Abstract
Silver nanoparticles (AgNPs) were green synthesized using leaf extract of the leaf blight disease (Rhizoctonia solani) susceptible red amaranthus (Amaranthus tricolor L.) and the disease-resistant green (A. dubius) and the wild amaranthus (A. viridis) genotypes, physically characterized, and assessed for their anti-fungal effects against R. solani. The green synthesized nanostructures showed an absorption maximum typical of silver nanoparticles in spectroscopy, and face-centered cubic structures in X-ray diffraction. Field Emission Scanning Electron Microscopic analysis and High-Resolution Transmission Electron Microscopy revealed the size range to be 35–45 nm for all the samples. In vitro mycelial growth inhibition of the pathogen occurred with 500 and 750 ppm concentrations of the nanoparticles in a poisoned-food assay. Further, detached leaves of red amaranthus variety were sprayed with the nanoparticles, and then challenged with the pathogen. There was significant difference in lesion development on leaves sprayed with Ad-AgNPs and Av-AgNPs compared to those treated with At-AgNPs. In the in vivo assay, challenging with the pathogen after spraying the foliage of the leaf blight susceptible red amaranthus variety with Ad-AgNPs at 750 ppm concentration recorded the lowest disease index (7.40) followed by that received Av-AgNPs spray at the same concentration (17.69), five days after inoculation. At-AgNPs treated plants had a disease index of 49.38. Our findings suggest that application of AgNPs green synthesized with leaf extract of disease-resistant genotypes of amaranthus effectively suppressed leaf blight disease incidence in a susceptible amaranthus genotype. To our knowledge, this is the first report on the improved plant pathogen-suppressive activity of any metal nanoparticle when biogenically synthesized using extracts from a disease-resistant plant genotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology has attracted interest and attention in recent years due to its potential applications in various scientific disciplines, including agriculture. The commercial application of nanoscale materials and structures, with a size range of 1 to 100 nm, is a promising aspect of nanoscience (Khan et al. 2019). Two essential components of nanotechnology are nanoparticle synthesis and characterization, and their application for specific purposes. Nano fertilizers, herbicides, fungicides, pesticides and nanosensors have been used in agriculture (Kaur et al. 2022). Several attempts have been made to use nanotechnology to manage diseases in various crop plants (Khan and Rizvi 2014; Elmer and White 2018; Worrall et al. 2018; Siddhartha et al. 2020).
Chemical synthesis of nanoparticles involves the use of toxic solvents, high pressure and energy, and requires high temperature for conversion. Among the various methods of synthesis of nanoparticles, the biogenic method is preferred due to the better yield, less expenditure incurred, and the eco-friendly methodology involved (Iravani et al. 2014; Dikshit et al. 2021). Green synthesis with the help of plant extracts is a bottom-up approach involving reduction/oxidation reaction, with very low toxicity compared to chemical methods, as it eliminates the use of harmful reagents (Kumar et al. 2021). It has been observed that green synthesized nanoparticles effectively reduce the severity of many plant diseases (Mishra et al. 2020; Ghazy et al. 2021; Ahmad et al. 2020). Various metals, including gold, silver, zinc, copper etc., are used for synthesizing nanoparticles. Silver is preferred to other metals due to its unique characteristics, including higher thermal and electrical conductivity. Also, silver has potential antibacterial, antioxidant, anticancer and wound healing properties (Flores‐Lopez et al. 2019). Various biological agents have been used for synthesizing silver nanoparticles, including fungi, bacteria and plant extracts (Qian et al. 2013; Wang et al. 2016; Meng et al. 2021; Roy et al. 2019). Efficient synthesis of silver nanoparticles has been reported using aqueous extracts of plant parts. Green synthesized silver nanoparticles exhibit potent anti-microbial activity, especially against bacteria, fungi (Al-Otibi et al. 2021; Singh et al. 2021) and algae (Bijula et al. 2022). We hypothesize that silver nanoparticles biosynthesized using extracts of disease-resistant plant cultivars or genotypes may have better disease-suppressive ability.
Amaranthus is a popular tropical leafy vegetable cultivated in Southern India. Leaf blight caused by soil-borne fungal pathogen Rhizoctonia solani Kühn is a devastating disease of amaranthus, especially in the popular red cultivars belonging to Amaranthus tricolor (Nayar et al. 1996; Gokulapalan et al. 2000). Symptoms appear as irregular light cream-white colored spots on the foliage, which further coalesce, forming shot holes. Leaves being the economic part, the disease greatly reduces the marketability of the produce, resulting in considerable financial loss. The chemical control measure recommended for managing the disease is the foliar application of the fungicide Mancozeb (0.4%) at fortnightly intervals (Gokulapalan et al. 1999). Even though chemical control is effective, there is profound demand for alternative methods to manage plant diseases (Panth et al. 2020; Zhou et al. 2022). Plant activators and biological control agents have been proposed for the management of the foliar blight disease of amaranthus (Nair and Anith 2009; Yashaswini et al. 2021, 2022). Another alternative is to cultivate disease-resistant varieties or genotypes of amaranthus. The green-leaved cultivar, A. dubius and the wild genotype, A. viridis are found to be resistant to the disease (Fig. 1). However, A. tricolor, the red-leaved cultivar, which is highly susceptible to the disease, has more consumer preference in South India. We wished to confirm if silver nanoparticles green synthesized with extracts from disease-resistant amaranthus genotypes have enhanced leaf blight suppressive effects than those synthezised with extracts from a disease-suceptible genotype. In the present study, leaf extracts of two leaf blight resistant genotypes, A. dubius and the wild genotype, A. viridis, and that from the susceptible genotype, A. tricolor were used for green synthesis of silver nanoparticles, and their relative efficacy in suppressing the disease in a susceptible red amaranthus cultivar was evaluated.
Leaf blight infection in amaranthus genotypes on artificial inoculation with Rhizoctonia solani. From left to right: Green amaranthus (Amaranthus dubius), wild amaranthus (Amaranthus viridis) and red amaranthus (Amaranthus tricolor). Photograph taken three days after artificial inoculation with R. solani mycelial disc on the lower side of the leaf
Materials and methods
Green synthesis of silver nanoparticles
Silver nanoparticles were green synthesized using aqueous leaf extracts of A. dubius, A. viridis and A. tricolor following the protocol by Alex et al. (2012). Fresh, healthy leaves were collected, and 5 g leaves were thoroughly washed in Labolene, followed by further washing in tap water and sterile deionized water twice each. The leaves were finely chopped, boiled in 100 ml sterile distilled water, filtered through Whatman No. 2 filter paper, and centrifuged at 10,000 rpm for 10 min to get the leaf extract. An aqueous solution of 1 mM silver nitrate (AgNO3) in double distilled water was prepared and mixed with the leaf extract in a ratio of 9:1 (V/V). It was heated for 40 s in a microwave oven and kept at room temperature under dark conditions for 1 h for the formation of silver nanoparticles. After incubation, a color change of the solution to dark brown indicated the reduction of silver ions into silver nanoparticles. The suspension was centrifuged at 10,000 rpm for 15 min. The pellet was resuspended in sterile deionized water, and once again centrifuged at 10,000 rpm for 15 min followed by drying in a hot air oven at 40 °C for 20 min, and the dry weight was noted. Finally, the pellet was resuspended in 1000 μL of sterile deionized water for future use. The bio reduction of silver ions was confirmed by adding 0.1 M aqueous sodium chloride into the supernatant obtained after centrifugation of the colloidal solution and noting the formation of precipitation, if any (Alex et al. 2012). The silver nanoparticles were designated as Ad-AgNPs, Av-AgNPs and At-AgNPs depending on the genotypes of the plants used for the green synthesis. Ad, Av and At correspond to Amaranthus dubius, Amaranthus viridis and Amaranthus tricolor respectively.
Physical characterization of silver nanoparticles
UV–visible spectroscopy
The bio reduction of silver ions into silver nanoparticles was monitored in the colloidal solution by measuring the absorption maxima using a UV Visible spectrometer (1900i, Shimadzu, Japan) by scanning at wavelengths from 300 to 600 nm in a quartz cuvette with sterile deionized water as reference.
X-ray diffraction analysis
The analysis for determining the crystalline nature of biosynthesized silver nanoparticles was carried out using an X-ray diffractometer (Bruker D8 ADVANCE with DAVINCI). The analysis was carried out with an operating voltage of 40 kV and a current of 30 mA. The diffraction patterns were recorded by Cu-Kα radiation of wavelength 1.54 Å in the region of 2θ from 20° to 120°.
Fourier transform infrared spectroscopy
The green synthesized silver nanoparticles were subjected to Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR) (Shimadzu, IR prestige 21, ZnSe crystal, KBr beam splitter) to analyse their spectra. The spectrum scan range was from 600 to 4000 cm−1.
Field emission scanning electron microscopy
The aqueous sample of silver nanoparticle suspension was drop-cast on to the FE-SEM sample stub using carbon tape and air dried. The Field Emission Scanning Electron Microscope (Tescan Brono s.r.o. Czech, MAIA3 XMH) analysis was performed with MAIA TC software.
High-resolution transmission electron microscopy
HRTEM analysis was carried out to elucidate the morphological characters of the green synthesized silver nanoparticles. 100 µL of the sample was made up to 1 mL with sterile distilled water, sonicated for 15 min and filtered (0.22 µm nylon syringe filter). A drop of the filtered sample was cast on to carbon-coated copper grids to create a thin film and air dried. The images were then developed using JEOL JEM 2100 with an acceleration voltage of 200 kV.
In vitro anti-fungal activity of silver nanoparticles
A virulent strain of the leaf blight pathogen, Rhizoctonia solani, previously isolated from the infected red amaranthus plants, and maintained at the Department of Microbiology, College of Agriculture, Vellayani, Thiruvananthapuram, Kerala, was used. Pathogenicity of the isolate was tested on detached leaves and intact plants of the disease-suceptible amaranthus cultivar Arun, and Koch’s postulates proved.
The anti-fungal potential of the biosynthesized silver nanoparticles was tested by the poisoned-food method described by Al-Othman et al. (2014). Ten mL of autoclaved double-strength PDA was mixed with an equal volume of each of the green synthesized AgNPs preparation to get a final concentration of either 500 or 750 ppm of the amendment and poured in to Petri plates of 10 cm dia. Plates were inoculated at the centre with a mycelial disc (5 mm dia) of R. solani, incubated at 28 °C for five days, and checked for mycelial growth inhibition. Commercially available silver nanoparticle suspension (Sigma-Aldrich, India) was also used at the same concentration as mentioned above. Filter sterilized (0.2 µm) leaf extracts of A. dubius, A. viridis and A. tricolor prepared for making the green synthesis of silver nanoparticles as described above were also amended in PDA medium at 50% concentration and inoculated with the pathogen to know the inhibitory action if any, of the extracts. Amendment of the medium with 0.4% Mancozeb was kept as the positive control. Absolute control plates had no amendments. Percent inhibition (PI) was calculated as follows:
where C is the diameter of mycelial growth (cm) in control plates, T that in the treatment plates.
Detached leaf assay with leaves of the red amaranthus variety Arun, as described elsewhere (Yashaswini et al. 2022) was carried out to evaluate the ability of green synthesized silver nanoparticles to suppress the infection by R. solani. Healthy leaves of red amaranthus (Variety-Arun) were collected and washed with sterile distilled water. Two different concentrations of silver nanoparticles, 500 ppm and 750 ppm were selected for the study and two sets of independent experiments were performed with three replications each. Green synthesized Ad-AgNPs, Av-AgNPs, At-AgNPs and commercially available AgNPs were prepared at concentrations mentioned above, and sprayed on individual detached leaves and allowed to air dry. Challenge inoculation with the pathogen was done by placing a mycelial disc (4 mm dia) of the pathogen on the lower side of the treated leaf after giving a single pinprick. The mycelial disc was covered with a thin layer of moist cotton to provide sufficient humidity. Leaves were kept in Petri dishes and incubated at 28 °C for three days. Leaves sprayed either with 0.4% Mancozeb solution or sterile water, challenged with the pathogen, and incubated under the same conditions served as controls. The development of lesions was assessed and scoring was done based on the scale developed by Nair and Anith (2009).
In vivo disease suppression in red amaranthus plants
The efficacy of green synthesized AgNPs on foliar blight disease suppression in the red amaranthus (A. tricolor) var. Arun was tested in a pot culture experiment in a naturally ventilated green house. Seedlings were grown in protrays with sterilized vermiculite as planting medium. Two seeds were sown per cavity of the portray, and a single seedling per cell was maintained after germination. Irrigation was given twice a day. After 15 days, seedlings were transplanted to plastic pots (17.5 cm dia) filled with one kg each of potting mixture (soil, coir pith and farm yard manure in the ratio 2:1:1). After 15 days of transplanting, plants were sprayed with nanoparticle suspensions or the fungicide. Two different concentrations of silver nanoparticles, 500 ppm and 750 ppm were used, and two independent sets of experiments were carried out. The experiments were set up as completely randomized design (CRD), with three replications having three plants each. Treatments included foliar spray with Ad-AgNPs, Av-AgNPs, At-AgNPs and commercially available AgNPs. Mancozeb (0.4%) spray was used as the chemical control. Pathogen inoculated and absolute controls were also maintained. Challenge inoculation with the pathogen was carried out on the top three fully unfurled, healthy, intact leaves of each plant in all treatments except the absolute control. Mycelial discs (4 mm dia) from five-day-old PDA plates of R. solani were used for inoculation. Inoculation was done on the lower leaf surface of intact leaves after giving a single pinprick. The mycelial bit was covered with a thin layer of moist cotton and the whole plant was covered with a polythene bag to maintain humidity. Observations on disease severity were recorded on the 3rd and 5th days after inoculation (DAI) of the pathogen. Disease severity was calculated for each plant inoculated with the pathogen. Severity of disease was scored using a 0–9 scale (Nair and Anith 2009).
Grade | Description |
|---|---|
0 | No infection |
1 | 1–10 percent of leaf area infected |
3 | 11–25 percent of leaf area infected |
5 | 26–50 percent of leaf area infected |
7 | 51–75 percent of leaf area infected |
9 | 76–100 percent of leaf area infected |
Percent disease index (PDI) was calculated using the formula:
Statistical analysis
Statistical analysis was done by one way Analysis of Variance (ANOVA) and the treatment means were compared using Duncan’s Multiple Range Test (DMRT) at 5% level of significance using the statistical package grapesAgri1, 1.1.0. in R version 4.2.3 (https://www.kaugrapes.com/home) (Gopinath et al. 2021).
Results
Green synthesis of silver nanoparticles
The formation of silver nanoparticles from AgNO3 solution, with the use of leaf extracts of Amaranthus spp. could be confirmed by the color change of the reaction mixture from pale yellow to dark brown. When leaf extract of A. tricolor was used, the color changed from reddish pink to dark brown (Fig. 2). The addition of sodium chloride to the supernatant of all the three samples did not produce any precipitate, whereas a white precipitate was formed in the control solution of AgNo3 where no leaf extract was used.
Physical characterization of silver nanoparticles
UV–visible spectroscopy
UV–visible spectroscopy confirmed the reduction of silver nitrate by the aqueous leaf extracts. Silver nitrate solution treated with the leaf extracts of A. dubius and A. viridis showed an absorption maximum at 420 nm, whereas the peak was at 440 nm when A. tricolor extract was used (Fig. 3A).
Characterization of green synthesized silver nanoparticles. A UV–visible absorption spectra (from 300 to 600 nm) of green synthesized AgNPs. (From top to bottom in the column; At-AgNPs, Av-AgNPs and Ad-AgNPs). B X-Ray Diffraction analytical pattern of green synthesized AgNPs. (From top to bottom in the column; At-AgNPs, Av-AgNPs and Ad-AgNPs). C Fourier Transform Infrared spectra of green synthesized AgNPs. (From top to bottom in the column; At-AgNPs, Av-AgNPs and Ad-AgNPs). Ad: Amaranthus dubius Av: Amaranthus viridis At: Amaranthus tricolor
X-ray diffraction analysis
The distinctive peaks in the XRD offered additional proof of the biosynthesized silver nanostructures made using the leaf extracts. The XRD pattern for green synthesized nanoparticles derived from the leaf extracts revealed strong peaks of 2 theta values of 27.44, 31.85, 37.77, 45.83, 54.47, 57.25, 64.42, and 76.79, which correspond to (210), (122), (101), (103), (142), (241), (220), and (201) planes based on its face-centered cubic structure, respectively (Fig. 3B).
Fourier transform infrared spectroscopy
FTIR studies of the prepared nanoparticles are shown in Fig. 3C. The peak at 750 cm−1 corresponds to out-of-plane bending vibrations of O–H groups (weak bending vibrations of alcohols and phenols). 1025–1375 cm−1 corresponds to N–H bonds/amides/C–N stretching vibrations of amines/C–O stretching alcohols/ethers/carboxylic acids or anhydrides. The nanoparticles exhibit stretching peaks at around 1632 cm–1, which is related to the C\(=\)C of alkenes. The slight peak at about 1374 cm−1 is related to C–H vibrations, contributing to the presence of alkanes. A broad peak at around 3282 cm−1 was attributed to stretching vibrations of the N–H and O–H groups.
Field emission scanning electron microscopy
The average size of Ad-AgNPs was found to be ~ 45 nm. For Av-AgNPs and At-AgNPs the size was ~ 35 nm. FESEM images also verified that the synthesized nanoparticles possess a uniform distribution (Fig. 4A).
Morphological analysis of green synthesized silver nanoparticles. A Field Emission Scanning Electron Microscopy (FE-SEM) images of green synthesized AgNPs. a Ad-AgNPs b Av-AgNPs c At-AgNPs. B Column 1: (From top to bottom) Tranmission Electrom Microscopy images of Ad-AgNPs, Av-AgNPs and At-AgNPs. Column 2: (From top to bottom) High-Resolution Transmission Electron Microscopy images of Ad-AgNPs, Av-AgNPs and At-AgNPs. Column 3: (From top to bottom) Selected Area Electron Diffraction pattern of Ad-AgNPs, Av-AgNPs, At-AgNPs. Ad: Amaranthus dubius Av: Amaranthus viridis At: Amaranthus tricolor
High-resolution transmission electron microscopy
The high-resolution TEM images showed the morphology of the synthesized nanoparticles and the average size of Ad-AgNPs was ~ 45 nm. For Av-AgNPs and At-AgNPs the size appeared to be ~ 35 nm. The SAED (Selected Area Electron Diffraction) pattern of the samples revealed the polycrystalline nature of the nanoparticles (Fig. 4B).
In vitro anti-fungal activity of green synthesized silver nanoparticles
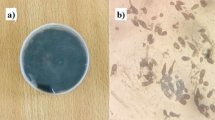
In poisoned-food assay, amendment with the chemical fungicide showed the highest inhibition up to 100% on the 5th day after inoculation. No fungal mycelial growth inhibition was noticed when commercial silver nanoparticle suspension at 500 ppm and 750 ppm and leaf extracts of A. dubius, A. viridis and A. tricolor were amended in the growth medium (Fig. 5). The percentage inhibition (PI) was 65.15 and 52.94, and 41.49 respectively, in medium amended with 500 ppm of Ad-AgNPs, Av-AgNPs and At-AgNPs. While the PI in plates amended with 750 ppm of green synthesized nanoparticles made from the leaf extracts of A. dubius, A. viridis and A. tricolor were 74.18 and 62.46 and 49.08 respectively. At a greater concentration, higher inhibition was observed and the test concentrations were set at 500 ppm and 750 ppm for future in vivo experiments (Table 1).
Mycelial growth of Rhizocotonia solani in poisoned PDA medium. A Ad-AgNPs (750 ppm) B Ad leaf extract (50%) C Av-AgNPs (750 ppm) D Av leaf extract (50%) E At-AgNPs (750 ppm) F At leaf extract (50%) G Control (No amendment) H Commercial AgNPs (750 ppm) I Mancozeb (0.4%). Amended Potato dextrose medium was inoculated with R. solani mycelial disc at the centre, incubated and observed after five days. Ad: Amaranthus dubius Av: Amaranthus viridis At: Amaranthus tricolor
In the detached leaf assay, inoculation of R. solani on untreated detached healthy leaves of red amaranthus variety Arun produced typical blight symptoms. The lesions appeared as translucent irregular green patches on the inoculated areas of leaves on the third day of inoculation with the pathogen. Later the patches enlarged, covered the entire leaf lamina and the leaves got dried up. Disease symptoms occurred in all leaves sprayed with silver nanoparticles as well. However, there was a significant difference between lesion development on leaves treated with Ad-AgNPs and Av-AgNPs compared to those treated with At-AgNPs (Fig. 6). The severity also differed significantly among the treatments.
Leaf blight incidence in A. tricolor var. Arun in the detached leaf assay. *Mean of nine independent observations. Columns of same color with the same superscripted letters/symbols do not differ significantly (p ≤ 0.05) according to Duncan’s multiple range test (DMRT). Ad: Amaranthus dubius Av: Amaranthus viridis At: Amaranthus tricolor
In vivo disease suppression
In the first set of experiments where nanoparticles of 500 ppm concentration were used, the pathogen-inoculated control had the highest disease severity. The disease severity in plants sprayed with Ad-AgNPs was 1.64 on 3 DAI, whereas in plants sprayed with At-AgNPs, a significantly high disease severity (16.46) was observed. A similar trend was observed five days after the challenge inoculation also. Disease severity was also less in plants sprayed with Av-AgNps. Occurrence and severity of disease were found to be less in plants sprayed with commercial silver nanoparticle dispersion and the chemical fungicide Mancozeb (Table 2).
In the second set of experiments with 750 ppm of silver nanoparticles also, the pathogen-inoculated treatment had the highest disease severity of 11.93 and 86.83, on 3 and 5 DAI respectively (Fig. 7). In contrast to plants sprayed with Ad-AgNPS, where the disease severity was 1.64, on 3 DAI, a significantly higher disease severity of 8.78 was observed in plants sprayed with At-AgNPs. The corresponding values 5 DAI were 7.41 in Ad-AgNPs and 49.38 in At-AgNPs treatments respectively. The disease severity in plants sprayed with Av-AgNps was also lesser than that observed in plants sprayed with commercial silver nanoparticle dispersion and Mancozeb (Table 2). In nutshell, disease severity was significantly lesser in plants treated with the silver nanoparticles synthesized using extracts from the resistant genotypes of amaranthus when compared to that sprayed with nanoparticles produced using extracts from the susceptible red amaranthus.
Leaf blight disease incidence in A. tricolor var. Arun treated with AgNPs (750 ppm) on 5th day after challenge inoculation with R. solani. Representative leaves from corresponding inoculated plants are also shown. A Ad-AgNPs B Av-AgNPs C At-AgNPs D Commercial AgNPs E Mancozeb (0.4%). F Pathogen control. Ad: Amaranthus dubius Av: Amaranthus viridis At: Amaranthus tricolor
Discussion
The use of plant extracts as reducing agents for the synthesis of metal nanoparticle is an innovative approach, avoiding the use of hazardous and non-renewable chemicals. The basic principle of such green synthesis is the capacity of flavonoids and alkaloids present in the extracts to reduce metal ion precursors. Phytochemicals in plants act as reducing, capping, and stabilizing agents (Afreen et al. 2020). The biosynthesis of nanoparticles primarily depends upon the type of plant extract, temperature, pH, and solvent used for extraction. Many studies have reported the characteristics of green synthesized nanoparticles as a function of the kind of plant extract, composition, and method of synthesis (Abou El-Nour et al. 2010).
The reduction and stabilization of silver ions by phytochemical components present in the extracts of plants having medicinal values have already been established well (Dikshit et al. 2021). Interestingly, nanoparticles synthesized with the leaf extract of medicinal plants like neem (Azadirachta indica), widely used in traditional medicine were proven to have enhanced antibacterial properties (Asimuddin et al. 2020).
Green synthesized AgNPs has been reported to have antibacterial, anti-fungal, anticancer and antioxidant activities (Valsalam et al. 2019; Wypij et al. 2021). There exist strong experimental data on the potential role of green synthesized silver nanoparticles in disease suppression (Castillo-Henríquez et al. 2020; Bawazeer et al. 2021; Singh et al. 2021). As characteristics of biogenically synthesized nanoparticles have been reported to be influenced by the kind of plant extract used, in the present study, we used leaf extracts from the leaf blight susceptible red Amaranthus (A. tricolor), and resistant genotypes A. dubius (Celine et al. 2013) and A. viridis for the green synthesis of AgNPs. Our aim was to check if the green synthesized metal nanoparticles using leaf extracts of disease-resistant amaranthus genotypes possess better disease-suppressive potential than those synthesized with the extract of a susceptible one. Even though a number of reports are available on the exploitation of green synthesized AgNPs for plant disease control, no studies have been carried out on the use of AgNPs synthesized using plant extracts of disease-resistant genotypes for managing any disease in a susceptible variety or cultivar.
Extracts from Amaranthus spp. have already been reported to serve as good biological agents for silver nanoparticle synthesis. All the three genotypes used in our study have already been used to green-synthesize silver nanoparticles (Fatimah and Aftrid 2019; Sigamoney et al. 2016). The formation of silver nanoparticles could be confirmed by the color change of the reaction mixture after 1 h of incubation. The color change is attributed to the excitation of surface plasmon vibrations in silver nanoparticles. Further, the addition of 0.1 M NaCl to the supernatant obtained after separating the green synthesized AgNPs verified the complete conversion of AgNPs, as no precipitate of silver chloride was formed in the supernatants indicating the complete conversion of Ag+ ions. The color change observed in the samples was confirmed by UV spectroscopy and found to be typical of AgNPs (Afreen et al. 2020). The characteristic surface plasmon resonance observed for AgNPs is the product of oscillation of free electrons in the AgNPs. The peak of UV–visible absorption spectra gives an indication of the uniformity of size of green synthesized AgNPs (Wei et al. 2021). Bioreduction, capping and stabilization of the nanoparticle assisted by components in the leaf extracts could be found out with FTIR. Analysis revealed that all three extracts used in the present study contained compounds that helped in the formation of metal nanoparticles. Phytochemicals present in the extracts act as capping agents and could provide specific and unusual properties to the nanoparticles (Sharma et al. 2019). FTIR results give a broad understanding of the kind of phytochemicals involved in the biogenesis of nanoparticles, but it would be difficult to decipher which component from the extract could have given the increased anti-fungal activity to the green synthesized AgNPs. X-ray diffraction (XRD) analysis and electron microscopy revealed the uniformity of the size and crystalline nature of all the three sets of green synthesized metal nanoparticles. Therefore, the difference in the anti-fungal activity of the nanoparticles towards R. solani may not be a function of the size or shape of the particles.
The toxicity of silver nanoparticles is reported to be due to their ability to form free radicals on contact with the cell surface that makes the cell wall porous, resulting in leakage of cell contents (Mikhailova 2020). Silver nanoparticles also cause interruptions in DNA replication and cessation of cell multiplication (Wen et al. 2023). Anti-microbial activity has been reported for silver nanoparticles synthesized using plant extracts of Amaranthus tricolor (Fatimah and Aftrid 2019), A. dubius (Sigamoney et al. 2016), A. viridis (Koyyati et al. 2014), A. retroflexus (Bahrami-Teimoori et al. 2017), A. gangeticus (Kolya et al. 2015) and A. spinosus (Preenanka and Sebastian 2022). The quick and easiest way to assess the anti-microbial activity of a nanoparticle suspension is by growth inhibition studies on agar plates. It gives insights into the direct action of the test materials on the target organism. The results of in vitro mycelial growth inhibition in the present study indicated that the green synthesized AgNPs using amaranthus leaf extract were effective in inhibiting the growth of R. solani, the leaf blight pathogen infecting amaranthus. Though green synthesized nanoparticles using extracts of all the three amaranthus genotypes had inhibitory effects on the fungal pathogen, it was evident that AgNPs synthesized from the resistant genotypes of amaranthus showed higher anti-fungal activity than the AgNPs synthesized with extract from the susceptible genotypes. Ad-AgNPs had better anti-fungal activity under in vitro conditions than Av-AgNPs. Anti-fungal activity of the leaf extracts from the amaranthus genotypes used in the study was tested to confirm whether the fungal suppressive effect was due to the contents of the leaf extracts. However, the addition of leaf extracts into the medium did not have any adverse effects on the growth of the fungus, suggesting that the extracts do not possess any direct inhibitory action.
Detached leaf assay, to some extent, mimics the real field situation as it involves the interaction of the host, fungal pathogen and the anti-fungal agent. Direct inhibitory activity of the anti-fungal agent could be measured by assessing the suppression of disease symptoms in this assay. The test was performed on the leaves of the highly blight susceptible red amaranthus. Detached leaf assay results were also in tune with the outcome of the in vitro plate assays. AgNPs produced with the extracts of disease-resistant genotypes suppressed the symptom development by R. solani on the detached leaves of susceptible red amaranthus. Since the test system does not contain whole plants, the probable biochemical elicitation of a typical defense response could be ruled out and direct toxic activity of the nanoparticle on the pathogen can only be the reason behind the suppression of the disease/symptom development.
The in vivo disease suppression assay, at both the concentrations tested, also gave similar results to the earlier in vitro results. Silver nanoparticles synthesized with extracts of the disease-resistant genotypes of amaranthus showed better disease-suppressive activity, and Ad-AgNPs suppressed the symptoms better than Av-AgNps. This was evident in the disease severity measured on the 3rd and 5th days of the challenge inoculation. The present study reports for the first time the increased disease-suppressive ability of the biogenic silver nanoparticles green synthesized using leaf extracts of a resistant plant genotype on the disease development by a plant pathogen in a susceptible variety/genotype. Our model could be extended to other pathosystems involving fungal, bacterial or viral pathogens in other crop plants for further validation. Another area that could be explored is the use of other metal nanoparticles, substituting silver. Copper and Zinc are two better candidates as they are environmentally safer than silver nanoparticles.
Conclusion
The present study adds to the evidence that the green synthesized nanoparticles from the leaf extracts of Amaranthus spp. exhibit remarkable anti-fungal activity, and it also helped reduce the incidence and severity of foliar blight disease in red amaranthus by R. solani. The most essential aspect that can be highlighted from our study is the remarkable effectiveness of silver nanoparticles produced with the extracts of disease-resistant amaranthus genotypes. Further research would help in utilizing the potential of the green synthesized nanoparticles from the disease-resistant genotypes in the disease management of a wide variety of crops. This concept can be exploited as a potential tool for managing pathogens in susceptible varieties of crop plants. Large-scale experiments are also required to resolve and validate the disease-suppressive potential of such nanoformulations under field conditions. The mechanism of action of green synthesized silver nanoparticles and molecular mechanisms of defense in plants also need to be investigated. Before a field-level recommendation is made, analysis of residue levels is also to be worked out in further experiments, especially in a leafy vegetable like amaranthus.
Data Availability
Data will be made available on request.
References
Abou El-Nour KM, Eftaiha AA, Al-Warthan A, Ammar RA (2010) Synthesis and applications of silver nanoparticles. Arab J Chem 3:135–140
Afreen A, Ahmed R, Mehboob S, Tariq M, Alghamdi HA, Zahid AA, Hasan A (2020) Phytochemical-assisted biosynthesis of silver nanoparticles from Ajuga bracteosa for biomedical applications. Mater Res Express 7:075404
Ahmad H, Venugopal K, Rajagopal K, De Britto S, Nandini B, Pushpalatha HG, Jogaiah S (2020) Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globules and their fungicidal ability against pathogenic fungi of apple orchards. Biomolecules 10:425
Alex S, Rani PMR, Soni KB, Nair DS, Reghunath BR (2012) Biosynthesis of silver nanoparticles with antibacterial activity using leaf extract of Michelia champaca. J Plant Sci Res 28:121–126
Al-Othman MR, Abd El-Aziz ARM, Mahmoud MA, Eifan SA, El-Shikh MS, Majrashi M (2014) Application of silver nanoparticles as anti-fungal and antiaflatoxin B1 produced by Aspergillus flavus. Dig J Nanomater Bios 9:151–157
Al-Otibi F, Alkhudhair SK, Alharbi RI, Al-Askar AA, Aljowaie RM, Al-Shehri S (2021) The anti-microbial activities of silver nanoparticles from aqueous extract of grape seeds against pathogenic bacteria and fungi. Molecules 26:6081
Asimuddin M, Shaik MR, Adil SF, Siddiqui MRH, Alwarthan A, Jamil K, Khan M (2020) Azadirachta indica based biosynthesis of silver nanoparticles and evaluation of their antibacterial and cytotoxic effects. J King Saud Univ Sci 32:648–656
Bahrami-Teimoori B, Nikparast Y, Hojatianfar M, Akhlaghi M, Ghorbani R, Pourianfar HR (2017) Characterisation and anti-fungal activity of silver nanoparticles biologically synthesized by Amaranthus retroflexus leaf extract. J Exp Nanosci 12:129–139
Bawazeer S, Rauf A, Shah SUA, Shawky AM, Al-Awthan YS, Bahattab OS, El-Esawi MA (2021) Green synthesis of silver nanoparticles using Tropaeolum majus: phytochemical screening and antibacterial studies. Green Process Synth 10:85–94
Bijula BL, Alex S, Soni KB, Anith KN, Joy M, Nair DS, Beena R, Benny A (2022) Algicidal effects of green synthesized silver nanoparticles using Tinospora cordifolia on Chlamydomonas reinhardtii. J Pure Appl Microbiol 16:1122–1129
Castillo-Henríquez L, Alfaro-Aguilar K, Ugalde-Alvarez J, Vega-Fernandez L, Montes de Oca-Vasquez G, Vega-Baudrit JR (2020) Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as anti-microbial agents in the agricultural area. Nanomaterials 10:1763
Celine VA, Girija VK, Sreelathakumary I, Vahab MA (2013) Selection of amaranth genotypes for resistance to Rhizoctonia solani. Int J Veg Sci 19:157–163
Dikshit PK, Kumar J, Das AK, Sadhu S, Sharma S, Singh S, Kim BS (2021) Green synthesis of metallic nanoparticles: applications and limitations. Catalysts 11:902
Elmer W, White JC (2018) The future of nanotechnology in plant pathology. Annu Rev Phytopathol 56:111–133
Fatimah I, Aftrid ZHVI (2019) Characteristics and antibacterial activity of green synthesized silver nanoparticles using red spinach (Amaranthus tricolor L) leaf extract. Green Chem Lett Rev 12:25–30
Flores-Lopez LZ, Espinoza-Gomez H, Somanathan RM (2019) Silver nanoparticles: electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects: mini review. J Appl Toxicol 39:16–26
Ghazy NA, Abd El-Hafez OA, El-Bakery AM, El-Geddawy DI (2021) Impact of silver nanoparticles and two biological treatments to control soft rot disease in sugar beet (Beta vulgaris L). Egypt J Biol Pest Control 31:1–12
Gokulapalan C, Reghunath P, Celine VA, Nair SR (1999) Managing leaf blight on amaranth. Indian J Hortic 44:33–33
Gokulapalan C, Kamala N, Umamaheswaran K (2000) Foliar blight of Amaranthus caused by Rhizoctonia solani Kuhn. J Mycol Plant Pathol 30:239–241
Gopinath PP, Parsad R, Joseph B, Adarsh VS (2021) GrapesAgri1: collection of shiny apps for data analysis in agriculture. J Open Source Softw 6:3437
Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9:385
Kaur S, Sharma K, Singh R, Kumar N (2022) Advancement in crops and agriculture by nanomaterials. In: Thakur A, Thakur P, Khurana SP (eds) Synthesis and applications of nanoparticles. Springer, Singapore. https://doi.org/10.1007/978-981-16-6819-7_14
Khan MR, Rizvi TF (2014) Nanotechnology: scope and application in plant disease management. Plant Pathol J 13:214–231
Khan I, Saeed K, Khan I (2019) Nanoparticles: Properties, applications and toxicities. Arab J Chem 12:908–931
Kolya H, Maiti P, Pandey A, Tripathy T (2015) Green synthesis of silver nanoparticles with anti-microbial and azo dye (Congo red) degradation properties using Amaranthus gangeticus Linn leaf extract. J Anal Sci Technol 6:1–7
Koyyati R, Nagati V, Nalvothula R, Merugu R, Kudle KR, Marx P, Padigya PRM (2014) Antibacterial activity of silver nanoparticles synthesized using Amaranthus viridis twig extract. Int J Res Pharm Sci 5:32–39
Kumar JA, Krithiga T, Manigandan S, Sathish S, Renita AA, Prakash P, Crispin S (2021) A focus to green synthesis of metal/metal-based oxide nanoparticles: various mechanisms and applications towards ecological approach. J Clean Prod 324:129198
Meng Y, Zhang H, Hu N, Zhang B, Qiu Z, Hu J, Xu X (2021) Construction of silver nanoparticles by the triple helical polysaccharide from black fungus and the antibacterial activities. Int J Biol Macromol 182:1170–1178
Mikhailova EO (2020) Silver nanoparticles: mechanism of action and probable bio-application. J Funct Biomater 11:84
Mishra AK, Tiwari KN, Saini R, Kumar P, Mishra SK, Yadav VB, Nath G (2020) Green synthesis of silver nanoparticles from leaf extract of Nyctanthes arbor-tristis L and assessment of its antioxidant, anti-microbial response. J Inorg Organomet Polym 30:2266–2278
Nair CB, Anith KN (2009) Efficacy of acibenzolar-S-methyl and rhizobacteria for the management of foliar blight disease of amaranth. J Trop Agric 47:43–47
Nayar K, Gokulapalan C, Nair MC (1996) A new foliar blight of amaranthus caused by Rhizoctonia solani. Indian Pyhtopathol 49:406–407
Panth M, Hassler SC, Baysal-Gurel F (2020) Methods for management of soilborne diseases in crop production. Agriculture 10:16
Preenanka R, Sebastian D (2022) Characterization of green synthesized antibacterial silver nanoparticles from Amaranthus spinosus L extract. BioNanoSci 12:502–511
Qian Y, Yu H, He D, Yang H, Wang W, Wan X, Wang L (2013) Biosynthesis of silver nanoparticles by the endophytic fungus Epicoccum nigrum and their activity against pathogenic fungi. Bioprocess Biosyst Eng 36:1613–1619
Roy A, Bulut O, Some S, Mandal AK, Yilmaz MD (2019) Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting anti-microbial activity. RSC Adv 9:2673–2702
Sharma D, Kanchi S, Bisetty K (2019) Biogenic synthesis of nanoparticles: a review. Arab J Chem 12:3576–3600
Siddhartha VA, Bashyal BM, Gogoi R, Kumar R (2020) New nano-fungicides for the management of sheath blight disease (Rhizoctonia solani) in rice. Int J Pest Manag 68:217–226
Sigamoney M, Shaik S, Govender P, Krishna SBN (2016) African leafy vegetables as bio-factories for silver nanoparticles: a case study on Amaranthus dubius C Mart. Ex Thell S Afr J Bot 103:230–240
Singh R, Patade VY, Sanchita Singh A (2021) Anti-microbial potential of silver nanoparticles biosynthesized using aerial yam bulbils for control of selected phytopathogens. Arch Phytopathol Plant Prot 54:2275–2293
Valsalam S, Agastian P, Arasu MV, Al-Dhabi NA, Ghilan AKM, Kaviyarasu K, Arokiyaraj S (2019) Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L and its enhanced in-vitro antibacterial, anti-fungal, antioxidant and anticancer properties. J Photochem Photobiol B Biol 191:65–74
Wang C, Singh P, Kim YJ, Mathiyalagan R, Myagmarjav D, Wang D, Yang DC (2016) Characterization and anti-microbial application of biosynthesized gold and silver nanoparticles by using Microbacterium resistens. Artif Cells Nanomed Biotechnol 44:1714–1721
Wei S, Wang Y, Tang Z (2021) A novel green synthesis of silver nanoparticles by the residues of Chinese herbal medicine and their biological activities. RSC Adv 11:1411–1419
Wen H, Shi H, Jiang N, Qiu J, Lin F, Kou Y (2023) Anti-fungal mechanisms of silver nanoparticles on mycotoxin producing rice false smut fungus. Iscience 26:105763
Worrall EA, Hamid A, Mody KT, Mitter N, Pappu HR (2018) Nanotechnology for plant disease management. Agronomy 8:285
Wypij M, Jędrzejewski T, Trzcinska-Wencel J, Ostrowski M, Rai M, Golinska P (2021) Green synthesized silver nanoparticles: antibacterial and anticancer activities, biocompatibility, and analyses of surface-attached proteins. Front Microbiol 12:632505
Yashaswini MS, Nysanth NS, Anith KN (2021) Endospore-forming bacterial endophytes from Amaranthus spp. improve plant growth and suppress leaf blight (Rhizoctonia solani Kühn) disease of Amaranthus tricolor L. Rhizosphere 19:100387
Yashaswini MS, Nysanth NS, Gopinath PP, Anith KN (2022) Endospore-forming phyllosphere bacteria from Amaranthus spp. suppress leaf blight (Rhizoctonia solani Kuhn) disease of Amaranthus tricolor L. J Trop Agric 60:95–108
Zhou H, Yuan X, Zhou H, Shen H, Ma L, Sun L, Fang G, Sun H (2022) Surveillance of pine wilt disease by high resolution satellite. J For Res 33:1401–1408
Acknowledgements
The authors are grateful to the Kerala Agricultural University for the facilities provided for undertaking the study. The help extended by Department of Science and Technology-Sophisticated Analytical Instumentation Facility, Mahatma Gandhi University, Kottayam, Kerala in physical characterization of the nanoparticles is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
SD: investigation, visualization, writing original draft. ARA: investigation, writing original draft. SV: investigation. SGJ: resources, visualization. PAD: investigation. ASRD: investigation. SST: resources. EMV: resources, visualization. PPG: formal analysis. KNA: conceptualization, methodology, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Divya, S., Anusree, A.R., Vigi, S. et al. Silver nanoparticles green synthesized with leaf extract of disease-resistant amaranthus genotypes effectively suppress leaf blight (Rhizoctonia solani Kühn) disease in a susceptible red amaranthus cultivar. 3 Biotech 13, 196 (2023). https://doi.org/10.1007/s13205-023-03614-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03614-y