 6(1), 2 (2015)
Liu, J., et al.: Nanoparticle cancer vaccines: design considerations and recent advances. Asian J. Pharm. Sci. 15(5), 576–590 (2020)
Aikins, M.E., Xu, C., Moon, J.J.: Engineered nanoparticles for cancer vaccination and immunotherapy. Acc. Chem. Res. 53(10), 2094–2105 (2020)
Byun, M.J., et al.: Advances in nanoparticles for effective delivery of RNA therapeutics. BioChip J. 16(2), 128–145 (2022)
Gong, N., et al.: Proton-driven transformable nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 15(12), 1053–1064 (2020)
Nam, J., et al.: Photothermal therapy combined with neoantigen cancer vaccination for effective immunotherapy against large established tumors and distant metastasis. Adv. Ther. (Weinh), 4(8), (2021)
Koshy, S.T., et al.: Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv. Biosyst. 1(1–2) (2017)
Grabowska, J., et al.: Liposome induction of CD8(+) T cell responses depends on CD169(+) macrophages and Batf3-dependent dendritic cells and is enhanced by GM3 inclusion. J. Control. Release 331, 309–320 (2021)
Xing, H., Hwang, K., Lu, Y.: Recent developments of liposomes as nanocarriers for theranostic applications. Theranostics 6(9), 1336–1352 (2016)
Chattergoon, M.A., Robinson, T.M., Boyer, J.D., Weiner, D.B.: Specific immune induction following DNA-based immunization. J. Immunol. 160, 5707–5718 (1998)
Yvonne Perrie, P.M.F.: Gregory Gregoriadis, Liposome-mediated DNA vaccination the effect of vesicle composition. Vaccines 19, 3301–3310 (2001)
Tarahovsky, Y.S., Koynova, R., MacDonald, R.C.: DNA release from lipoplexes by anionic lipids: correlation with lipid mesomorphism, interfacial curvature, and membrane fusion. Biophys. J. 87(2), 1054–1064 (2004)
Huang, T., et al.: Lipid nanoparticle-based mRNA vaccines in cancers: current advances and future prospects. Front. Immunol. 13, 922301 (2022)
Woo, S.R., et al.: STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41(5), 830–842 (2014)
Fu, J., et al.: STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 7(283), 283–352 (2015)
Baird, J.R., et al.: Radiotherapy combined with novel STING-targeting oligonucleotides results in regression of established tumors. Cancer Res. 76(1), 50–61 (2016)
Affandi, A.J., et al.: Selective tumor antigen vaccine delivery to human CD169(+) antigen-presenting cells using ganglioside-liposomes. Proc. Natl. Acad. Sci. U. S. A. 117(44), 27528–27539 (2020)
Zhao, Y., et al.: Mannose-modified liposome co-delivery of human papillomavirus type 16 E7 peptide and CpG oligodeoxynucleotide adjuvant enhances antitumor activity against established large TC-1 grafted tumors in mice. Int. J. Nanomed. 15, 9571–9586 (2020)
Alhamhoom, Y., et al.: Recent advances in the liposomal nanovesicles based immunotherapy in the treatment of cancer: a review. Saudi Pharm. J. 31(2), 279–294 (2023)
Hao, Y., et al.: Lipid-based nanoparticles as drug delivery systems for cancer immunotherapy. MedComm 4(4), e339 (2023)
Acknowledgements
This research was supported by the Chung-Ang University Graduate Research Scholarship in 2022 (Eunseo Jeong), the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (No. 2020R1A5A1018052), and the Korea Environment Industry & Technology Institute (KEITI) funded by the Korea Ministry of Environment (MOE) (No. 2022002980003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Dr. Jonghoon Choi is the CEO/Founder; Dr. Yonghyun Choi is the CTO; and Jayoung Chae, Suyeon Ahn, and Heejin Ha are researchers at the Feynman Institute of Technology at the Nanomedicine Corporation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, G., Choi, Y., Hong, J. et al. All-Rounder Liposomes in Cancer Immunotherapy: Strategies and Design Applications of Engineered Liposomal Nanomaterials. BioChip J 18, 211–232 (2024). https://doi.org/10.1007/s13206-024-00147-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-024-00147-1