Abstract
In this study, bacterial strains isolated from olive oil mill wastewater assigned to Bacillus (n = 4) and Klebsiella (n = 1) genera, were evaluated for their ability to accumulate intracellular PHA granules using Sudan Black staining. A maximum PHA production of 0.14 g/L (i.e., 30.2% wt./wt. in dry biomass) was observed in Bacillus amyloliquefaciens strain OM81 after 72 h of incubation in the presence of 2% glucose (synthetic medium). To reduce bioplastic production costs and recover a polluting product, olive mill wastewater was tested as a carbon source. In this context, the maximum growth (1.45 g/L) was observed in the presence of 50% olive mill wastewater. After extracting the biopolymers with chloroform, quantitative and qualitative analyses were conducted using Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), and thermogravimetric analysis (TGA). FTIR showed an absorption band at 1730 cm−1 assigned to the elongation of the PHB carbonyl groups. This approach offers a dual benefit of reducing pollution and bioplastic production costs. The Bacillus amyloliquefaciens strain OM81 showed promising results for PHAs production, making it a potential candidate for further investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The excessive usage of petroleum-based plastics for single-use items has led to their accumulation in the environment. As these plastics do not biodegrade, they accumulate in both terrestrial and marine environments. Which causes annually the death of numerous animals, as well as the contamination of soils and waterways with the chemicals they release, and the micro and nanoparticles they accumulate (Chang et al. 2022; Issac and Kandasubramanian 2021). Furthermore, the production of petrochemical plastics not only relies on non-renewable fossil fuels, but also results in significant energy consumption and greenhouse gas emissions. In fact, abandoned plastics in nature have been found to emit methane and ethylene, two potent greenhouse gases (Singh and Sharma 2016).
To address these challenges and explore alternative materials, researchers have developed biodegradable plastics made from biopolymers such as polyhydroxyalkanoates (PHA) (Alsafadi et al. 2020; Bhargava et al. 2020; Mannina et al. 2020; Naser et al. 2021; Park et al. 2021). These biopolymers are extracted from bacteria and have similar material properties to polypropylene (McAdam et al. 2020; Meng et al. 2021). Compared to petrochemical plastics, biopolymers offer several advantages, including being renewable, biobased, and biocompatible. The existence of PHA in bacteria has been known since the 1920s when Maurice Lemoige observed the formation of polyhydroxybutyrate (PHB) inside the bacterium Bacillus megaterium (Lemoigne 1926). PHA are synthesized by microorganisms that can activate a survival mechanism when they lack certain nutrients or when they detect an excess of carbon in their nutrient medium (Jung et al. 2019; Lee et al. 2021; Tarrahi et al. 2020). The physical and chemical properties of PHAs vary significantly based on their monomer composition, leading to a broad range of characteristics. Their hydrophobicity, melting point, glass transition temperature, and degree of crystallinity are all influenced by the type of monomers they contain (Sehgal and Gupta 2020; Naser et al. 2021). Moreover, PHAs display a diverse array of mechanical characteristics, ranging from hard and crystalline to elastic. Wild and recombinant strains of Ralstonia eutropha, formerly known as Alcaligenes eutrophus or Cupriavidus necator (Biglari et al. 2018), are best known for accumulating high levels of PHA. However, recent reports have drawn attention to the significant production of PHA by various bacterial species. One such species is Aneurinibacillus sp. H1, an innovative Gram-positive bacterium exhibiting moderate thermophilicity. This microorganism is a promising route for PHA biosynthesis, as it enables the monomer composition to be adapted to suit specific needs (Sedlacek et al. 2020).
Other noteworthy strains that have been studied over the past few years include Bacillus sp. (Kanjanachumpol et al. 2013), Pseudomonas sp. (Kanavaki et al. 2021), Burkholderia sp. (Keenan et al. 2006), Azotobacter sp. (Urtuvia et al. 2022), Halomonas sp. (Thomas et al. 2020), Haloferax sp. (Singh and Singh 2018), Acetobacter sp. (Chang et al. 2021) and recombinant E. coli (Ray et al. 2012). During nutritional deficiencies, various Bacillus species were found to produce up to 90% (w/w) PHA in dry cells (Anjum et al. 2016). Due to their genetic stability, these microorganisms have emerged as model organisms in industry and science (Biedendieck et al. 2007).
Bacillus species have the capability of producing PHA copolymers utilizing simple, affordable, and structurally unbound carbon sources, in addition to having a faster growth rate than other bacteria. Moreover, the isolates have the potential to secrete various hydrolytic enzymes that can be utilized to produce PHA at a low cost, using agro-industrial and other waste materials (Israni and Shivakumar 2013). However, the high production cost and the widespread availability of low-cost petrochemical-derived plastics have hindered the development of bioplastics for packaging applications. To make the process economically attractive, several studies have demonstrated that organic-rich agricultural, agro-industrial, and domestic wastes can be employed as new raw materials for PHA production (Sehgal and Gupta 2020).
Olive mill wastewater (OMW), a by-product of the olive oil industry, has the potential to be a low-cost substrate for PHA production. OMW is rich in easily digestible carbon sources, including carbohydrates, lipids, and volatile fatty acids, which serve as direct substrates for PHA production (Alsafadi and Al-Mashaqbeh 2017). Mediterranean countries, particularly Tunisia, generate large quantities of OMW annually, with an average production of 1 million tons of olive oil mill wastewater (Marks et al. 2020). This results in serious environmental concerns such as changes in soil microbial populations, threats to surface and groundwater sources, and air pollution from phenol and sulfur dioxide emissions (Albalasmeh and Mohawesh 2023).
The manufacture of PHAs from OMW currently involves multi-stage processes, which include pretreatment to remove polyphenols, acidogenic fermentation to generate volatile fatty acids, and the accumulation of PHAs using pure or mixed culture cell methods. This approach has a high cost and requires significant resources (Alsafadi and Al-Mashaqbeh 2017). This work represents the first attempt to produce PHAs from untreated OMW using the genus Bacillus. We developed a new culture method for the production of PHA from OMW in order to reduce its production cost, while helping to reduce pollution.
Materials and methods
Materials
The strains Bacillus thioparans OM39, Bacillus thuringiensis OM55, Bacillus cereus OM75, Bacillus amyloliquefaciens OM81, and Klebsiella oxytoca OM91 were retrieved from the Laboratory Bioresources, Environment and Biotechnologies (BeB, LR22ES04) at the Higher Institute of Applied Biological Sciences of Tunis (ISSBAT). In a previous study of Arous et al. (2018), these strains were isolated and identified from a sequential batch reactor (SBR) that utilized OMW as a substrate. Fresh OMW was collected from a three-phase decanter olive mill located in the Nabeul region of Tunisia and stored in clean 1.5-l bottles at 4 °C until use. Prior to use, the OMW was decanted and filtered. The OMW was then subjected to various analyses (Table 1), including assessment of pH, electrical conductivity (EC), total solids (TS), volatile solids (VS), chemical oxygen demand (COD), total phenols, ammoniacal nitrogen (NH4-N), total sugars, and total phosphorus (Fleyfel et al. 2022).
Growth conditions
PHAs are produced by bacteria only under nitrogen-limited conditions (Naser et al. 2021). The PHA production process involved two steps: (i) the bacteria were grown in 250-mL Erlenmeyer flasks containing 50 mL of Brain Heart Infusion (BHI) medium. The flasks were inoculated with 1% (v/v) of the inoculum and then incubated at 37 °C with constant shaking at 120 rpm for 24 h. (ii) Once an optimal cell concentration was reached, the cell mass was collected by centrifugation at 6000 rpm for 20 min, washed with sterile distilled water, and inoculated into nitrogen-limited medium (NLM). The culture was incubated at 37 °C on a rotatory shaker (120 rpm) for 72 h. After selecting the best PHA-producing strain, the same fermentation protocol was repeated, except that glucose was replaced by OMW at different concentrations (25%, 50%, 75%, 100% (v/v) diluted in pure water).
Screening for PHAs producing strains
The PHA-producing bacteria were identified using a nitrogen-limiting medium (NLM) agar and incubated for 2 days at 37 °C. The composition of the medium was according to Chaijamrus and Udpuay (2008) and contained (in g/L): 20 glucose (as the sole carbon source), 0.5 NH4Cl, 2.3 KH2PO4, 2.3 Na2HPO4, 0.5 MgSO4·7H2O, 0.01 CaCl2, and 20 agar, supplemented with 5 mL/L trace element solution containing (in g/L): 0.2 ZnSO4·7H2O, 1.8 MnCl2·4H2O, 2.8 H3BO4, and 0.1 CuSO4. The pH was adjusted to 7 and the medium was autoclaved for 15 min at 121 °C. This medium was used for all subsequent experiments in this study.
Sudan Black B plate assay
PHA production potential of all isolates was evaluated qualitatively using Sudan Black B dye (Liu et al. 1998). An ethanolic solution of Sudan Black B (0.05%, w/v) was added to the colonies, and the plates were incubated for 30 min. Colonies that turned dark blue in color after rinsing with 96% ethanol were considered positive for PHA production (Phanse et al. 2011).
Sudan Black B staining
The bacterial smears were first air-dried and then fixed on glass slides. They were then stained with Sudan Black B (0.3% in 70% ethanol) for 15 min, followed by washing with ethanol, rinsing, and staining with safranin for 5 min (Wei et al. 2011). Finally, the stained smears were observed under an optical microscope with a 100× oil-immersion objective.
Determination of cell weight
The culture medium for PHA production was centrifuged at 4 °C for 20 min at 6000 rpm. The resulting pellets were washed with distilled water and transferred into pre-weighed sterile glass flasks. The flasks were then oven-dried at 55 °C for 24 h, cooled down in a desiccator, and weighed to determine the PHA yield (Wang and Lee 1997). The experiment was performed in triplicate. The supernatant was collected to measure the biochemical parameters such as glucose abatement rate (which confirms its consumption by bacteria), phenolic compounds, and soluble COD.
Extraction of the accumulated PHA
The dried biomass was collected, and the cellular granules were weighed and crushed using a mortar. Then, 10 mL of chloroform were added to the flasks, which were sealed and kept at room temperature for 24 h to facilitate the release of intracellular PHAs. The mixture was filtered through Whatman No. 1 paper and collected in a pre-weighed flask. The solvent was evaporated using a rotavapor, and the PHA obtained was weighed. The biopolymer content was calculated as a percentage of cellular dry weight using Eq. 1. The experiment was performed in triplicate:
A film of PHA was produced and characterized.
PHA characterization
UV–Vis spectroscopy
The extracted PHA was dissolved in chloroform and its spectra were scanned in the range of 200–320 nm using a Spectrum Instruments SP-UV 300RB. The spectra were analyzed for a net peak at 240 nm, and chloroform blank was used for comparison (Selvakumar et al. 2011).
FTIR spectroscopy
The PHA film was characterized via Fourier Transform Infrared (FTIR) spectrometry in the range of 4000 to 400 cm−1 according to Beji et al. (2023) using the PerkinElmer Frontier IR/NIR systems (USA).
Differential scanning calorimetry (DSC)
The thermal properties of the PHA film were studied using differential scanning calorimetry with a DSC Q2000 and an RCS90 cooling system (TA Instruments). Samples of PHA weighing 5–10 mg were placed in aluminum pans and heated from − 40 °C to 400 °C at a rate of 10 °C per minute in a nitrogen atmosphere (García-Quiles et al. 2019).
Thermogravimetric analysis (TGA)
The thermogravimetric analysis (TGA) of the PHA sample was conducted using a Mettler Toledo TGA/DSC 3 + Equipment. The sample was heated from 25 to 800 °C at a rate of 10 °C/min under a N2 flux (30 mL/min) (Beji et al. 2023).
Statistical analyses
The mean values of three analyses were calculated and expressed as mean ± standard deviation using Origin Pro 2018 software. Statistical analysis was performed using a Student’s t-test to determine the differences among means at a 95% confidence level using Statgraphics software.
Results and discussion
There is a growing interest in finding sustainable alternatives to petroleum-based plastics which pose a significant environmental threat. This study explores the potential of using OMW as an economical carbon source for producing bacterial PHAs, utilizing B. thioparans OM39, B. thuringiensis OM55, B. cereus OM75, B. amyloliquefaciens OM81, and K. oxytoca OM91 previously isolated from OMW (Arous et al. 2018).
Confirmation of PHA production
The five strains were screened for their ability to accumulate PHA granules using the Sudan Black B (SB) plate test, which is a rapid and viable colony staining method. The test distinguishes between PHA-accumulating bacteria, which appeared blue-black, and non-PHA producers, which appeared white. Upon staining with SB, all five isolates appeared blue-black, indicating the presence of PHA granules (Supplementary Data 1). The SB-stained bacterial cells were examined under a microscope, revealing the presence of vesicles like granules (look like holes) within pink-colored cells, as shown in Supplementary Data 2. This staining method indicated the presence of PHA granules in the cells (Wei et al. 2011). The staining also enabled a comparison of the PHA production ability among the bacterial strains, revealing that Cupriavidus taiwanensis 184 had a significantly higher amount of PHA granules (black region) compared to the other strains.
Bacteria growth and accumulation of PHA
All five bacterial strains showed a significant ability to produce PHAs, ranging from 13.6% of dry cell weight for B. thuringiensis to 30.2% for B. amyloliquefaciens (Table 2). These findings are consistent with those reported by Chen et al. (1991) when cultivating various Bacillus strains using glucose as the carbon source. The reduction in residual sugars (from 20.0 g/L to values between 18.5 and 7.8 g/L) and the increase in glucose abatement rate (reaching 61.2%) indicate that these strains can use glucose as a carbon source and convert it into PHA in the form of reserve granules in their cells. The obtained bioplastics are displayed in Supplementary Data 3. B. amyloliquefaciens OM81 exhibited the highest biomass and PHA yields when grown on nitrogen-limiting medium supplemented with glucose as a carbon source, among the five strains tested. The residual glucose concentration was reduced to 7.8 g/L, with a glucose abatement rate of 61.2%, and the PHA yield was 0.14 g/L of dry weight, corresponding to a production rate of 30.2%. Due to its excellent ability to convert glucose into PHA-rich biomass, this strain was selected for further investigation. The extracted PHA (Supplementary Data 3A) was subjected to extensive physicochemical (UV–visible), structural (FTIR), and thermal (DSC and TGA) characterizations.
The biomass and PHA production of B. amyloliquefaciens OM81 were determined in media with varying concentrations of untreated OMW (25%, 50%, 75%, and 100% (v/v) diluted in water). The results presented in Table 3 show that growth was observed in all media, including 100% OMW, indicating that the strain was not inhibited by the raw OMW. The best biomass production was obtained in the medium with 100% OMW (2.81 g/L), which could be attributed to the strain’s prior acclimation to high OMW concentrations in a batch reactor (SBR) and the presence of low levels of toxic compounds, such as tannins and simple phenolic compounds in the raw OMW. The highest yield of PHAs (11.2%) was obtained in the presence of 50% OMW concentration, which can be attributed to the dilution of OMW, leading to a decrease in both COD and phenolic compounds of the raw OMW, thus promoting cell proliferation and PHA production. However, these yields are relatively low compared to those observed in synthetic medium (glucose). Alsafadi and Al-Mashaqbeh (2017) reported that the optimal cell growth of Haloferax mediterranei was achieved at 5% of OMW, with growth being inhibited by increasing concentrations of OMW from 50 to 75%.
UV–visible spectrophotometric analysis
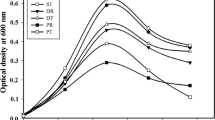
PHAs produced by the various bacterial strains were analyzed using UV–visible spectrophotometry. The bacterial cultures were scanned, and a peak was observed at 243 nm with four strains, as shown in Fig. 1. This peak was particularly prominent in OM39, OM81, and OM91, suggesting that the PHA produced by these bacteria is likely to be the polyhydroxybutyrate biopolyester (Selvakumar et al. 2011). This analysis was conducted on extracts obtained from the culture of OM81, which yielded the highest amount of PHA, grown in media with increasing OMW concentrations. Spectra obtained from 25%, 50%, 75% and 100% OMW concentrations also showed a peak at 243 nm, corresponding to the PHB peak, as depicted in Fig. 2. Selvakumar et al (2011) and Getachew and Woldesenbet (2016) obtained similar results in their studies on Haloarcula marismortui MTCC 1 and Bacillus spp., respectively.
Structural and thermal characterization of PHA produced by B. amyloliquefaciens strain OM81
FTIR spectrophotometric analysis
The FTIR spectrum of PHA produced by strain OM81 using glucose as a carbon substrate (Fig. 3) revealed the presence of various absorption peaks. The peak at 2917–2849 cm−1 was assigned to the presence of an alkyl-CH3 group, while the absorption bands at 1730 and 1600 cm−1 indicated the elongation of the carbonyl groups C=O (a distinctive PHB peak) and −C=C, respectively (Guo et al. 2019; Miranda et al. 2023; Mohapatra et al. 2017). Peaks at 1492 and 1451 cm−1 were assigned to the asymmetrical deformation of the C–H bond in the group CH2, and the peak at 1366 cm−1 indicated the methyl group CH3 (Valdez-Calderón et al. 2022). The vibrations between 1250 and 1027 cm−1 corresponded to the elongation of the C–O–C bond of the ester function (Muneer et al. 2022). These FTIR spectrum results were consistent with those found in the literature for PHB (Mohapatra et al. 2017).
Differential scanning calorimetry and thermogravimetric analyses
The PHA extracted from OM81 bacterial strain, produced in the presence of excess glucose in synthetic medium, was subjected to thermal analyses using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). The resulting DSC thermogram exhibited two peaks: the first peak at 69 °C was attributed to the boiling point of residual chloroform, while the second peak at 147.3 °C corresponded to the melting of the produced PHA (Alsafadi and Al-Mashaqbeh 2017; Kim et al. 2020). Mohapatra et al. (2017) found similar results for PHB homopolymer, showing a dual melting point at temperatures of 120.6 °C and 145.9 °C attributed to the re-crystallization followed by cross-linking isomerization reactions. In our case, only the second peak was observed. These findings indicate the potential of B. amyloliquefaciens strain OM81 to produce PHA as a suitable material for bioplastic manufacture, especially for food packaging applications as confirmed by Tripathi et al. (2019). A lower melting temperature (112.8 °C) was observed by Choi et al. (2021) on short-chain-length PHA copolymer produced by Pseudomonas sp. B14-6 b.
Thermal stability analysis of the produced PHA was conducted using TGA. The TGA and DTG curves are shown in Fig. 4. The first weight loss observed in the 25–75 °C temperature range was attributed to the evaporation of residual chloroform. The second weight loss was observed in the temperature range of 210–355 °C, and the third weight loss was observed in the temperature range of 360–460 °C. The observed phenomenon could be attributed to the cross-linking isomerization process that takes place before the degradation as stated before by Mohapatra et al. (2017). However, it is challenging to determine if the cross-linking process follows a distinct mechanism for the various groups in PHB. These results are consistent with previous studies, which suggested that cross-linking isomerization can improve the polymer’s thermal stability (Mohapatra et al. 2017). Further research has indicated that introducing a (4HB) monomer into the polymer composition substantially reduced the melting temperature and concurrently resulted in a gradual improvement in thermal decomposition resistance (Sedlacek et al. 2020). The first derivative TGA profile revealed a degradation temperature (Td) of 420 °C (Fig. 4), lower than the one observed by Mohapatra et al. 2017 (466.8 °C), but higher than the degradation temperature (288 °C) observed by Miranda et al. (2023).
Conclusions
The present study revealed that all five bacterial strains tested were capable of synthesizing PHA in a nitrogen-limited environment. Among them, B. amyloliquefaciens strain OM81 exhibited the highest biomass and PHA yield of 0.14 g/L of dry weight, corresponding to a production rate of 30.2%, a residual glucose concentration reduced to 7.8 g/L, and a glucose abatement rate of 61.2%. The synthesized PHA was subjected to structural and thermal characterization. FTIR spectrum revealed an absorption band at 1730 cm−1 assigned to the elongation of the PHB carbonyl groups. Additionally, we investigated the suitability of using OMW (11.8%) as a carbon source for PHA synthesis by this strain. The study underscores the potential of utilizing OMW to produce biodegradable polymers, while also reducing production costs. However, further optimization of the biopolyester production process is needed. Future research should also investigate a low-cost pretreatment of OMW and the use of other waste streams as potential carbon sources for PHA synthesis.
Data availability
Not applicable in this case.
References
Albalasmeh AA, Mohawesh OE (2023) Effects of olive mill wastewater on soil physical and hydraulic properties: a review. Water Air Soil Pollut 234(1):42. https://doi.org/10.1007/s11270-022-06055-0
Alsafadi D, Al-Mashaqbeh O (2017) A one-stage cultivation process for the production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) from olive mill wastewater by Haloferax mediterranei. New Biotechnol 34:47–53. https://doi.org/10.1016/j.nbt.2016.05.003
Alsafadi D, Ibrahim MI, Alamry KA, Hussein MA, Mansour A (2020) Utilizing the crop waste of date palm fruit to biosynthesize polyhydroxyalkanoate bioplastics with favorable properties. Sci Total Environ 737:139716. https://doi.org/10.1016/j.scitotenv.2020.139716
Anjum A, Zuber M, Zia KM, Noreen A, Anjum MN, Tabasum S (2016) Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: a review of recent advancements. Int J Biol Macromol 89:161–174. https://doi.org/10.1016/j.ijbiomac.2016.04.069
Arous F, Hamdi C, Kmiha S, Khammassi N, Ayari A, Neifar M, Mechichi T, Jaouani A (2018) Treatment of olive mill wastewater through employing sequencing batch reactor: performance and microbial diversity assessment. 3 Biotech 8(11):481. https://doi.org/10.1007/s13205-018-1486-6
Beji A, Keshk SMAS, Douiri S, Charradi K, Ben Hassen R, Gtari M, Attia H, Ghorbel D (2023) Bioactive film based on chitosan incorporated with cellulose and aluminum chloride for food packaging application: fabrication and characterization. Food Biosci 53:102678. https://doi.org/10.1016/j.fbio.2023.102678
Bhargava N, Sharanagat VS, Mor RS, Kumar K (2020) Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: a review. Trends Food Sci Technol 105:385–401. https://doi.org/10.1016/j.tifs.2020.09.015
Biedendieck R, Gamer M, Jaensch L, Meyer S, Rohde M, Deckwer WD, Jahn D (2007) A sucrose-inducible promoter system for the intra- and extracellular protein production in Bacillus megaterium. J Biotechnol 132(4):426–430. https://doi.org/10.1016/j.jbiotec.2007.07.494
Biglari N, Ganjali Dashti M, Abdeshahian P, Orita I, Fukui T, Sudesh K (2018) Enhancement of bioplastic polyhydroxybutyrate P(3HB) production from glucose by newly engineered strain Cupriavidus necator NSDG-GG using response surface methodology. 3 Biotech 8(8):330. https://doi.org/10.1007/s13205-018-1351-7
Chaijamrus S, Udpuay N (2008) Production and characterization of polyhydroxybutyrate from molasses and corn steep liquor produced by Bacillus megaterium ATCC 6748. Agric Eng. X, 1–12.
Chang YC, Reddy MV, Imura K, Onodera R, Kamada N, Sano Y (2021) Two-stage polyhydroxyalkanoates (PHA) production from cheese whey using Acetobacter pasteurianus C1 and Bacillus sp. CYR1. Bioengineering 8(11):157. https://doi.org/10.3390/bioengineering8110157
Chang X, Fang Y, Wang Y, Wang F, Shang L, Zhong R (2022) Microplastic pollution in soils, plants, and animals: a review of distributions, effects and potential mechanisms. Sci Total Environ 850:157857. https://doi.org/10.1016/j.scitotenv.2022.157857
Chen GQ, König KH, Lafferty RM (1991) Occurrence of poly-D(-)-3-hydroxyalkanoates in the genus Bacillus. FEMS Microbiol Lett 84(2):173–176. https://doi.org/10.1111/j.1574-6968.1991.tb04592.x
Choi TR, Park YL, Song HS, Lee SM, Park SL, Lee HS, Kim HJ, Bhatia SK, Gurav R, Choi KY, Lee YK, Yang YH (2021) Fructose-based production of short-chain-length and medium-chain-length polyhydroxyalkanoate copolymer by Arctic pseudomonas sp. B14–6. Polymers 13:1398. https://doi.org/10.3390/polym13091398
Fleyfel LM, Leitner NKV, Deborde M, Matta J, El Najjar NH (2022) Olive oil liquid wastes-characteristics and treatments: a literature review. Process Saf Environ Prot 168:1031
García-Quiles L, Cuello ÁF, Castell P (2019) Sustainable materials with enhanced mechanical properties based on industrial polyhydroxyalkanoates reinforced with organomodified sepiolite and montmorillonite. Polymers 11(4):696. https://doi.org/10.3390/polym11040696
Getachew A, Woldesenbet F (2016) Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res Notes 9(1):1–9. https://doi.org/10.1186/s13104-016-2321-y
Guo Y, Wang L, Chen Y, Luo P, Chen T (2019) Properties of luffa fiber reinforced PHBV biodegradable composites. Polymers 11(11):1765
Israni N, Shivakumar S (2013) Combinatorial screening of hydrolytic enzyme/s and PHA producing Bacillus spp. for cost effective production of PHAs. Int J Pharma Bio Sci 4(3):934–945
Issac MN, Kandasubramanian B (2021) Effect of microplastics in water and aquatic systems. Environ Sci Pollut Res 28:19544–19562
Jung HR, Yang SY, Moon YM, Choi TR, Song HS, Bhatia SK, Gurav R, Kim EJ, Kim BG, Yang YH (2019) Construction of efficient platform Escherichia coli Strains for polyhydroxyalkanoate production by engineering branched pathway. Polymers 11:509. https://doi.org/10.3390/polym11030509
Kanavaki I, Drakonaki A, Geladas ED, Spyros A, Xie H, Tsiotis G (2021) Polyhydroxyalkanoate (Pha) production in pseudomonas sp. phdv1 strain grown on phenol as carbon sources. Microorganisms 9(8):1636. https://doi.org/10.3390/microorganisms9081636
Kanjanachumpol P, Kulpreecha S, Tolieng V, Thongchul N (2013) Enhancing polyhydroxybutyrate production from high cell density fed-batch fermentation of Bacillus megaterium BA-019. Bioprocess Biosyst Eng 36(10):1463–1474. https://doi.org/10.1007/s00449-013-0885-7
Keenan TM, Nakas JP, Tanenbaum SW (2006) Polyhydroxyalkanoate copolymers from forest biomass. J Ind Microbiol Biotechnol 33(7):616–626. https://doi.org/10.1007/s10295-006-0131-2
Kim HS, Chen J, Wu LP, Wu J, Xiang H, Leong KW, Han J (2020) Prevention of excessive scar formation using nanofibrous meshes made of biodegradable elastomer poly (3-hydroxybutyrate-co-3-hydroxyvalerate). J Tissue Eng 11:2041731420949332. https://doi.org/10.1177/2041731420949332
Lee SM, Lee HJ, Kim SH, Suh MJ, Cho JY, Ham S, Jeon JM, Yoon JJ, Bhatia SK, Gurav R, Lee EY, Yang YH (2021) Screening of the strictly xylose-utilizing Bacillus sp. SM01 for polyhydroxybutyrate and its co-culture with Cupriavidus necator NCIMB 11599 for enhanced production of PHB. Int J Biol Macromol 181:410–417. https://doi.org/10.1016/j.ijbiomac.2021.03.149
Lemoigne M (1926) Products of dehydration and of polymerization of β-hydroxybutyric acid. Bull Soc Chem Biol 8:770–782
Liu M, González JE, Willis LB, Walker GC (1998) A novel screening method for isolating exopolysaccharide-deficient mutants. Appl Environ Microbiol 64(11):4600–4602. https://doi.org/10.1128/aem.64.11.4600-4602.1998
Mannina G, Presti D, Montiel-Jarillo G, Carrera J, Suárez-Ojeda ME (2020) Recovery of polyhydroxyalkanoates (PHAs) from wastewater: a review. Biores Technol 297:122478. https://doi.org/10.1016/j.biortech.2019.122478
Marks EA, Kinigopoulou V, Akrout H, Azzaz AA, Doulgeris C, Jellali S, Jeguirim M (2020) Potential for production of biochar-based fertilizers from olive mill waste in Mediterranean Basin countries: an initial assessment for Spain. Sustainability 12(15):6081. https://doi.org/10.3390/su121560816081
McAdam B, Fournet MB, McDonald P, Mojicevic M (2020) Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 12:2908. https://doi.org/10.3390/polym12122908
Meng D, Gong C, Sukumaran RK, Dionysiou DD, Huang Z, Li R, Li Q (2021) Production of polyhydroxyalkanoates from propylene oxide saponification wastewater residual sludge using volatile fatty acids and bacterial community succession. Bioresourc Technol 329:124912. https://doi.org/10.1016/j.biortech.2021.124912
Miranda DA, Marín K, Sundman O, Hedenström M, Quillaguaman J, Gorzsás A, Broström M, Carlborg M, Lundqvist J, Romero-Soto L, Jönsson LJ, Carrasco C, Martín C (2023) Production and characterization of poly(3-hydroxybutyrate) from Halomonas boliviensis LC1 cultivated in hydrolysates of quinoa stalks. Fermentation 9:556. https://doi.org/10.3390/fermentation9060556
Mohapatra S, Sarkar B, Samantaray DP, Daware A, Maity S, Pattnaik S, Bhattacharjee S (2017) Bioconversion of fish solid waste into PHB using Bacillus subtilis based submerged fermentation process. Environ Technol 38(24):3201–3208. https://doi.org/10.1080/09593330.2017.1291759
Muneer F, Rasul I, Qasim M, Sajid A, Nadeem H (2022) Optimization, production and characterization of polyhydroxyalkanoate (PHA) from indigenously isolated novel bacteria. J Polym Environ 30(8):3523–3533
Naser AZ, Deiab I, Darras BM (2021) Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: a review. RSC Adv 11(28):17151–17196. https://doi.org/10.1039/d1ra02390j
Park YL, Song HS, Choi TR, Lee SM, Park SL, Lee HS, Kim HJ, Bhatia SK, Gurav R, Park K, Yang YH (2021) Revealing of sugar utilization systems in Halomonas sp. YLGW01 and application for poly(3-hydroxybutyrate) production with low-cost medium and easy recovery. Int J Biol Macromol 167:151–159. https://doi.org/10.1016/j.ijbiomac.2020.11.163
Phanse N, Chincholikar A, Patel B, Rathore P, Vyas P, Patel M (2011) Screening of PHA (poly hydroxyalkanoate) producing bacteria from diverse sources. Int J Biosci 1:27–32
Ray AK, Ghosh K, Ringø E (2012) Enzyme-producing bacteria isolated from fish gut: a review. Aquac Nutr 18(5):465–492. https://doi.org/10.1111/j.1365-2095.2012.00943.x
Sedlacek P, Pernicova I, Novackova I, Kourilova X, Kalina M, Kovalcik A, Obruca S (2020) Introducing the newly isolated bacterium Aneurinibacillus sp. H1 as an auspicious thermophilic producer of various polyhydroxyalkanoates (PHA) co copolymers–2. Material study on the produced copolymers. Polymers 12:1298
Sehgal R, Gupta R (2020) Polyhydroxyalkanoate and its efficient production: an eco-friendly approach towards development. 3 Biotech 10(12):1–14. https://doi.org/10.1007/s13205-020-02550-5
Selvakumar K, Srinivasan G, Baskar V, Madhan R (2011) Production and isolation of polyhydroxyalkanoates from Haloarcula marismortui MTCC 1596 using cost effective osmotic lysis methodology. Eur J Exp Biol 1(3):180–187
Singh P, Sharma VP (2016) Integrated plastic waste management: environmental and improved health approaches. Procedia Environ Sci 35:692–700. https://doi.org/10.1016/j.proenv.2016.07.068
Singh A, Singh AK (2018) Isolation, characterization and exploring biotechnological potential of halophilic archaea from salterns of western India. 3 Biotech 8:45. https://doi.org/10.1007/s13205-017-1072-3
Tarrahi R, Fathi Z, Seydibeyoğlu MÖ, Doustkhah E, Khataee A (2020) Polyhydroxyalkanoates (PHA): from production to nanoarchitecture. Int J Biol Macromol 146:596–619. https://doi.org/10.1016/j.ijbiomac.2019.12.181
Thomas T, Sudesh K, Bazire A, Elain A, Tan HT, Lim H, Bruzaud S (2020) PHA production and PHA synthases of the halophilic bacterium Halomonas sp. SF2003. Bioengineering 7(1):29
Tripathi AD, Raj Joshi T, Kumar Srivastava S, Darani KK, Khade S, Srivastava J (2019) Effect of nutritional supplements on bio-plastics (PHB) production utilizing sugar refinery waste with potential application in food packaging. Prep Biochem Biotechnol 49(6):567–577
Urtuvia V, Ponce B, Andler R, Peña C, Diaz-Barrer A (2022) Extended batch cultures for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) production by Azotobacter vinelandii OP growing at different aeration rates. 3 Biotech 12:304. https://doi.org/10.1007/s13205-022-03380-3
Valdez-Calderón A, Barraza-Salas M, Quezada-Cruz M, Islas-Ponce MA, Angeles-Padilla AF, Carrillo-Ibarra S, Rivas-Castillo AM (2022) Production of polyhydroxybutyrate (PHB) by a novel Klebsiella pneumoniae strain using low-cost media from fruit peel residues. Biomass Convers Biorefinery 12(11):4925–4938
Wang F, Lee SY (1997) Poly (3-hydroxybutyrate) production with high productivity and high polymer content by a fed-batch culture of Alcaligenes latus under nitrogen limitation. Appl Environ Microbiol 63(9):3703–3706
Wei YH, Chen WC, Huang CK, Wu HS, Sun YM, Lo CW, Janarthanan OM (2011) Screening and evaluation of polyhydroxybutyrate-producing strains from indigenous isolate Cupriavidus taiwanensis strains. Int J Mol Sci 12(1):252–265. https://doi.org/10.3390/ijms12010252
Acknowledgements
The authors gratefully acknowledge all the team of Packtek for their precious collaboration, especially Mrs. Eya Turki, Imen Ben Khalifa and Khira Lassoued.
Funding
There is no funding source for this study.
Author information
Authors and Affiliations
Contributions
S. Bacha: Formal analysis, investigation, methodology, data curation, writing—original draft. F. Arous: data curation, formal analysis, investigation, methodology, validation, writing—review and editing. E. Chouikh: data curation, writing—original draft. A. Jaouani: supervision, validation. M. Gtari: supervision, writing—review and editing. K. Charradi: formal analysis. H. Attia: supervision, funding acquisition. D. Ghorbel: project administration, methodology, supervision, validation, funding acquisition, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare that they have no conflict of interest in the publication.
Consent for publication
All the authors mutually agreed that the work should be published in the 3 Biotech.
Ethical approval
This article does not have any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13205_2023_3808_MOESM1_ESM.docx
Supplementary file1 (DOCX 8614 KB). Supplementary Data 1. Initial screening of PHA producers by Sudan Black B staining and selective plate method. (A) Klebsiella oxytoca OM91, (B) Bacillus amyloliquefaciens OM81, (C) Bacillus cereus OM75, (D) Bacillus thioparans OM39, (E) Bacillus thuringiensis OM55. Supplementary Data 2. Microscopic observations of Sudan Black B stained PHA producers. (A) Klebsiella oxytoca OM91, (B) Bacillus amyloliquefaciens OM81, (C) Bacillus cereus OM75, (D) Bacillus thioparans OM39, (E) Bacillus thuringiensis OM55. Supplementary Data 3. Appearance of PHA produced in glucose-based medium: (A) obtained from Bacillus amyloliquefaciens OM81, (B) obtained from the four other strains.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bacha, S., Arous, F., Chouikh, E. et al. Exploring Bacillus amyloliquefaciens strain OM81 for the production of polyhydroxyalkanoate (PHA) bioplastic using olive mill wastewater. 3 Biotech 13, 415 (2023). https://doi.org/10.1007/s13205-023-03808-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03808-4