Abstract
Lasiodiplodia species commonly thrive as endophytes, saprobes, and plant pathogens in tropical and subtropical regions. Association of Lasiodiplodia species causing stem rot in dragon fruit in the coastal belt of Odisha, eastern India, has been illustrated here. The stem rot disease was characterized by yellowing of the stem, followed by softening of the stem tissues with fungal fructifications of the pathogen in the affected tissues. On the basis of macro- and micromorphological characteristics, the four fungal isolates recovered from diseased stems were identified initially as Lasiodiplodia species. By comparing DNA sequences within the NCBI GenBank database as well as performing a multigene phylogenetic analysis involving the internal transcribed spacer region (ITS-rDNA), β-tubulin (β-tub), and elongation factor-alpha (EF1-α) genes, the identity of Lasiodiplodia isolates was determined. The isolate CHES-21-DFCA was identified as Lasiodiplodia iraniensis (syn: L. iranensis) and the remaining three isolates, namely CHES-22-DFCA-1, CHES-22-DFCA-2, and CHES-22-DFCA-3, as L. theobromae. Although pathogenicity studies confirmed both L. iraniensis and L. theobromae were responsible for stem rot in dragon fruit, L. iraniensis was more virulent than L. theobromae. This study established the association of Lasiodiplodia species with stem rot in dragon fruit using a polyphasic approach. Further investigations are required, particularly related to on host–pathogen–weather interaction and spatiotemporal distribution across the major dragon fruit–growing areas of the country to formulate prospective disease management strategies. This is the first report on these two species of Lasiodiplodia inflicting stem rot in Hylocereus species in India.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dragon fruit (Selenicereus spp., formerly Hylocereus) is a perennial cactus belonging to family Cactaceae. It has drawn attention in many countries during recent years as an edible fruit. This fruit is native to Central America, Mexico, and South America (Barthlott and Hunt 1993). The former genus Hylocereus comprised 14 species and among them, H. undatus, H. polyrhizus, H. costaricencis, and H. megalanthus were the most cultivated species in dragon fruit–growing countries (Tel-Zur et al. 2011). This fruit is rich in vitamin C, phosphorus, calcium, salt, potassium, and vitamin A, and contains up to 16.6% of total solids (Tel Zur 2015). Vietnam is one among the leading producers of dragon fruit and is the foremost exporter in the world (Mercado-Silva 2018). Hylocereus species were introduced to India in the late 1990s (Karunakaran et al. 2019). Dragon fruit is known by numerous vernacular names such as pitaya, pitahaya, strawberry pear, and buahnaga. This tropical fruit crop starts bearing fruits in the year following planting and attains maximum yield potential in 5 years (Hamidah and Zainuddin 2007). It has great potential as a new, water-efficient, and very adaptable crop for India. Cultivation of dragon fruit is witnessing a momentum in many states of India, including the east Indian states such as Odisha. The ever-increasing demand for healthy and nutritious fruit is favoring the expansion of dragon fruit cultivation. However, major constraints for dragon fruit cultivation are diseases and insect pests. Numerous plant pathogens were reported to cause diseases in dragon fruit (Balendres and Bengoa 2019). Although dragon fruit is cultivated and thrives well in diverse tropical regions, under newly introduced environmental conditions, diseases may be a bottleneck for its successful production if not managed well in time. Dragon fruit diseases so far reported from India include anthracnose from Andaman and Nicobar Islands (Abirami et al. 2019), viral disease from Telangana state (Parameswari et al. 2021), and stem canker caused by Neoscytalidium dimidiatum from Pune, Satara, and Solapur districts of Maharashtra (Salunkhe et al. 2022).
In 2018, in an experimental farm at Bhubaneswar, in the coastal belt of the Odisha state, eastern India, red and white pulp varieties of dragon fruit exhibited stem rot symptoms characterized by yellowing of the stem, followed by softening of stem tissues. In most cases, rotting typically initiated along the stem, from growing tips and stem margins, but it was also observed in the middle of the stem involving part of, or the entire section of, the stem. As rotting progressed, fungal fructifications appeared on the cankers. Finally, the rotten portion detached from stem leaving behind only the central core. On both red and white pulp varieties, the incident of rotting was more prominent during summer. Previous studies in different countries indicated that Lasiodiplodia and other species in the Botryosphaeriaceae family were responsible for stem rot of dragon fruit (Mohd et al. 2013; Briste et al. 2019, 2022; Serrato-Diaz and Goenaga 2021; de Mello et al. 2022). A similar stem canker of cactus pear (Opuntia ficus-indica), another member of the family Cactaceae, was caused by Neofusicoccum batangarum, a fungus that like Lasiodiplodia species belongs to the family Botryosphaeriaceae (Aloi et al. 2020) and toxins produced by this fungus are hypothesized to be responsible for the symptoms (Masi et al 2020). As morphological features between Lasiodiplodia species and other Botryosphaeriaceae overlap in some cases, molecular methods have been used to identify these fungi and, in a broader context, to elucidate the phylogeny of the Botryosphaeriaceae (Slippers et al. 2005, 2017). Botryosphaeriaceae is the largest family within the order Botryosphaeriales, which encompasses at least 24 major genera including Diplodia, Lasiodiplodia, Botryosphaeria, Dothiorella, and Neofusicoccum (Burgess et al. 2018; Phillips et al. 2019; Zhang et al. 2021). Among them, Lasiodiplodia spp. are cosmopolitan and known to be associated with approximately 500 hosts, mostly woody plants, and different fruit trees in subtropical and tropical zones where they cause numerous diseases like cankers, die-back, fruit rot, and root rots in tree species such as mango, citrus, eucalyptus, neem, avocado, apple, pear, and peach (Punithalingam 1980; Alves et al. 2008; Rodríguez-Galvez et al. 2015). Lasiodiplodia species have different lifestyles, traversing from saprophytic to endophytic and to pathogenic roles (Slippers and Wingfield 2007; Abdollahzadeh et al. 2010; Liu et al. 2012; Chen 2015; Dissanayake 2015).
Botryosphaeriaceae in general survives in a latent condition as endophytes for extended time, but under the influence of stress factors, may cause disease (Slippers and Wingfield 2007). The external stimuli in the form of high temperature or drought stress trigger these fungi to transition into potential pathogens, which ultimately cause the disease (Aloi et al. 2021). The accurate identification of the causative agent of a disease is critical for developing appropriate disease management strategies. This investigation was carried out with the objective to study the symptomatology of stem rot of dragon fruit observed at Bhubaneswar as well as identify and characterize the causal agent of the disease.
Material and methods
Sampling and isolation
Dragon fruit stems with rotting symptoms were collected from the experimental orchard located at Bhubaneswar, Odisha, eastern India (20°14ʹ N, 85°46ʹ E). They were carried to a research laboratory for pathogen isolation and subsequent identification of the causative agent. Briefly, three 5 × 5 mm2 pieces of symptomatic tissue along with bordering uninfected healthy portion were cut from progressing edge of the lesions. Further, the tissues were surface sterilized with 1% sodium hypochlorite for 1 min and washed consecutively three times with sterilized distilled water. The tissue bits were allowed to air-dry on sterilized blotting paper and transferred aseptically to Petri dishes on a fresh potato dextrose agar (PDA) medium amended with streptomycin sulfate (100 ppm) to avert bacterial contamination. The dishes were incubated at 28 ± 2 °C under room condition with continuous illumination and observed periodically for fungal growth. The emerging hyphal tips from the infected tissues were aseptically transferred to new PDA dishes and incubated at 28 ± 2 °C with a 12 h photoperiod. Pure cultures of the isolates were coded as CHES-21-DFCA, CHES-22-DFCA-1, CHES-22-DFCA-2, and CHES-22-DFCA-3, and the initial identification was carried out based on macro- and micro-morphological features. Pure cultures of the isolates were maintained at 4 °C on PDA slants for further study.
Pathogenicity
The pathogenicity of fungal isolates was evaluated on mature and healthy, uniformly sized dragon fruit stems, with a total of 10 stem segments allocated per isolate. In brief, healthy stems were washed under flowing tap water, followed by disinfection with 70% ethanol. They were rinsed twice with sterile distilled water. After air-drying in a laminar flow chamber, the stems were wounded with a sterilized needle at regular intervals (2.5–5 cm) depending on the stem size. An 8 mm diameter mycelial plug, punched from the margin of a 7-day-old colony in a PDA dish, was placed on each wound, with the mycelial surface facing down. To prevent desiccation, the mycelial plug was covered with wet sterile cotton. The stems were placed in plastic containers lined with two layers of sterilized wet blotting papers and incubated at 28 ± 2 °C and more than 80% relative humidity with a 12 h light and dark cycle at ambient room conditions. The inoculated stems were observed daily up to 15 days post inoculation. The experiment was carried out twice. Tissues from the margins of the lesions were picked up and placed into PDA plates and incubated for a week to recover the inoculated fungi. The isolates recovered from artificially induced lesions were identified and compared with the original isolates, fulfilling Koch’s postulates. To know the ability of pathogenic fungi in causing rot at different temperatures, tests were also conducted. These involved inoculating the highly virulent isolate CHES-21-DFCA and incubating the stem segments at different temperatures ranging from 10 to 40 °C. The severity of symptom was rated at several time intervals after inoculation (2, 4, 6, 8, 10, 12, 14, and 16 days post inoculation).
Phenotypic characterization
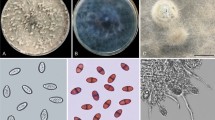
The isolates were cultured on PDA medium to examine the colony characteristics. Three 8 mm discs were cut out from 7-day-old colonies of each isolate, and these mycelial discs were transferred to PDA dishes. The dishes were incubated at 28 ± 2 °C for 1 week. The mean radial growth (mm per day) and colony color of each isolate were determined. Sterilized toothpicks were kept near to the fungal discs on water agar (2%) and incubated at 28 ± 2 °C for 2–3 weeks for sporulation. Observations on conidial characters were recorded under an Olympus Bx53 microscope equipped with a digital camera. Conidia were mounted in lactic acid (100%). The dimensions of conidia and pycnidia were also recorded. Preliminary identification of isolates was done based on colony color and morphology and color, as well as shape, size, septation, and striations of conidia, according to the criteria described by Phillips et al. (2013).
Molecular characterization
All four isolates characterized in the current study were grown in potato dextrose broth (PDB) at 28 ± 2 ºC for 12 days. The mat of mycelium was separated from the broth by filtering through sterilized filter paper (Whatman No.1) and washed with sterile distilled water. A fungal DNA purification kit (HiPurATM; HiMedia, Maharashtra, India) was used to extract the total genomic DNA of the isolates according to the manufacturer’s instructions. The respective primer pairs used for amplification of ITS region of ribosomal DNA (ITS-rDNA), partial elongation factor 1-alpha (EF-1α), and β-tubulin (β-tub) genes were ITS1/ITS4 (5′-TCCGTAGGTGAACCTGCGG-3′/5′-TCCTCCGCTTATTGATATGC-3′), EF1-688F/EF1-1251R (5′-CGGTCACTTGATCTACAAGTGC-3′/5′-CCTCGAACTCACCAGTACCG-3′), and Bt2a/Bt2b (5′-GGTAACCAAATCGGTGCTGCTTTC-3′/5′-ACCCTCAGTGTAGTGACCCTTGGC-3′), according to White et al. (1990), Alves et al. (2008), and Glass and Donaldson (1995), respectively. PCR reaction mixtures consisted of green dye-added 25 μl of 2 × PCR MAX Master Mix (Takara Bio Inc.), 1 μl of each primer (10 mM), and 2 μl of DNA template. The volume was brought to 50 μl using sterile nuclease-free water. The PCR amplification cycles were as follows: an initial denaturation at 94 ºC for 4 min; 35 cycles of denaturation at 94 ºC for 15 s; annealing at 52 ºC (ITS1/4), 55 ºC (EF1-688F/1251R), and 65 ºC (Bt2a/Bt2b) for 40 s; elongation at 72 ºC for 1 min, followed by a final elongation step at 72 ºC for 5 min. The PCR products were separated by gel electrophoresis in agarose gel (1.2%) stained with ethidium bromide (EtBr) viewed under ultraviolet light and photographed in a gel documentation system (Vilber, Marne-la-Vallée, France). The target amplicon was eluted from gel using a gel extraction kit (QIAquick; Qiagen India, New Delhi, India) following the manufacturer’s instructions and sequenced by Sanger sequencing method (Eurofins India Pvt Ltd, Karnataka, India). The resultant sequences were edited and assembled with the BioEdit software, V.7.0.9.0 (Hall 1999). Nucleotide sequences were assembled and deposited in the GenBank database (http://www.ncbi.nlm.nih.gov).
Phylogenetic analysis
For each of the three loci, sequences of reference strain and Lasiodiplodia species homologous to the isolates of the present study were retrieved from the NCBI GenBank database via Basic Local Alignment Search Tool (BLAST) (Altschul et al. 1990) and aligned with the isolates of this study for phylogenetic analysis (Table 1). The concatenated sequences of three genes (ITS, EF1-α, and β-tub) of the present isolates (both pathogenic and nonpathogenic isolates) were included as well as additional selected reference sequences of Lasiodiplodia species were constructed using the Sequence Matrix software, version 1.7.8 (Vaidya et al. 2011). The maximum parsimonious tree was constructed through Phylogenetic Analysis Using Parsimony (PAUP), v. 4.0b10, with 1000 random stepwise addition and TBR (tree bisection and reconnection) as branch swapping algorithms. To understand the robustness of the tree, the tree length (TL), retention index (RI), homoplasy index (HI), consistency index (CI), and rescaled consistency index (RC) and homoplasy index were calculated along with bootstrap analysis involving 1000 bootstrap replications. The phylogenetic tree was rooted through Diplodia mutila CBS230.30.
Results
Symptomatology
The dragon fruit stem rot was detected in the experimental orchard at Bhubaneswar throughout the year with different degrees of severity spanning from partial to complete rotting of the stem. Observations were made in three randomly selected distinct plots, each comprising 100 plants aged between 3 and 5 years for each season. Each plot included both red and white pulp dragon fruit types. During the summer months of 2018, a few dragon fruit plants showed symptoms of stem rot, which did not result in considerable crop loss. However, by mid-May 2019, the stem rot incidence was observed in a high proportion, affecting 26% of red-fleshed plants and 34% of white-fleshed plants. Although the disease appeared throughout the year, the manifestation of symptoms peaked during April–June, which correspond to summer months. Usually, symptoms initiated along the margin or tip of the stem, although instances were also observed in the middle of the stem without any physical injury or wounds. The typical symptom of stem rot was characterized by yellowing and softening of the stem, followed by rotting, involving partial or complete length of the stem (Fig. 1A–H). Subsequently, circular to irregular cankerous lesions were observed in the middle or at the margin of the lesions. When the rot became old, the cankerous growth containing fungal fructifications involving host tissues was also seen (Fig. 2A–E). The cankerous grayish lesions contained black-headed pycnidia arranged in a circular to irregular manner, which expanded overtime, covering the entire lesion In advanced stages of the disease, the host epidermis became dried and shredded, leaving characteristic circular holes if rotting occurred on the stem margin. In some cases, rotten portion detached from the stem leaving behind only the central core (Fig. 2F). When the rot was confined on the margin of stems, the infected host tissues along with fungal structures separated in a semi-circular shape. The stem rot disease occurred in the middle of stem without any physical damage or wound. The symptoms later led to a general decline in the vigor of the plant. Eventually, the severely infected plants become less productive in subsequent seasons.
Isolation and pathogenicity
Isolation was carried out from the infected symptomatic stem and four representative isolates (CHES-21-DFCA, CHES-21-DFCA-1, CHES-22-DFCA-2, and CHES-22-DFCA-3) were further selected for pathogenicity evaluation and characterization. In pathogenicity assays, two isolates viz., CHES-21-DFCA and CHES-22-DFCA-2, produced disease symptoms comparable to those observed in the field. Other two isolates did not show any symptoms. Upon artificial inoculation, CHES-21-DFCA induced a yellow discoloration around the inoculation site, which appeared 3 days post inoculation. Then, yellowing enlarged rapidly, leading to stem softening and rotting within 10 days (Fig. 3). Conversely, the isolate CHES-22-DFCA-2 showed very mild symptoms, with rotting confined to the site of inoculation and not progressing further. No rotting symptoms were observed in stems inoculated with isolates CHES-21-DFCA-1 and CHES-22-DFCA-3 even after 15-day incubation. The isolates recovered from artificially inoculated symptomatic stems were identical to CHES-21-DFCA and CHES-22-DFCA-2, fulfilling Koch’s postulates. Notably, the symptoms produced by the isolate CHES-21-DFCA were much more severe than those incited by the isolate CHES-22-DFCA-2. Similar kind of extensive rotting symptoms were observed under field conditions more often than milder rotting patterns associated with the isolate CHES-22-DFCA-2. The ability of the isolate CHES-21-DFCA to induce stem rot was tested at temperatures ranging from 10 to 40 °C. The pathogen was able to induce rotting within a week at temperatures ranging from 15 to 40 °C. Maximum severity of rotting was recorded at 35 and 40 °C (Fig. 4A–F). Although rotting expanded slowly at temperature between 15 and 30 °C, all stem portions were rotten completely at the site of inoculation within 2 weeks. In contrast, at 10 °C, it took at least 35 days for the rot to reach a diameter of 8 mm.
Phenotypic characterization
The colonies of all four fungal isolates had a grayish-black cottony growth texture, accompanied by an olivaceous green-to-black color on the reverse/bottom side. The diameter of the colony grew to 90 mm after 4–5 days of incubation at 28 ± 2 °C and a 12:12 h (light:dark) photoperiod. The isolates were identified as Lasiodiplodia species based on morphological and conidial characteristics.
Lasiodiplodia iraniensis
The colony of the L. iraniensis isolate CHES-21-DFCA was initially grayish white with fluffy aerial mycelia on the PDA medium and became olivaceous gray at the surface whereas the reverse showed greenish gray after 2 weeks of incubation at 28 ± 2 °C. Blister-like, thick-walled, globose, dark brown pycnidia were produced on the sterilized toothpicks within 3 weeks of incubation. These pycnidia measured 230–535 μm in diameter. The isolates lacked conidiophores whereas paraphyses were cylindrical and hyaline. Initially aseptate, they became septate when matured and very rarely branched. Conidia, which were initially hyaline, aseptate, and ovoid with blunt/rounded ends, became dark brown, thick-walled, with one middle septum and longitudinal striations when matured. The conidia measured 17.5–24 × 11–14.5 μm size (n = 50) (Table 2). The teleomorph form remained unknown.
Lasiodiplodia theobromae
The colonies of the L. theobromae isolates CHES-21-DFCA-1, CHES-22-DFCA-2, and CHES-22-DFCA-3 were initially white with fluffy aerial mycelia on the PDA medium. They became smoky gray after 15 days when incubated at 28 ± 2 °C. All three isolates later produced abundant black pigmentation, which was visible from the reserve side of the PDA media. The morphological features of all three L. theobromae isolates were similar in nature. Dark gray to black color knot-like pycnidia were produced on water agar overlaid with sterilized darbha grass (Desmostachya bipinnata) within 2 weeks. The pycnidia were solitary, globose, uniloculate, and thick-walled. They were semi- or fully immersed, measuring 250–550 μm. Conidiophores were cylindrical, hyaline, rarely septate, and branched. Fungal paraphyses were hyaline and aseptate. Young conidia were hyaline and aseptate, and mature conidia were dark brown, striated, with one middle septation. Conidial measurements (n = 50) of all three isolates are given in Table 2. The teleomorph form remained unknown.
Molecular characterization
For molecular characterization, the nucleotide sequences of ITS, EF1-α, and β-tub genes were generated and used to identify the Lasiodiplodia isolates at the species level. The amplified PCR products of ITS (~ 550 bp), EF1-α (~ 500 bp), and β-tub (~ 450 bp) genes were sequenced using the custom sanger sequencing services and were subjected to NCBI Blastn analysis. Both the forward and reverse sequences were compiled and submitted to NCBI GenBank and accession numbers were obtained (Table 1). The BLAST searches of the isolate CHES-21-DFCA in the NCBI GenBank database showed that the ITS sequence of this isolate exhibited 98.55–98.76% similarity with L. pseudotheobromae (MN341226, MN887206) and 98.55% similarity with L. theobromae (MK166047, MH865367) and L. iranensis (MK282705). The EF1-α gene showed 100% similarity with L. hyalina with 91% query cover (KX499917, KY751302), 98.19%–99.42% similarity with L. iranensis isolates with 100% query cover (MF580812, MW725045, OL455942), 95%–96% similarity with L. theorbromae isolates (OL455945, MK570085, MF580814), and more than 97% similarity with L. thailandica (KY751303) and L. jatrophicola (KT325583, KT325582, KU507447). Notably, a stretch of 8 nucleotides (AGCGCTGC) found in the EF1-α gene sequences of all the L. thailandica isolates was missing in the examined isolates (L. iranensis, L. hyalina, and L. jatrophicola) (Table 1). The β-tub gene sequence of this isolate exhibited more than 99% similarity with L. pseudotheobromae (MN867365, MN243787), L. theobromae (KR260823, KR260821), L. iranensis (MK294103, MK294101), L. jatrophicola (MH251965, MH251964), L. lignicola (KT852958), and L. hormozganensis (OL405589, OL405587) isolates.
The ITS sequences of isolates CHES-22-DFCA-1, CHES-22-DFCA-2, and CHES-22-DFCA-3 displayed 99%–100% similarity with different L. theobromae isolates (MN831964, MN831965, MK584592, MT103322) and > 99% similarity with L. hormozganensis (JX464085, JX464085). Likewise, the EF1-α gene of these three isolates, viz., CHES-22-DFCA-1, CHES-22-DFCA-2, and CHES-22-DFCA-3, was 100% similar to L. theobromae isolates HB2 and ML1001 (MF580814, JN542563) and 98.85% similar to L. mahajangana isolates (OL455923, OL455922). The β-tub gene of these three isolates was also 100% identical to L. theobromae isolates (MN172230, MK587448, MW118596). Hence, multigene phylogenetic analysis was carried out by combining all three gene sequences, in order to establish the identity of all four isolates to the species level.
Multigene phylogenetic analysis
Owing to conflicts observed in single-gene phylogenies in case of all the four isolates (data not shown), the three genes were concatenated. The combined dataset comprised 623 characters of ITS (1–623), 792 characters of EF1-α (624–1416), and 680 characters of β-tub (1420–2100) genes. A multigene phylogenetic analysis was carried out using the ITS, β-tub, and EF1-α genes combined dataset of Lasiodiplodia species, including 43 isolates from GenBank corresponding to a wide range of known Lasiodiplodia species and one outgroup taxa (Diplodia mutila-CBS230.30) and 4 isolates (CHES-21-DFCA, CHES-22-DFCA-1, CHES-22-DFCA-2, and CHES-22-DFCA-3) from this study (Table 1). The maximum parsimonious tree is shown in Fig. 5. The resultant phylogenetic tree is given with bootstrap support values above the nodal branches. The isolate CHES-21-DFCA represented a distinct lineage from L. hyalina, L. thailandica, and L. jatrophicola and clustered along with L. iranensis isolates. Thus, it was identified as L. iranensis. The remaining three isolates were identified as L. theobromae as they clustered with L. theobromae reference strains. The maximum parsimony analysis constructed a single most parsimonious tree with TL = 1019, CI: 0.745, RI: 0.776, RC: 0.578, and HI: 0.255. There were 214 parsimony informative characteristics among the 1203 constant characters. The combined dataset analysis improved phylogenetic resolution. Thus, the pathogenic isolates CHES-21-DFCA and CHES-22-DFCA-2 were identified as L. iraniensis and L. theobromae, respectively.
Discussion
The symptoms of stem rot were observed in 2018 on the newly introduced crop in the state of Odisha, located in the eastern part of India. Because dragon fruit is a low-maintenance crop, stem rot has been found to have a significant impact on crop health. Over the 3-year investigation period, the disease increased significantly and rendered the plants less productive. Hence, this study was aimed to identify the causal agent of the stem rot disease of dragon fruits and to develop suitable management strategies. Investigations based on morphology, pathogenicity assessment, and multiple gene phylogenetic analysis revealed the association of Botryosphaeriaceae fungi in the stem rot disease of dragon fruit. To the best of our knowledge, this is the first report of L. iraniensis and L. theobromae as the causal agents of stem rot in dragon fruit plants in India. Among the two Lasiodiplodia species, L. iraniensis proved to be more virulent than L. theobromae. However, out of three L. theobromae isolates, only one isolate (CHES-21-DFCA-2) was able to cause rotting at the site of inoculation (only) whereas other two isolates (CHES-22-DFCA-1 and CHES-22-DFCA-3) were not able to cause any kind of rotting symptom on artificial inoculation. Hence, it can be inferred that in nature nonpathogenic strains of L. theobromae coexist as endophytes along with pathogenic strains. Similar observations were made by Sosa et al. (2016) in the cacao cushion galls disease caused by L. theobromae and Fusarium decemcellulare.
In the current study, L. iraniensis was found to cause a variety of rotting symptoms on the stems of dragon fruits including, marginal rotting, tip rot, partial, or complete stem rot. The symptoms were more severe in white-pulped varieties. In several dragon fruit–growing countries such as Israel, Taiwan, Malaysia, China, and the United States, canker disease affecting dragon fruit has been attributed to another member of the Botryosphaeriaceae family, N. dimidiatum. Moreover, canker disease caused by N. dimidiatum was devastating in Vietnam, which is a leading global exporter of dragon fruits. In Vietnam, this disease affected approximately more than 10,000 ha area and yield losses ranged from 30 to 70% in individual fields. Stem canker incited by N. dimidiatum has also been reported from India in commercially grown dragon fruit orchards of Pune, Solapur, and Satara districts of Maharashtra with a disease incidence of approximately 40% (Salunkhe et al. 2022). Additionally, stem rot and canker diseases of dragon fruit caused by Diaporthe phaseolorum and L. theobromae, respectively, have been reported from Bangladesh (Karim et al. 2019; Briste et al. 2022).
Detailed species-level morphological characterization of Lasiodiplodia isolates using morphology is nearly impossible as the size of the spores is extremely variable. In the past, when identification was based on morphology, many Lasiodiplodia species were identified as L. theobromae (Punithalingam 1976). Until 2000, species within the Botryosphaeriaceae family were identified exclusively by their morphological features (Denman et al. 2000). As conidial septation and pigmentation are strongly affected by cultural conditions, misidentification has become common in literature (Alves et al. 2006). Many new species of Lasiodiplodia have been described since 2004 based on DNA sequencing together with morphological features. As pointed out by Phillips et al. (2013), morphology of spores should only be used to distinguish between genera as it is not appropriate for identifying the species in Lasiodiplodia. To precisely identify the Lasiodiplodia species, multigene phylogenetic analysis is crucial, as suggested by Phillips et al. (2013).
Sequences of ITS, EF-1α, and β-tub regions are commonly used to differentiate the Lasiodiplodia species (Slippers et al. 2014; Bautista-Cruz et al. 2019). Alves et al. (2008) used morphological data together with ITS and EF-1α to characterize a group of Lasiodiplodia isolates that were earlier identified as L. theobromae. In Peru, sequence data of ITS and EF-1α were used to establish the association of five Lasiodiplodia species causing die-back of mango, which were earlier described as L. theobromae (Rodriguez-Galvez et al. 2017). Lasiodiplodia theobromae, L. pseudotheobromae, L. subglobosa, L. brasiliense, L. iraniensis, and L. citricola were reported to cause canker and die-back symptoms in Persian lime wherein the identity of organisms was confirmed by molecular tools and multigene phylogeny (Bautista-Cruz et al. 2019). In the current study, we used ITS, β-tub, and EF-1α sequence data to identify and investigate the phylogenetic relationships of L. iraniensis and L. theobromae with other closely related Lasiodiplodia species. L. jatrophicola, L. hyalina, and L. thailandica are phylogenetically close to but clearly distinct from L. iraniensis. However, a few researchers believe that L. iraniensis and L. jatrophicola have to be regarded as synonyms, as these two species cannot be divided into different species by using ITS and EF1-α sequence data (Rodriguez-Galvez et al. 2017; Cruywagen et al. 2017). However, in the present study, the combined gene such as ITS, β-tub, and EF-1α could clearly separate L. iraniensis from L. jatrophicola. Lasiodiplodia iraniensis was first reported from Iran on Salvadora persica, Eucalyptus sp., Juglans sp., mango, Citrus sp., and almond (Abdollahzadeh et al. 2010). Later, it was reported from various countries on economically important trees, such as mandarin (Al-Sadi et al. 2013), mango (Marques et al. 2013; Al-Sadi et al. 2013; Rodriguez-Galvez et al. 2017), Anacardium occidentale (Netto et al. 2017), and Bougainvillea (Li et al. 2015). Wither tip in citrus (Citrus reticulata cv. Kinnow) caused by Colletotrichum siamense and L. iraniensis has been reported from Pakistan, causing 40% yield loss (Fayyaz et al. 2018). Recently, L. iraniensis has been identified as the causal agent of rotting of Yam (Dioscorea spp.) in Florida (Jibrin et al. 2022). Several studies indicate that Botryosphaeriaceae fungi shows endophytic behavior within healthy tissues of plants. They shift into a pathogenic lifestyle, implying that these fungi become aggressive when plants are stressed (Schoeneweiss 1981; Blodgett and Stanosz 1995; Jami et al. 2013). This occurs in nonoptimal disturbed environments (Slippers and Wingfield 2007). Abiotic factors such as severe sunburn, drought, and freezing predispose plants, including citrus, to xylem dysfunction, which results in branch canker and die-back (Raimondo et al. 2010; Khanchouch et al. 2017; Aloi et al. 2021). In dogwoods (Cornus florida), plant stress has been demonstrated to be a crucial factor in triggering the pathogenic behavior of L. theobromae, and this was corroborated by the failure of artificial inoculations in pathogenicity trials (Mullen et al. 1991).
Although dragon fruit is a crassulacean acid metabolism (CAM) plant, in our study, the symptoms were more pronounced during summer months (March–May; data not shown). The fungi belonging to the Botryosphaeriaceae family grow well within the temperature range of 15–37 °C. In spite of having the ability to grow between 9 and 39 °C, the optimal temperature of 27–33 °C has been reported for the fungi (D’souza and Ramesh 2002). The extracellular enzymatic activity of the fungi also varies according to the temperature, which was confirmed by pathogenicity test on dragon fruit stems infected by L. iraniensis. Symptoms were more severe when stems were incubated at 30, 35, and 40 °C compared to those incubated at 15, 20, and 25 °C. On Chinese hackberry, canker disease outbreak caused by L. pseudotheobromae occurred between July and August (Liang et al. 2020; Zhang 2012).
Although dragon fruit adapt and thrives well in diverse tropical regions, infections by new pathogens can pose a challenge to its successful production. In Vietnam, an unproductive diseased dragon fruit orchard was rejuvenated into a healthy and high yielding productive one by implementing strict field sanitation and effective fungicide programs (Fullerton et al. 2018). However, management of stem rot/canker caused by Lasiodiplodia species is challenging because of limited information about this new pathogen on this new host plant and nonavailability of registered fungicides for this newly introduced crop. Currently, pruning and destruction of the infected stem is the best management strategy available. Hence, the stem rot disease should be monitored closely in various dragon fruit growing regions of the country to tackle this disease effectively.
Data availability
Data will be made available on request.
Change history
27 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s13205-023-03800-y
References
Abdollahzadeh J, Javadi A, Goltapeh EM, Zare R, Phillips AJL (2010) Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia 25:1–10. https://doi.org/10.3767/003158510X524150
Abirami K, Sakthivel K, Sheoran N, Baskaran V, Gutam K, Jerard BA, Kumar A (2019) Occurrence of anthracnose disease caused by Colletotrichum siamense on dragon fruit (Hylocereus undatus) in Andaman Islands. India Plant Dis 103(4):768. https://doi.org/10.1094/PDIS-09-18-1489-PDN
Aloi F, Giambra S, Schena L, Surico G, Pane A, Gusella G, Stracquadanio C, Burruano S, Cacciola SO (2020) New insights into scabby canker of Opuntia ficus-indica, caused by Neofusicoccum batangarum. Phytopathol Mediterr 59:269–284. https://doi.org/10.14601/Phyto-11225
Aloi F, Riolo M, Parlascino R, Pane A, Cacciola SO (2021) Bot Gummosis of Lemon (Citrus × limon) Caused by Neofusicoccum parvum. J Fungi 7:294. https://doi.org/10.3390/jof7040294
Al-Sadi AM, Al-Wehaibi AN, Al-Shariqi RM, Al-Hammadi MS, Al-Hosni IA, Al-Mahmooli IH, Al-Ghaithi AG (2013) Population genetic analysis reveals diversity in Lasiodiplodia spp. infecting date palm Citrus, and mango in Oman and the UAE. Plant Dis 97:1363–1369. https://doi.org/10.1094/PDIS-03-13-0245-RE
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J of Mol Biol 215(3):403–410. https://doi.org/10.1006/jmbi.1990.9999
Alves A, Correia A, Phillips AJL (2006) Multi-gene genealogies and morphological data support Diplodia cupressi sp. nov., previously recognized as D. pinea f. sp. cupressi, as a distinct species. Fungal Divers 23(1):1–15
Alves A, Crous PW, Correia A, Phillips AJL (2008) Morphological and molecular data reveal cryptic species in Lasiodiplodia theobromae. Fungal Divers 28:1–13
Balendres M, Bengoa J (2019) Diseases of dragon fruit (Hylocereus species): etiology and current management options. Crop Prot 126:104920. https://doi.org/10.1016/j.cropro.2019.104920
Barthlott W, Hunt DR (1993) Cactaceae. In: Kubitzki K (ed) The families and the genera of vascular plants. Springer-Verlag, Berlin, pp 161–196. https://doi.org/10.2307/25065357
Bautista-Cruz MA, Almaguer-Vargas G, Leyva-Mir SG, Colinas-Leon MT, Correia KC, Camacho-Tapia M, Robles-Yerena L, Michereff SJ, Tovar-Pedraza JM (2019) Phylogeny, distribution and pathogenicity of Lasiodiplodia Species associated with cankers and dieback symptoms of Persian lime in Mexico. Plant Dis 103:1156–1165. https://doi.org/10.1094/PDIS-06-18-1036-RE
Blodgett JT, Stanosz GR (1995) Sphaeropsis sapinea and host water stress in a red pine plantation in central Wisconsin. Phytopathology 85:1044. https://doi.org/10.1094/phyto.1997.87.4.429
Briste PS, Bhuiyan MAHB, Akanda AM, Hassan O, Mahmud NU, Kader MA, Chang T, Islam MT (2019) First report of dragon fruit stem canker caused by Lasiodiplodia theobromae in Bangladesh. Plant Dis 103:2686. https://doi.org/10.1094/PDIS-03-19-0619-PDN
Briste PS, Akanda AM, Bhuiyan Md, Abdullahil BB, Mahmud NU, Islam T (2022) Morphomolecular and cultural characteristics and host range of Lasiodiplodia theobromae causing stem canker disease in dragon fruit. J Basic Microbiol 62(689–700):62. https://doi.org/10.1002/jobm.202100501
Burgess TI, Tan YP, Garnas J, Edwards J, Scarlett KA, Shuttleworth LA (2018) Current status of the Botryosphaeriaceae in Australia. Australas Plant Pathol 48(1):35–44. https://doi.org/10.1007/s13313-13018-10559-13317
Chen S (2015) β-Resorcylic acid derivatives with α-glucosidase inhibitory activity from Lasiodiplodia sp. ZJ-HQ1, an endophytic fungus in the medicinal plant Acanthus ilicifolius. Phytochem Lett 13:141–146. https://doi.org/10.1016/j.phytol.2015.05.019
Cruywagen EM, Slippers B, Roux J, Wingfield MJ (2017) Phylogenetic species recognition and hybridisation in Lasiodiplodia: a case study on species from baobabs. Fungal Biol 121(4):420–436. https://doi.org/10.1016/j.funbio.2016.07.014
de Mello JF, de Queiroz Brito AC, dos Santos Oliveira da Silva E et al (2022) First report of Lasiodiplodia pseudotheobromae causing cladode rot in Hylocereus sp. in Brazil. J Plant Pathol 104:899. https://doi.org/10.1094/PDIS-05-22-1192-PDN
Denman S, Crous PW, Taylor JE, Kang JC, Pascoe I, Wingfield MJ (2000) An overview of the taxonomic history of Botryosphaeria and a re-evaluation of its anamorphs based on morphology and ITS rDNA phylogeny. Stud Mycol 45:129–140
Dissanayake AJ (2015) Lasiodiplodia pseudotheobromae causes pedicel and peduncle discolouration of grapes in China. Australas Plant Dis Notes 10:21. https://doi.org/10.1007/s13314-015-0170-5
Dsouza AD, Ramesh M (2002) Senescence in fungi. Resonance 7:51–55. https://doi.org/10.1007/BF02896308
Fayyaz A, Bonello P, Tufail M, Amrao L, Habib A, Gai Y, Sahi ST (2018) First report of citrus wither tip (Tip Dieback), a disease complex caused by Colletotrichum siamense and Lasiodiplodia iraniensis on Citrus reticulata cv. Kinnow in Punjab Pakistan. Plant Dis. https://doi.org/10.1094/PDIS-04-18-0576-PDN
Fullerton RA, Sutherland PA, Rebstock RS, Nguyen TH, Nguyen NAT, Dang TL, Ngo TKT, Nguyen VH (2018) The Life Cycle of Dragon Fruit Canker Caused by Neoscytalidium dimidiatum and implications for control. Proceedings of dragon fruit regional network initiation workshop. FFTC, Taipei, pp 71–80
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330. https://doi.org/10.1128/aem.61.4.1323-1330.1995
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic acids symposium series [London]. Information Retrieval Ltd Springer, pp 1979–2000. https://doi.org/10.12691/ajmr-3-2-1
Hamidah S, Zainuddin M (2007) Disease of dragon fruit: Hylocereus sp. National Horticulture Conference of Malaysia
Jami F, Slippers B, Wingfield MJ, Gryzenhout M (2013) Greater Botryosphaeriaceae diversity in healthy than associated diseased Acacia karroo tree tissues. Aust Plant Pathol 42:421–430. https://doi.org/10.1007/s13313-013-0209-z
Jibrin M, Qingchun L, Yi H, Urbina H, Gazis R, Zhang S (2022) Lasiodiplodia iraniensis, a new causal agent of tuber rot on yam (Dioscorea species) imported into the United States and implications for quarantine decisions. Plant Dis. https://doi.org/10.1094/PDIS-11-21-2421-SC.10.1094/PDIS-11-21-2421-SC
Karim MM, Rahman MM, Islam MN, Akhter MS, Khatun F, Rahman ML, Goswami BK (2019) Occurrence of stem rot disease of Hylocereus undatus in Bangladesh. Indian Phytopathol 72:545–549. https://doi.org/10.1007/s42360-019-00166-1
Karunakaran G, Arivalagan M, Sriram S (2019) Dragon fruit country report from India. FFTC Agricultural Policy Platform (FFTC-AP), pp 1–8
Khanchouch K, Pane A, Chriki A, Cacciola SO (2017) Major and emerging fungal diseases of Citrus in the Mediterranean region. Citrus Pathology. https://doi.org/10.5772/66943
Li G, Arnold R, Liu F, Li J, Chen S (2015) Identification and Pathogenicity of Lasiodiplodia Species from Eucalyptus urophylla × grandis, Polyscias balfouriana and Bougainvillea spectabilis in Southern China. J Phytopathol 163(11/12):956–967. https://doi.org/10.1111/jph.12398
Liang L, Li H, Zhou L, Chen F (2020) Lasiodiplodia pseudotheobromae causes stem canker of Chinese hackberry in China. J for Res 31:2571–2580. https://doi.org/10.1007/s11676-019-01049-x
Liu JK, Phookamsak R, Doilom M (2012) Towards a natural classification of Botryosphaeriales. Fungal Divers 57:149–210. https://doi.org/10.1007/s13225-012-0207-4
Marques MW, Lima NB, Morais MA Jr, Barbosa MAG, Souza O, Michereff SJ, Phillips AJL, Camara MPS (2013) Species of Lasiodiplodia associated with mango in Brazil. Fungal Divers 61:181–193. https://doi.org/10.1007/s13225-013-0231-z
Masi M, Aloi F, Nocera P, Cacciola SO, Surico G, Evidente A (2020) Phytotoxic metabolites isolated from Neufusicoccum batangarum, the causal agent of the scabby canker of cactus pear (Opuntia ficus-indica L.). Toxins 12:126. https://doi.org/10.3390/toxins12020126
Mercado-Silva EM (2018) Pitaya- Hylocereus undatus (Haw). Exotic Fruits Reference Guide. https://doi.org/10.1016/B978-0-12-803138-4.00045-9
Mohd MH, Salleh B, Zakaria L (2013) Identification and molecular characterizations of Neoscytalidium dimidiatum causing stem canker of red-fleshed dragon fruit (Hylocereus polyrhizus) in Malaysia. J Phytopathol 161:841–849. https://doi.org/10.1111/jph.12146
Mullen JM, Gilliam CH, Hagan AK, Morgan-Jones G (1991) Canker of dogwood caused by Lasiodiplodia theobromae, a disease influenced by drought stress or cultivar selection. Plant Dis 75:886–889. https://doi.org/10.1094/PD-75-0886
Netto MSB, Lima WG, Correia KC, DaSilva CFB, Thon M, Martins RB, Miller RNG, Michereff SJ, Ca mara MPS, (2017) Analysis of phylogeny, distribution, and pathogenicity of Botryosphaeriaceae species associated with gummosis of Anacardium in Brazil, with a new species of Lasiodiplodia. Fungal Biol 121:437–451. https://doi.org/10.1016/j.funbio.2016.07.006
Parameswari B, Bajaru B, Sivaraj N (2021) First record of cactus virus X in dragon fruit (Hylocereus spp.) in India. Indian Phytopathol. https://doi.org/10.1007/s42360-021-00421-4
Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ (2013) The Botryosphaeriaceae: genera and species known from culture. Stud Mycol 76(1):51–167. https://doi.org/10.3114/sim0021
Phillips AJL, Hyde KD, Alves A, Liu JK (2019) Families in botryosphaeriales: a phylogenetic, morphological and evolutionary perspective. Fungal Divers 94:1–22. https://doi.org/10.1007/s13225-018-0416-6
Punithalingam E (1976) Botryodiplodia theobromae. CMI descriptions of pathogenic fungi and bacteria, No. 519. Commonwealth Mycological Institute, Kew. https://doi.org/10.1079/DFB/20056400519
Punithalingam E (1980) Plant diseases attributed to Botryodiplodia theobromae Pat. Cramer, Vaduz
Raimondo F, Nardini A, Salleo S, Cacciola SO, Gullo MAL (2010) A tracheomycosis as a tool for studying the impact of stem xylem dysfunction on leaf water status and gas exchange in Citrus aurantium L. Trees 24:327–333. https://doi.org/10.1007/s00468-009-0402-4
Rodriguez-Galvez E, Maldonado E, Alves A (2015) Identification and pathogenicity of Lasiodiplodia theobromae causing dieback of table grapes in Peru. Eur J Plant Pathol 141:477–489. https://doi.org/10.1007/s10658-014-0557-8
Rodriguez-Galvez E, Guerrero P, Barradas C, Crous PW, Alves A (2017) Phylogeny and pathogenicity of Lasiodiplodia species associated with dieback of mango in Peru. Fungal Biol 121(4):452–465. https://doi.org/10.1016/j.funbio.2016.06.004
Salunkhe VN, Bhagat YS, Chavan SB, Lonkar SG, Kakade VD (2022) First report of Neoscytalidium dimidiatum causing stem canker of dragon fruit (Hylocereus spp.) in India. Plant Dis. https://doi.org/10.1094/PDIS-04-22-0909-PDN
Schoeneweiss DF (1981) The role of environmental stress in diseases of woody plants. Plant Dis 65:308–314. https://doi.org/10.1094/PD-65-308
Serrato-Diaz LM, Goenaga R (2021) First report of Neoscytalidium dimidiatum causing stem canker on dragon fruit (Hylocereus spp.) in Puerto Rico. Plant Dis 105:2728. https://doi.org/10.1094/PDIS-10-20-2265-PDN
Slippers B, Wingfield MJ (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol Rev 21:90–106. https://doi.org/10.1016/j.fbr.2007.06.002
Slippers B, Johnson GI, Crous PW, Coutinho TA, Wingfield BD, Wingfield MJ (2005) Phylogenetic and morphological re-evaluation of the Botryosphaeria species causing diseases of Mangifera indica. Mycologia 97(1):99–110. https://doi.org/10.3852/mycologia.97.1.99
Slippers B, Roux J, Wingfield MJ, van der Walt FJJ, Jami F, Mehl JWM, Marais GJ (2014) Confronting the constraints of morphological taxonomy in the Botryosphaeriales. Persoonia 33:155–168. https://doi.org/10.3767/003158514X684780
Slippers B, Crous PW, Jami F, Groenewald JZ, Wingfield MJ (2017) Diversity in the Botryosphaeriales: Looking back, looking forward. Fungal Biol 121:307–321. https://doi.org/10.1016/j.funbio.2017.02.002
Sosa D, Parra F, Noceda C, Pérez-Martínez S (2016) Co-occurrence of pathogenic and not pathogenic Fusarium decemcellulare and Lasiodiplodia theobromae isolates within cushion galls disease of cacao (Theobroma cacao L.). J Plant Prot Res. https://doi.org/10.1515/jppr-2016-0020
Tel Zur NY (2015) R&D of Pitahayas - dragon fruit – vine cacti: limitations and challenges and the current global market. Acta Hortic 1067:365–370. https://doi.org/10.17660/ActaHortic.2015.1067.50
Tel-Zur NY, Mizrahi A, Mouyal CJ, Schneider B, Doyle JJ (2011) Phenotypic and genomic characterization of vine cactus collection (Cactaceae). Genet Resour and Crop Evol 58:1075–1085. https://doi.org/10.1007/s10722-010-9643-8
Vaidya G, Lohman DJ, Meier R (2011) Sequence matrix: concatenation software for the fast 735 assembly of multi-gene datasets with character set and codon information. Cladistics 27:171–736. https://doi.org/10.1111/j.1096-0031.2010.00329.x
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Zhang L (2012) Global Forest pest health profile: a case study under the global forest resources assessment 2005. China Agricultural Press, Beijing
Zhang Y, Zhou Y, Sun W, Zhao L, Pavlic-Zupanc D, Crous PW, Slippers B, Dai Y (2021) Toward a natural classification of Botryosphaeriaceae: a study of the type specimens of Botryosphaeria sensulato. Front Microbiol 12:737541. https://doi.org/10.3389/fmicb.2021.737541
Acknowledgements
The authors extend sincere thanks to the Director, ICAR-IIHR, Bengaluru, India, for the facilities provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Ethical approval
This article does not contain any studies with human participants or animals (vertebrates) performed by any of the authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ganesan, S., Kumari, N., Sahu, S. et al. Identification of Lasiodiplodia species inciting stem rot of dragon fruit in India through polyphasic approach. 3 Biotech 13, 333 (2023). https://doi.org/10.1007/s13205-023-03754-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03754-1