Abstract
Tensile stress is one of the most common mechanical stresses on the connective tissues of human organs. Cell stretching devices have been developed to study the effects of tensile stress on cells and tissues. In this study, we review how these devices function mechanically and apply them to biological research. To this end, we technically evaluate the four types of actuation processes used in cell stretching devices, including electric motor-driven and electromagnetic actuation, along with their pros and cons. For example, these cell stretching devices have shortcomings including large size, a complicated system, and generation of heat and shock, which hinder the real-time imaging of cells during stretching in high-resolution microscopes. We also describe the effects of tensile stress on cellular and tissue homeostasis. With this review, we seek to explore future directions for development of cell tensioning devices to understand mechanobiological responses to mechanical stress in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cells in normally functioning organs are exposed to various kinds of mechanical stresses such as tensile stress, compressive stress, and shear stress [1,2,3,4,5] (Fig. 1). For instance, epithelial cells in the intestine are subjected to tensile stress due to the peristaltic movement of the intestine, and endothelial cells in blood vessels experience shear stress due to blood flow [4,5,6]. These mechanical stresses induce intracellular biochemical signals that are essential for cell growth, proliferation, and migration [7,8,9]. In addition, it has been recently reported that mechanical stresses can drive cancer invasion [10]. Once a mechanical stress is sensed by a cell, it is converted into biological responses through various signaling processes, known as mechanotransduction [11, 12] (Fig. 2). Mechanotransduction plays an important role in regulating cellular activities, and defects in the process can lead to severe diseases such as cancer and heart failure [1, 2, 13].
Three types of mechanical stresses on a cell. A typical demonstration of the impact of different stresses on an imaginary square. Shear stress produces an angle of difference between opposing sides while tension and compression act normally toward the cell membrane. The drawing is modified from the source [5]
A schematic describing the effects of tensile stress on cell proliferation, apoptosis, migration, and self-renewal. The drawing is modified from the source [11]
Tensile stress is one of the most common mechanical stresses experienced by connective tissues in human organs [1]. In the in vivo context, cells in deforming organs, such as heart, lung, and intestine, are surrounded by various mechanical cues, especially those mediated by tensile stress [1]. Thus, it is important to mimic such environments for studying the functions and behaviors of cells in deforming organs; however, traditional cell culture methods cannot simulate such conditions due to the complexity of in vivo tissue environments [14]. To overcome this limitation, researchers have tried to mimic the cellular environment in which tensile stress is subjected in vitro, and various cell stretching devices have been developed [14,15,16,17]. These cell stretching devices have been widely used for studying the effects of tensile stress on the morphological characteristics of cells, integrity of cell–cell junctions, cytoskeletal structure reorganization, cell proliferation, division, and apoptosis [15, 18,19,20,21,22].
In this study, various kinds of cell stretching devices will be reviewed based on their mechanical actuation and application for biological studies. Different types of actuation processes used in cell stretching devices will be technically evaluated to ascertain their advantages and disadvantages. Studies performed with such devices to investigate the effects of tensile stress on cells will also be introduced. This review will broaden our insights into cell stretching devices and their biological implications.
2 Actuating Types of Cell Stretching Devices
2.1 Electric Motor-Driven Actuation
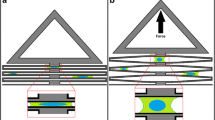
Electric motors have been used as actuators in many cell stretching devices because they can precisely lengthen the connected object to the desired amount [14]. Most cell stretching devices use a method of culturing cells on a flexible substrate and indirectly stretching the cells by elongating the substrate. The type of cell stretching driven by electric motors is typically divided into two methods. One is a horizontal stretching method in which a flexible membrane for cell culture is fixed on one side and laterally elongated to the other side by the motor. Chang et al. [23] connected a micropatterned flexible membrane between two stages on a basal plate, which then uniaxially elongated the membrane by translating one stage through a linear motor while fixing the other stage (Fig. 3a) [23]. A similar methodology is found elsewhere [24]. Kaunas et al. [25] fixed a silicon rubber membrane for a cell culture on a polycarbonate square frame, and an I-shaped Teflon indenter was lifted from under the membrane by the motor to produce uniaxial stretching (Fig. 3b) [25]. Similarly, Huang et al. [26] fixed the membrane for a cell culture to a ring-shaped holder and connected it to a movable stage. The stage vertically translated the membrane by a servo motor on a fixed circular indenter to elongate the membrane equi-biaxially (Fig. 3c) [26]. These methods have the advantages that both static and cyclic stretch can be applied to cells, while the stretch magnitude including stretching ratio, time, and frequency can be easily controlled through computer programming. However, these devices have disadvantages, in that the entire system is too large and complex because a precise motor controller, stages, and computer must be installed for operating motor-based actuation. Additionally, these methods carry a risk of cell contamination because the cell culture location is close to the motor, which can rust due to the humid cell culture environment [14].
2.2 Electromagnetic Actuation
An electromagnet-based cell stretching device of relatively small size was developed to compensate for the disadvantages of electric motor-based cell stretching devices, which can be imprecise when measuring the very large and complex response of the entire system [27]. In this device, a permanent magnet was inserted into a polydimethylsiloxane (PDMS) chamber where cells can be cultured, and the chamber was placed and fixed next to the electromagnet. The permanent magnet was translated back and forth toward the electromagnet by the electromagnetic force, uniaxially elongating the PDMS chamber. Simultaneously, the membrane for the cell culture attached to the bottom of the PDMS chamber was uniaxially stretched. In a similar way, a wrinkled-skin-on-a-chip cell configuration was developed using a permanent magnet and electromagnet [28]. In this device, a porous membrane existed in the PDMS chip, and skin tissue was cultured on the upper part of the porous membrane, while a medium for tissue culture was supplied on the lower part of the porous membrane. Then, the permanent magnet inserted into the chip was translated back and forth by the electromagnet elongating the chip repeatedly, resulting in uniaxial stretch to the skin tissue and accumulation of wrinkles and damage (Fig. 4a). This stretching method has the advantages of device fabricated at a relatively small size, applicability of real-time imaging via microscope, and easy control of the stretch magnitude by adjusting the strength and period of the electromagnetic force. However, electromagnets emit heat as well as electromagnetic force, and this heat can damage the cells or increase the temperature in the cell incubator. In addition, since the permanent magnet is translated at a very high speed when the electromagnetic force is generated, there is a risk of shock transfer, which can damage the cells. In addition, it is difficult to consistently apply a precise stretch magnitude to the cells because translation of the permanent magnet induced by the electromagnetic does not follow a linear path.
2.3 Pneumatic Actuation
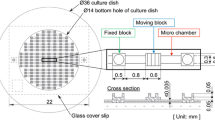
The pneumatic actuation method has been widely used in cell stretching devices based on microelectromechanical systems (MEMS). This actuation type can be divided into two methods. One is to apply pneumatic pressure onto a PDMS chip for expanding the built-in membrane for cell culture, like inflating a balloon [15, 16]. Then, the membrane is equi-biaxially stretched. We fabricated a three-layered cell stretching device using soft lithography [15]. Three PDMS layers, including the cell culture chamber, loading post, and pneumatic chamber, were fabricated and bonded layer-by-layer. When pneumatic pressure was applied to the pneumatic chamber, it lifted the loading post to elevate the membrane of the cell culture to produce equi-biaxial stretch (Fig. 4b).
The other method uses a vacuum inside the hollow chambers located on both sides of the membrane of the cell culture [6]. When a vacuum is generated in the hollow chambers, they contract, which laterally elongates the membrane between the chambers to produce uniaxial stretch. Huh et al. [6] fabricated three chambers arranged in a horizontal direction inside a PDMS chip [6]. When the two chambers on both sides were contracted by vacuum generation, the membrane for the cell culture in the central chamber was laterally elongated to cause uniaxial stretch. This pneumatic actuation type has the advantages that the device can be fabricated in a relatively very small size through the MEMS technology, and the stretch can be simultaneously applied to multiple sample groups by placing several membranes and chambers in a single chip and connecting them to the inlet for simultaneous application of pneumatic pressure. However, MEMS-based cell stretching devices have the disadvantage that direct imaging through a microscope is incredibly difficult because the membrane for the cell culture is located inside the device and the pneumatic chamber or loading post is located in the space under the membrane.
2.4 Thermomechanic and Hydraulic Actuation
Although not mainly used in cell stretching studies, a device using thermomechanical actuation was developed. This type applies the thermal expansion properties of shape memory alloy (SMA) to cell stretching. Iwadate et al. [29] connected the two sides of the membrane for cell culture to the fixed holder and the SMA coil, respectively, and then applied a current to the SMA coil so that the membrane could be uniaxially elongated by contraction and expansion of the SMA coil [29]. This method can precisely control the stretch magnitude but has a disadvantage that the heat generated when applying an electric current to the SMA coil can damage cells.
A cell stretching device using hydraulic actuation was also developed. Wang et al. [30] fabricated a microfluidic device composed of three PDMS layers [30]. The membranes for the cell cultures were equi-biaxially elongated by hydraulic force when water was injected into the fluidic channel by a pump and lifted the membranes. This method, like pneumatic actuation, can stretch a number of cell sample groups simultaneously, and the stretch magnitude can be precisely controlled because water can accurately deliver hydraulic force. However, due to the gas permeability of the PDMS membrane, the existing pneumatic actuation type has a risk that the stretch magnitude might not be maintained when the stretch is longitudinally applied. To its credit, this hydraulic actuation type uses water instead of air when applying the pressure, so the stretch magnitude is stably maintained for a long time [14]. However, similar to the pneumatic actuation type, this method has a disadvantage that direct imaging through a microscope would be very costly and technically challenging and has yet to be achieved.
3 Effects of Tensile Stress on Tensional Homeostasis of Cells
3.1 Cell Proliferation, Division, and Apoptosis
Cells in the body are often subjected to tensile stress and they must proliferate, differentiate, and modulate gene expressions to maintain their tissue homeostasis and prevent injuries or detrimental pathology [1, 17, 31]. In this regard, various studies about the effects of tensile stress on cell proliferation, division, and apoptosis have been conducted. We reported that cyclic stretching induced cell spreading and growth on a soft substrate [15]. Primary mouse embryonic fibroblasts (PMEFs) were seeded on soft pillars, but they could not normally spread and form stress fibers. When the 5% cyclic stretch over a frequency of 0.1 Hz was applied to the cells on the soft pillars for 6 h, cell spreading and stress fiber formation significantly increased, inducing cell proliferation. When subjected to cyclic stretch (5%, 0.1 Hz), the myocardin-related transcription factor-A (MRTF-A) and yes-associated protein 1 (YAP1) [9, 11, 32,33,34] started to be localized into the nucleus. MRTF-A was fully localized in the nucleus after 2 h but moved back to the cytoplasm when the stretch stopped (Fig. 5). These results suggest that repetition of tensile stress could be a stimulation for cell proliferation, and MRTF-A and YAP1 were acting like sensors for mechanical stimuli.
An equi-biaxial cell stretching device actuated by pneumatic pressure and nuclear localization of MRTF-A by cyclic stretching over time [15]
Recently, Gudipaty et al. [21] reported that the stretch triggers rapid epithelial cell division [21] using a uniaxial cell stretching device actuated by linear motor, which is similar to the device as shown in Fig. 3a [23]. The stretch can induce rapid cell division because it can lower the cell density in the cell monolayer. To test if the stretch accelerates the cell division in the cell monolayer, kidney epithelial cells were prepared and 40% static stretch was applied. They found that the stretch induced an approximately fivefold increase in cell division in 1 h. Piezo1, which is a stretch-activated channel for cell death induced by either cell crowding or extrusion [35, 36], was localized in the plasma membrane in the sparse regions where cells were divided by the stretch to form cytoplasmic aggregates in the dense regions in which the cells were extruded. These results suggest that piezo acts as a homeostatic sensor, inducing cell extrusion and apoptosis in the dense regions and cell division in the sparse regions, to control the cell number of the monolayer.
3.2 Cell Shape and Cytoskeleton
Tensile stress can determine cell shape through rearrangement of the cytoskeletal structure [18]. Cell shape is a fundamental signal for cell proliferation and regulates cell growth and physiology [37, 38]. Since tensile stress directly affects cell shape, the effects of tensile stress on cell shape and cytoskeletal structure of cells have been studied in various ways. We reported that the shapes of retinal pigmented epithelial (RPE-1) cells were elongated, showing a high cell aspect ratio when subjected to cyclic stress, and the cell division axis was aligned perpendicular to the elongating axis [18]. The aspect ratio of the cells significantly increased after 10% equi-biaxial cyclic stretch (0.1–10 Hz) for 6 h, but there was no significant change in cell area, except under 10 Hz frequencies. Since cells are divided along the axis perpendicular to their long axis, it was suggested that tensile stress induces elongation of a cell and thus determines its cell division axis.
3.3 Cell–Cell Junctions
In the intestinal epithelium, cell–cell junctions are mainly composed of tight junctions (TJs) and adherens junctions (AJs), and the integrity of TJs is regulated by AJs [39]. Since TJs act as a physical and functional barrier to prevent diffusion of pathogens and toxins, disruption of TJs can cause the pathogenesis of various gastrointestinal diseases [40, 41]. Since the intestine is often subjected to mechanical stresses, especially to the tensile stress induced by peristalsis [4], the effect of tensile stress on disruption of TJs needs to be thoroughly investigated to understand gastrointestinal diseases. In this regard, Samak et al. [19] reported that a cyclic stretch disrupted the cell–cell junctions in the intestinal cell monolayers [19]. A 12% cyclic stretch (0.1 Hz) caused TJ proteins (occludin and ZO-1) and AJ proteins (E-cadherin and β-catenin) to be deformed. In addition, the cyclic stretch increased the paracellular permeability of the intestinal cell monolayer. After application of the stretch for 2 h, the junctional distribution of tight junction proteins was highly deformed and showed wavy structure and disrupted after 6 h. In case of adherens junction proteins, they did not show wavy structure but were dissociated over time. To determine if JNKs, c-Src, and MLCK, which were known to be activated by the tensile stress mediate the disruption of cell–cell junctions [42,43,44,45], inhibitors (SP600125, PP2, ML-7) were treated before application of the stretch. With the treatments of all three inhibitors, the cell–cell junctions were not disrupted after the stretch. In addition, the cyclic stretch increased paracellular permeability of Caco-2 cell monolayer by JNK, c-Src, and MLCK. These results suggest that excessive tensile stress can disrupt cell–cell junctions and increase paracellular permeability.
3.4 Epithelial–Mesenchymal Transition
During the epithelial–mesenchymal transition (EMT), epithelial cells lose their polarity and cell–cell adhesion and become mesenchymal stem cells, gaining migratory and invasive properties [46, 47]. EMT also contributes to aberrant wound healing and fibrosis, especially when the lungs sustain epithelial injuries, including acute respiratory distress syndrome, pulmonary fibrosis, and iatrogenic lung injury [48]. Since the lung epithelium is subjected to tensile stresses due to expansion and contraction of the lung during respiration, tensile stress is assumed to be highly related to EMT, but the effect of tensile stress on lung epithelial injuries is often underappreciated [48]. To reveal the importance of tensile stress in lung epithelial injuries and EMT, Heise et al. [48] investigated if cell stretch can induce EMT in alveolar epithelia [48]. In their study, the expression of EMT markers, including E-cadherin, vimentin, and \(\mathrm{\alpha }\)-smooth muscle actin (SMA), was analyzed after transforming growth factor-β 1 (TGF-β 1), which is known to induce EMT, and cellular stretch were applied to alveolar type II epithelial (AT2) cells. With treatment of TGF-β 1 (10 ng/mL) and application of 15% cyclic stretch (0.86 Hz) for 4 days, expression of E-cadherin significantly decreased, and those of vimentin and \(\mathrm{\alpha }\)-SMA were significantly increased compared to non-stretched cells. Since the decreased expression in E-cadherin and the increased expression of vimentin and \(\mathrm{\alpha }\)-SMA indicate the inducement of EMT, these results confirmed that tensile stress could induce EMT in alveolar epithelial cells. In wound healing and fibrosis of epithelial cells, it was soundly demonstrated that the tensile stress could induce EMT; however, whether tensile stress can induce EMT in the epithelial cell monolayer showing invasive properties has not yet been studied.
4 Conclusion
Cell stretching devices have been developed using various actuation types and have been used to study the effects of tensile stress on cells. With the development, many remarkable new findings and results have been reported, but there are still remaining issues to be solved for more detailed studies. However, there are remaining technical issues that require improvement. For example, previous studies about the effect of tensile stress on cells were mainly performed by end-point experiments, analyzing only the changes between the starting point and endpoint of the stretch. To truly reveal how tensile stress affects cells, the responses of cells to tensile stresses should be analyzed in real-time with high-resolution imaging. However, the existing cell stretching devices have shortcomings including large size, a complicated system, and generation of heat and shock. These factors hinder the real-time imaging of cells during stretching in high-resolution microscopes. Once these issues are solved in the future, the relationship between cells and tensile stress will be thoroughly revealed.
References
Wang, J.H.C., Thampatty, B.P.: An introductory review of cell mechanobiology. Biomech. Model. Mechanobiol 5, 1–16 (2006)
Ingber, D.E.: Cellular mechanotransduction: putting all the pieces together again. FASEB J. 20, 811–827 (2006)
Butcher, D.T., Alliston, T., Weaver, V.M.: A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108–122 (2009)
Sanderson, I.R.: The physicochemical environment of the neonatal intestine. Am. J. Clin. Nutr. 69, 1028s–1034s (1999)
Wang, L., et al.: Biomechanical studies on biomaterial degradation and co-cultured cells: mechanisms, potential applications, challenges and prospects. J. Mater. Chem. B 7, 7439–7459 (2019)
Huh, D., et al.: Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668 (2010)
Hoffman, B.D., Crocker, J.C.: Cell mechanics: dissecting the physical responses of cells to force. Annu. Rev. Biomed. Eng. 11, 259–288 (2009)
Happe, C.L., Engler, A.J.: Mechanical forces reshape differentiation cues that guide cardiomyogenesis. Circ. Res. 118, 296–310 (2016)
Aragona, M., et al.: A mechanical checkpoint controls multicellular growth through YAP1/TAZ regulation by actin-processing factors. Cell 154, 1047–1059 (2013)
Labernadie, A., et al.: A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 19, 224–237 (2017)
Cai, X., Wang, K.C., Meng, Z.: Mechanoregulation of YAP and TAZ in cellular homeostasis and disease progression. Front. Cell Dev. Biol. 9, 673599 (2021)
Silver, F.H., Siperko, L.M.: Mechanosensing and mechanochemical transduction: how is mechanical energy sensed and converted into chemical energy in an extracellular matrix? Crit. Rev. Biomed. Eng. 31, 255–331 (2003)
DuFort, C.C., Paszek, M.J., Weaver, V.M.: Balancing forces: architectural control of mechanotransduction. Nat. Rev. Mol. Cell. Biol. 12, 308–319 (2011)
Kamble, H., et al.: Cell stretching devices as research tools: engineering and biological considerations. Lab Chip 16, 3193–3203 (2016)
Cui, Y., et al.: Cyclic stretching of soft substrates induces spreading and growth. Nat. Commun. 6, 1–8 (2015)
Chiu, C.H., et al.: Osteogenesis and chondrogenesis of primary rabbit periosteal cells under non-uniform 2-axial tensile strain. BioChip J. 14, 438–446 (2020)
Trepat, X., et al.: Universal physical responses to stretch in the living cell. Nature 447, 592–595 (2007)
Kim, E.H., et al.: Effect of cyclic stretching on cell shape and division. BioChip J. 9, 306–312 (2015)
Samak, G., et al.: Cyclic stretch disrupts apical junctional complexes in Caco-2 cell monolayers by a JNK-2-, c-Src-, and MLCK-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 306, 947–958 (2014)
Lee, C.-F., et al.: Cyclic stretch-induced stress fiber dynamics–dependence on strain rate, Rho-kinase and MLCK. Biochem. Biophys. Res. Commun. 401, 344–349 (2010)
Gudipaty, S.A., et al.: Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 543, 118–121 (2017)
Wyatt, T.P., et al.: Emergence of homeostatic epithelial packing and stress dissipation through divisions oriented along the long cell axis. Proc. Nat’l. Acad. Sci. U. S. A. 112, 5726–5731 (2015)
Chang, Y.J., et al.: Micropatterned stretching system for the investigation of mechanical tension on neural stem cells behavior. Nanomedicine 9, 345–355 (2013)
Ahn, J., et al.: A microfluidic stretch system upregulates resistance exercise-related pathway. BioChip J. (2022). https://doi.org/10.1007/s13206-022-00051-6
Kaunas, R., et al.: Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc. Nat’l. Acad. Sci. U. S. A. 102, 15895–15900 (2005)
Huang, L., et al.: A stretching device for high-resolution live-cell imaging. Ann. Biomed. Eng. 38, 1728–1740 (2010)
Harshad, K., et al.: An electromagnetic cell-stretching device for mechanotransduction studies of olfactory ensheathing cells. Biomed. Microdevices 18, 45 (2016)
Lim, H.Y., et al.: Development of wrinkled skin-on-a-chip (WSOC) by cyclic uniaxial stretching. J. Ind. Eng. Chem. 68, 238–245 (2018)
Iwadate, Y., et al.: Cyclic stretch of the substratum using a shape-memory alloy induces directional migration in Dictyostelium cells. Biotechniques 47, 757–767 (2009)
Wang, Q., et al.: A microscale mechanical stimulator for generating identical in-plane surface strains toward live cells on multiple loading sites. Sens. Actuators B Chem. 194, 484–491 (2014)
Gopalan, S.M., et al.: Anisotropic stretch-induced hypertrophy in neonatal ventricular myocytes micropatterned on deformable elastomers. Biotechnol. Bioeng. 81, 578–587 (2003)
Zhao, X.-H., et al.: Force activates smooth muscle α-actin promoter activity through the Rho signaling pathway. J. Cell Sci. 120, 1801–1809 (2007)
McGee, K.M., et al.: Nuclear transport of the serum response factor coactivator MRTF-A is downregulated at tensional homeostasis. EMBO rep. 12, 963–970 (2011)
Dong, J., et al.: Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 (2007)
Eisenhoffer, G.T., et al.: Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484, 546–549 (2012)
Coste, B., et al.: Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010)
Singhvi, R., et al.: Engineering cell shape and function. Science 264, 696–698 (1994)
Folkman, J., et al.: Role of cell shape in growth control. Nature 273, 345–349 (1978)
Matter, K., et al.: Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr. Opin. Cell Biol. 17, 453–458 (2005)
Madara, J.L.: Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol. 60, 143–159 (1998)
DeMeo, M.T., et al.: Intestinal permeation and gastrointestinal disease. J. Clin. Gastroenterol. 34, 385–396 (2002)
Bogoyevitch, M.A., et al.: Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. 70, 1061–1095 (2006)
Bogoyevitch, M.A., et al.: c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim. Biophys. Acta Proteins Proteom. 1804, 463–475 (2010)
Chaturvedi, L.S., et al.: Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J. Biol. Chem. 282, 14–28 (2007)
Li, W., et al.: Integrin and FAK-mediated MAPK activation is required for cyclic strain mitogenic effects in Caco-2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G75–G87 (2001)
Thiery, J.P.: Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2, 442–454 (2002)
Greenburg, G., et al.: Epithelia suspended in collagen gels can lose characteristics of migrating mesenchymal cells. J. Cell Biol. 95, 333–339 (1982)
Heise, R.L., et al.: Mechanical stretch induces epithelial-mesenchymal transition in alveolarepithelia via hyaluronan activation of innate immunity. J. Biol. Chem. 286, 17435–17444 (2011)
Acknowledgements
This research was equally supported by the Fostering Global Talents for Innovative Growth Program (P0008746) supervised by the Korean Institute for Advancement of Technology (KIAT) and by the Technology Innovation Program (Industrial Strategic Technology Development Program-Development of disease models based on 3D microenvironmental platforms mimicking multiple organs and evaluation of drug efficacy) (20008413) funded by the MOTIE (Ministry of Trade, Industry, and Energy) in Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, J., Kim, S., Uddin, S. et al. Microfabricated Stretching Devices for Studying the Effects of Tensile Stress on Cells and Tissues. BioChip J 16, 366–375 (2022). https://doi.org/10.1007/s13206-022-00073-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-022-00073-0