Abstract
During our previous study, the mixed infection of canna yellow mottle virus (CaYMV), bean yellow mosaic virus (BYMV), and cucumber mosaic virus (CMV) was identified in a Black Knight cultivar of canna exhibiting severe yellow streak and mottling symptoms. Before the development of the virus-free plants, the ability of callogenesis and organogenesis from the ovary, stalk, and rhizome explants was tested on different concentrations and combinations of TDZ, NAA, BAP, and Ads growth regulators. The performance of rhizome explants was above all the explant types and 33.33 ± 1.67 rhizomes (out of 50 placed) showed callus development on ME medium (MS supplemented with 0.8 mg/L TDZ and 0.25 mg/L NAA) and further on a refined M4 medium (MS supplemented with 4.0 mg/L BAP, 1.0 mg/L NAA and 50 mg/L Ads) produced 4.06 ± 0.16 shoots per explant. The development of virus-free plants was attempted by in vitro chemotherapy using ribavirin. Not only in callogenesis and shoot development but also in the ribavirin treatments, rhizomes developed about 3.78 ± 0.68 shoots per explant on 40 mg/L ribavirin in the ME medium. These optimizations suggested that ME medium for callogenesis, M4 medium for shoot development and the treatment of 40 mg/L ribavirin for 30 days at M4 medium was effective. The elimination of coinfection of all three viruses from rhizome explants of 0.5 cm2 of the Black Knight cultivar was attempted. Consequently, a total of 53.33% of plants free from all three viruses (48 out of the 90 plants developed) were obtained when screened by RT-PCR and PCR for their absence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Canna (Canna generalis L.) of the family Cannaceae (order Zingiberales) is an annual herb. Due to its eye-catching foliage and flowers, canna has socioeconomic importance and is disseminated over the world (Tanaka 2008). Not only as flower beds but also it is used at borders in landscapes of lawns and parks where attractive foliage and flowers provide an interesting layout (Roy 2009). Canna has remarkable medicinal value too (Taiba and Arkan 2020) and contains polyphenolic secondary metabolites, flavonoids, isoprene polymers, terpenoids, basic nitrogen-containing organic molecules, alkaloids, proteins, carbohydrates, steroids, glycosides, oils, triterpene, glycosides, saponins, tannins, and pigments in good proportions (Pandey and Bhandari 2021). The phytoextract is used in traditional medicine as a diuretic for fever and dropsy, as a demulcent, to treat suppuration, and rheumatism, and to regain energy (Chigurupati et al. 2021; Duke and Ayensu 1985). More, antitumor (Pornsiriprasert et al. 1986), cytotoxic (Darsini et al. 2015), human immunodeficiency virus type 1 reverse transcriptase inhibitory activity (Woradulayapinij et al. 2005), and antibacterial properties (Patil 2021) has also shown by phytoextract. These attributes forced the development of new varieties using disease-free canna and a large number of hybrids have been generated and cultivated around the globe (Tanaka 2008).
Considering the immense potential of canna as a multifaceted plant, the Protection of Plant Varieties and Farmers' Rights Authority, Ministry of Agriculture and Farmers Welfare, India has designated CSIR-NBRI, Lucknow as the Distinctness, Uniformity, and Stability (DUS) test center (Singh et al. 2014). The institute conserves and maintains more than 50 cultivars of canna as germplasm (Roy 2007). Earlier, several cultivars of canna were found to exhibit severe yellow streak and mottling symptoms on leaves (Kumari et al. 2014, 2015). The co-infection of canna yellow mottle virus (CaYMV, genera Badnavirus), bean yellow mosaic virus (BYMV, Potyvirus), and cucumber mosaic virus (CMV, Cucumovirus) in Allegheny, Black Knight, and Eileen Gallo cultivars were found with disease (Kumari et al. 2016). Our DUS center is dedicated to the production of canna and therefore the disease-free planting material is a prerequisite.
The development of virus-free plants through in vitro chemotherapy has been demonstrated as a successful tool for eliminating viruses in plants that are propagated by vegetative cutting like orchids, lily, chrysanthemum, begonia, gerbera, and gladiolus (Kumar et al. 2009; Panattoni et al. 2013; Gautam et al. 2017). Various explanations are suggested for the elimination of the virus including the action of growth regulators, particularly cytokinin (Barlass and Skene 1982), phenol-amines (Martino 1985), loss of enzymes necessary for viral replication, and RNA degradation due to cell injury during explant excision (Mellor and Stace-Smith 1977). The use of the antiviral compound, ribavirin [1-(β-d-Ribofuranosyl)-1"H"-1,2,4-triazole-3-carboxamide], a nucleoside inhibitor that suppresses the replication as well as multiplication of virus in infected cells, has provided a better strategy for virus elimination (de Fazio et al. 1978; Panattoni et al. 2013). However, the concentration to be used in chemotherapy is tissue or plants specific as reported for banana (Lassois et al. 2013), chrysanthemum (Ram et al. 2005; Kumar et al. 2009), gladiolus (Aminuddin and Singh 1993), lily (Xu et al. 2000), orchid (Loi et al. 1991) etc. In the present study, in vitro regeneration and development of virus-free canna plants of the Black Knight cultivar were attempted for the production and availability of the disease-free healthy planting material for the first time. Because, no study is reported for the development of virus-free canna plants through in vitro chemotherapy using ribavirin and therefore the protocol was first optimized for high-efficiency shoot regeneration using the ovary, stalk, and rhizome of the Black Knight cultivar. The best responsive explant type was then used for the development of virus-free plants and details are discussed.
Materials and methods
Virus culture
The newly emerging young suckers of the Black Knight cultivar exhibiting symptoms of severe yellow streak and mottling on leaves and plant stunting (Kumari et al. 2016) were dug out from the repository of the DUS center. Leafy green portions were removed leaving the basal rhizome with axillary buds and transferred to the earthen pots filled with soil, soilrite, and vermiculite in a 2:1:1 ratio. Similarly, the healthy rhizomes of the Black Knight cultivar were also maintained and used as a control for comparison during further experiments. All potted plants were maintained in the insect-proof glasshouse under natural illumination and 25 ± 2 °C temperature.
Virus detection
To ascertain the presence of CaYMV, BYMV, and CMV in the ovary, stalk, and rhizome plants parts of the Black Knight cultivar, all PCR and Reverse transcription-PCR (RT-PCR) assays were performed following the conditions of Kumari et al. (2016). Briefly, 100 mg tissues were used for the genomic DNA and total RNA isolation independently using the GenElute Plant Genomic DNA Miniprep Kit (Sigma-Aldrich, MO, USA) and RNeasy Plant Mini Kit (Sigma-Aldrich, MO, USA), respectively, following the manufacturer’s instructions. Samples from the healthy Black Knight cultivar were treated as negative control and the known infected plants of the same cultivar as the positive control. The primers of Momol et al. (2004) capable of amplifying about 565 bp DNA band were used for the detection of CaYMV in PCR. While primers of Gibbs and Mackenzie (1997) capable of amplifying about 800 bp DNA band and primers of Choi et al. (1999) capable of amplifying about 900 bp DNA band were used for detection of BYMV and CMV, respectively, in RT-PCR. The DNA preparations were subjected directly to PCR amplification. The RNA preparations were first subjected to cDNA synthesis with respective downstream primers independently using GoScript™ Reverse Transcription System (Promega Corporation, Madison, USA) following their instructions. The synthesized complementary DNA was then used as a template for PCR amplification in a thermal cycler (PTC200, MJ Research). The conditions for the amplification of target genes of viruses were described elsewhere (Kumari et al. 2016). The amplified products were electrophoresed on 1% agarose gel in 1X TAE buffer (40 mM Tris-acetate and 1 mM EDTA) and a current of 5 V/cm.
Explants and surface sterilization
For the establishment of in vitro regeneration protocol, the ovary, stalk (5 cm above the rhizome), and rhizome of the Black Knight cultivar of canna were selected as explant types.
All explants were first roughly excised to 2.5 cm pieces and subjected to surface wash under running tap water for 30 min. Explants were treated with 1% (w/v) carbendazim (Bavestin, BASF India Limited, India) in the beakers with continuous stirring for 10 min to eliminate any fungal contaminations. Then washed in 5% (v/v) Teepol detergent (Orpington, Kent, UK) for 5 min and rinsed four times with sterilized distilled water. Under the aseptic condition in a laminar airflow hood, the explants were reduced to 0.5 cm2 sizes and quickly immersed in 70% (v/v) alcohol for 30 s following to 1.0% (w/v) HgCl2 solution for 10 min and finally rinsed four times in sterilized distilled water. After blot drying, these explants were used for callogenesis (callus development) and organogenesis (shoot development) by culturing in basal Murashige and Skoog's medium (Murashige and Skoog 1962) supplemented with different concentration and combinations of plant growth regulators.
Media composition and optimization of regeneration protocol
The plant growth regulators used for callogenesis and organogenesis include α-naphthalene acetic acid (NAA), 6-benzyl amino purine (BAP), adenine sulfate (Ads) and thidiazuron (TDZ) as suggested by Wafa et al. (2016) and were procured from Sigma-Aldrich (MO, USA). Different concentrations of TDZ ranging from 0.2 to 1.0 mg/L (with a gradient of 0.2 mg/L) supplemented to basal MS medium were used for callus formation, while the concentration of 0.25 mg/L NAA was common to all treatments (denoted as MA, MB, MC, MD, ME and MF medium). The combinations and concentrations of NAA ranging from 0.25 to 1.5 mg/L (with a gradient of 0.25 mg/L) and BAP from 1.0 to 6.0 mg/L (with a gradient of 1.0 mg/L) supplemented to basal MS medium were used for direct organogenesis or shoot regeneration while the concentration of 50 mg/L Ads was common to all treatments (denoted as M1 to M6 medium).
For callogenesis, 0.5 cm2 size explants of the ovary, stalk, and rhizome, obtained from the healthy Black Knight cultivar of canna (10 explants per plate with 5 replicates) were placed on MA to MF medium. For organogenesis, the rhizome explants showing callus formation at their ends were selected for further experimentations. The rhizome explants (10 explants per plate with 3 replicates) were placed on M1to M6 medium. All the explants were incubated at 25 ± 2 °C under 16/8 h light/dark period and subcultured every fortnight to their respective fresh medium and responses were recorded for the statistical analysis. Light at 60 μmol photon m2/s was supplied by fluorescent tube lights fitted on culture racks (Chen 2005). The young developed shoots were subcultured onto half-strength basal MS medium with 0.6% agar–agar for rooting. The best combination of medium with the highest efficiency was used for in vitro virus elimination therapy.
In vitro chemotherapy
The virus-infected rhizomes of the Black Knight cultivar of canna were used for the production of virus-free canna plants through in vitro chemotherapy with different concentrations (20, 30, 40, 50, and 60 mg/L) of ribavirin (Duchefa Biochemie, The Netherlands). The 0.5 cm2 rhizome explants were surface sterilized following the aforementioned procedures, blot dried, and placed onto the media plates containing optimized concentrations of TDZ, BAP, NAA, and Ads in MS medium. Cultures were incubated under 16 h light and 8 h dark period at 25 ± 2 °C and with a light intensity of 60 μmol photon m2/s at the culture level and subcultured every fortnight.
Screening of regenerated plants
The 1–2 leaf stage shoots were indexed by PCR/RT-PCR assays to ascertain the absence of all three viruses (CaYMV, BYMV and CMV) in them. Briefly, 100 mg leaf tissue from the developing shoots along with the young leaf samples from healthy (as negative control) and infected Black Knight cultivar (as positive control) plants were used for genomic DNA and total RNA isolation independently following the aforementioned procedures. The rest of the PCR and RT-PCR conditions were as described above.
Rooting and acclimatization
The 1–2 leaf stage shoots were subcultured onto a half-strength basal MS medium containing 0.6% agar–agar for rooting. The well-grown plantlets were hardened in Hoagland solution (Hoagland and Arnon 1950) under a glasshouse in a hardening chamber with 80–90% relative humidity. Post hardening for 20 days, regenerated plants were potted in vermiculite containing 6-in. pots and maintained in an insect-proof glasshouse. The developed plants were finally transferred to 12-in. earthen pots filled with a mixture of soil, soilrite, and vermiculite in a ratio of 2:1:1.
Statistical analysis
Ten explants of stalk, ovary, and rhizome in three replicates were used for the callogenesis study. To obtain the direct shoots, ten responding explants of rhizome (during callogenesis) were used and repeated three times for each treatment. The proliferating shoots with distinctly visible apical meristem were counted and subjected to analysis of variance. The values of data are presented as the mean SE (±) of different replicates. The critical value was obtained from the t test at 0.05% significance level (P = 0.05). All statistical analyses were performed using Statistical for Windows (version 5.1, StatSoft, Inc., Tulsa, OK) and SPSS (SPSS Inc. version 16.0. Chicago, SPSS Inc.). For rooting and acclimatization, five replicates were used and efficiency was calculated accordingly.
Results
The canna plants of the Black Knight cultivar in which coinfection of CaYMV, BYMV, and CMV identified earlier were selected for the elimination of these viruses through in vitro chemotherapy with an aim to optimize a method for the development of virus-free canna plants. These plants were exhibiting symptoms of yellow streak and mottling on leaves and stunting, resulting in quality deterioration of the plants. Before starting the optimization protocols, the presence of these three viruses was confirmed in the ovary, stalk and rhizome explants of the Black Knight cultivar. PCR with CaYMV-specific primers and RT-PCR with BYMV and CMV-specific primers resulted in the amplification of expected 563 bp, 800 bp, and 900 bp DNA bands, respectively, in all tissues. Whereas, the ovary, stalk, and rhizome explants of healthy Black Knight cultivar plants did not produce any amplicon with the aforementioned primers and were used for the optimization of regeneration conditions.
Optimization of plant growth regulators and regeneration protocol
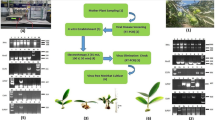
The ovary of the developed flower, stalk (5 cm above the rhizome), and newly emerged rhizomes of healthy Black Knight cultivar were used for the optimization of callogenesis and organogenesis (Fig. 1). All explants responded to their best efficiency on the ME medium (MS supplemented with 0.8 mg/L of TDZ and 0.25 mg/L NAA), however, higher callus formation was achieved with rhizome explants (Table 1; Figs. 2a, 3a). More than 66% of the rhizome explants (33.33 ± 1.67 out of the 50 placed) produced yellowish-green callus from their excised ends, whereas it was 40% with ovary explants (20.00 ± 1.15 out of the 50 placed) and only 12% of the stalk explants (6.00 ± 0.57 out of the 50 placed) produce such callus (Table 1). The callogenesis in the rhizome explants though was significant but shoots were very small, mal-developed, and were less in numbers during the later stages of regeneration on the ME medium, hence the organogenesis (direct shoot regeneration) medium was developed. Contrary, the ovary and stalk explants did not produce any shoot during the later stages of regeneration on the ME medium.
Graphs showing a response of growth regulators on explants. a Response of rhizome, ovary and stalk (stem) explants on MS medium supplemented with TDZ (0.2–1.0 mg/L) for callus formation (callogenesis) while the concentration of 0.25 mg/L NAA was common to all treatments. b Effected of BAP, NAA and Ads on direct shoot development from rhizome explants. Data represent the callus formation (a) and average number of shoots per explant (b) with standard error (SE) obtained from respective treatments
Image showing the callus development from rhizome (a), ovary (b) and stalk (c) explants of canna cv. Black Knight in ME medium (MS medium supplemented with 0.8 mg/L TDZ and 0.25 mg/L NAA). Response of rhizome explants on M4 medium (MS medium amended with 4.0 mg/L of BAP, 1.0 mg/L of NAA and 50.0 mg/L of Ads) for shoot development (d–e). Initiation of direct shoot regeneration on M4 medium (d) after 15 days of incubation, multiple shoot formation (3–4 shoots) per explant after 30 days (e) and shoot development stage after 40 days (f)
The lethal dose of the ribavirin, (on a gradient of 10 mg/L supplemented in the ME medium), was observed on each explant type for 30 days (Table 2) and explants were subcultured every fortnight on fresh medium. Results revealed that the concentration of 50 mg/L and 60 mg/L were lethal. Though 1–2 small malformed shoots developed in some of the explants but they turned brown and eventually died during subsequent subcultures. Among all the explant types, the number of shoots that emerged per rhizome was higher at 20 mg/L and 30 mg/L concentration of ribavirin but the number of virus-free plants was less as compared to the ribavirin-treated explants at 40 mg/L which produced an optimum (3.7 ± 0.68) number of virus-free shoots (Table 2). Therefore, 40 mg/L ribavirin was considered optimum for the development of virus-free canna plants from rhizome explants.
For organogenesis, the combination of 4.0 mg/L BAP, 1.0 mg/L NAA, and 50.0 mg/L Ads (M4 medium, Table 3) was found optimum for direct shoot regeneration (Figs. 2b, 3b, c) and a maximum of 4.06 ± 0.16 shoots per explant were obtained. Only 1–2 shoots per rhizome explant were obtained on the M1, M3 and M5 medium, whereas M2 and M6 media were least responsive and had single or no shoots (Table 3). The growth of the developed shoots was apparently normal on the M4 medium and therefore used for further experimentation. More than 98% rooting was achieved in all developed shoots within 15 days on a half-strength basal MS medium solidified with 0.6% agar.
Development of virus-free plant by in vitro chemotherapy
A total of 90 virus-infected rhizomes (30 infected rhizomes in each treatment and in three replicates) of the Black Knight cultivar were excised to 0.5 cm2 after surface sterilization and placed on ME medium with 40 mg/L ribavirin for 30 days of treatment (subcultured on fresh medium every fortnight). After 15 days of incubation, the yellowish-green callus started developing at the excised ends and a significant amount developed in 30 days. The 28 rhizome explants that produced callus were further placed on M4 medium without ribavirin (Fig. 3d–f) and a total of 90 shoots were obtained with an efficiency of 3–4 shoots per explant (Fig. 3e,f).
Validation of the regenerated shoots
The regenerated shoots were validated in two successive stages of development to ascertain the absence of all three viruses. Stage 1 considered was the day on which shoots were excised from the developing callus and placed on the rooting medium after chemotherapy. Stage 2 was considered at 90 days from stage 1. Screening by PCR at stage 1 revealed the absence of CaYMV in 75 shoots (out of the 90 tested) with 83.33% (75/90) virus elimination efficiency. Whereas screening by RT-PCR revealed the absence of BYMV and CMV in the 72 and 78 shoots with 80.0% (72/90) and 86.66% (78/90) virus elimination efficiency, respectively. Cumulatively, during stage 1, a total of 51 shoots were found free from infection of all three viruses with 56.66% (51/90) elimination efficiency. At stage 2, by and large, a total of 48 shoots tested either by PCR or RT-PCR were found free from all three viruses (Fig. 4). The optimized method could successfully eliminate the mixed infection of CaYMV, BYMV, and CMV from the Black Knight cultivar of canna with more than 50% virus elimination efficiency (53.33%, 48 virus-free plants out of the total 90 developed). Such a developed 48 virus-free plants were hardened without any further plant loss and finally potted in 12-in. earthen pots, and maintained at the glasshouse. These virus-free plants were phenotypically alike to the control plants (healthy plants of the Black Knight cultivar).
Discussion
Among the canna cultivars having coinfection (of CaYMV, BYMV and CMV), the Black Knight cultivar was selected randomly to first establish the regeneration and then the development of virus-free plants. It is pertinent to mention here that not only the infection of these viruses in canna but also Canna yellow streak virus (CaYSV, genus Potyvirus) and Tomato aspermy virus (TAV, genus Cucumovirus) have been reported (Momol et al. 2004; Rajakaruna et al. 2014). These viruses are transmitted through vegetative propagation in canna and have been reported to deteriorate the ornamental quality (Monger et al. 2007; Kumari et al. 2016). Canna plants are mostly propagated in the nursery through vegetative rhizomes (Hosoki and Sasaki 1991) because the seed coat of canna is extremely hard (Joshi and Pant 2010). The rhizome has also been used for the regeneration and development of transgenic canna plants (Purshottam et al. 2019; Rani et al. 2019). The vegetative propagation, however, has its own drawback that if the mother culture is virus-infected, it will disseminate the virus to all progenies, unavoidably. As no chemical means are available to eliminate the virus from the infected plant in field conditions, therefore the present study aimed at the development of virus-free canna plants of the Black Knight cultivar which is always a prerequisite for further multiplication in the repository of the DUS center at our institute. In the present study, rhizomes along with the ovary and stalk explants of the Black Knight cultivar were used for the development of virus-free canna plants.
During regeneration, it was observed that on the optimized ME media, all three explant types: ovary, stalk, and rhizome showed callogenesis after four weeks. A study with rhizome explants of canna cv. Trinacriavariegata cultured on MS supplemented with 6-BAP, TDZ, and kinetin has shown that TDZ inhibits the shoot development whereas increases callogenesis (Prestowitz 2007; Rani et al. 2019). It was also observed in the present study that the rate of callus formation was high, but the length of the developed shoots was small and less in numbers on the ME medium and therefore required another medium wherein direct shoot regeneration could be achieved from the callus. The combination where a higher concentration of NAA was coupled with a lower concentration of BAP induced the formation of only roots in canna, whereas increasing the concentrations of NAA led to a reduction in both shoot and root formation (Wafa et al. 2016). They achieved a high number of shoot formation on MS medium supplemented with 3.0 mg/L of BAP and 1.5 mg/L of NAA after 15 days of incubation. Our optimized protocol denoted as M4 (MS supplemented with 4.0 mg/L of BAP, 1.0 mg/L of NAA and 50 mg/L of Ads) has shown similar results and a high number of direct shoot formation was achieved after two weeks of incubation of callusing rhizome explants.
Among the reported antiviral compounds against plant virus eradication, ribavirin is the most frequently used (Panattoni et al. 2013) which suppresses the replication as well as multiplication of virus in the infected cells (de Fazio et al. 1978). Inhibition of viral RNA synthesis decreases the number of virus particles released from the infected cells to the newly dividing cells, thus resulting in the reduction of virus titers in the explants and helping virus eradication (Simpkins et al. 1981; Fraser and Gerwitz 1984). As no study is reported to date for the development of virus-free canna plants through in vitro chemotherapy and hence before starting the virus elimination, the lethal concentration of ribavirin was optimized for the rhizome explants. The concentrations of ribavirin being used for virus eradication ranged between 20 and 50 mg/L (Laimer and Barba 2011) depending on the types of virus and hosts, and the virus-host combinations (Panattoni et al. 2013). The low regeneration rate with a high concentration is due to the phytotoxic effect of ribavirin (Agroambientali and Re 2002). In the present study, we used 20–60 mg/L concentration of ribavirin and observed that at the 40 mg/L concentration the highest virus-free plants with maximum plant survival can be achieved. Using 40 mg/L of ribavirin, a total of 56.6% plants (51/90 assayed by PCR/RT-PCR) were found free from CaYMV, BYMV and CMV in stage 1, while the independent analysis for each virus showed 83.3% (75/90) shoots were free from CaYMV, 80.0% (72/90) from BYMV, and 86.6% (78/90) from CMV. The variation in the coinfection elimination efficiency for different viruses may be their localization as reported in the case of rose, apple, and potato (Hu et al. 2015; Chahardehi et al. 2016). Studies for in vitro virus elimination in potatoes revealed that high concentrations of ribavirin successfully eliminated most of the prevalently known potato viruses like potato virus A, potato virus M, potato virus S, potato virus X, and potato virus Y but failed to eliminate potato leaf roll virus (Agroambientali and Re 2002). Herein, when screening of the well-established plants was done at stage 2, the efficiency decreased to 53.3% and 48 developed plants were found free from infection of all three viruses out of the 90 assayed. Studies revealed the reduction of virus titer during the elimination but not complete virus eradication (Gallard et al. 2011; Silva et al. 2011) and this may one of the possible explanations for the reduction in the virus elimination efficiency in stage 2. To the best of our knowledge, this is the first report of the development of virus-free canna plants through in vitro chemotherapy of rhizome explants. The optimized protocol has the potential to be used in the development of virus-free plants of other canna cultivars.
Conclusions
This is the first report of the development of virus-free canna cultivar Black Knight plants through in vitro chemotherapy of 0.5 cm2 rhizome explants in which coinfection of CaYMV, BYMV, and CMV was eliminated using 40 mg/L of ribavirin treatment for 30 days with a significant efficiency of 53.33% (48 developed plants were virus-free out of the 90 developed plants). Following this protocol, the virus-free canna plants of other cultivars may also be developed as disease-free planting material for germplasm conservation.
References
Agroambientali T, Re V (2002) Eradication of potato virus Y and potato leafroll virus by chemotherapy of infected potato stem cuttings. Phytopathol Mediterr 41:76–78
Aminuddin S, Singh BP (1993) Multiplication of gladiolus cultivars for producing virus-free propogules. Indian J Virol 9:74–77
Barlass M, Skene KGM (1982) In vitro plantlet formation from Citrus species and hybrids. Sci Hortic 17:333–341. https://doi.org/10.1016/0304-4238(82)90114-5
Chahardehi A, Rakhshandehroo F, Mozafari J, Mousavi L (2016) Efficiency of a chemo-thermotherapy technique for eliminating Arabis mosaic virus (ArMV) and Prunus necrotic ringspot virus (PNRSV) from in vitro rose plantlets. J Crop Prot 5(4):497–506
Chen C (2005) Fluorescent lighting distribution for plant micropropagation. Biosyst Eng 90:295–306. https://doi.org/10.1016/j.biosystemseng.2004.10.005
Chigurupati S, Alharbi N, Sharma A, Alhowail A, Vardharajula V, Vijayabalan S, Das S, Fathema K, Amin E (2021) Pharmacological and pharmacognostical valuation of Canna indica leaves extract by quantifying safety profile and neuroprotective potential. Saudi J Biol Sci 28:5579–5584. https://doi.org/10.1016/j.sjbs.2021.05.072
Choi SK, Choi JK, Park WM, Ryu KH (1999) RT-PCR detection and identification of three species of cucumoviruses with a genus-specific single pair of primers. J Virol Methods 83:67–73. https://doi.org/10.1016/S0166-0934(99)00106-8
Darsini IP, Shamshad S, Paul M (2015) Canna Indica (L.): a plant with potential healing powers: a review. Int J Pharma Bio Sci 6:1–8
Duke JA, Ayensu ES (1985) Medicinal plants of China. Reference Publications Inc., Algonac
de Fazio G, Caner J, Vicente M (1978) Inhibitory effect of virazole (ribavirin) on the replication of tomato white necrosis virus (VNBT). Arch Virol 58:153–156. https://doi.org/10.1007/BF01315408
Fraser RSS, Gerwitz A (1984) Effects of 2-α-hydroxybenzylbenzimidazole on Tobacco mosaic virus and host RNA synthesis in tobacco leaf disc plants. Plant Sci Lett 34:111–117. https://doi.org/10.1016/0304-4211(84)90133-0
Gallard A, Mallet R, Chevalier M, Grapin A (2011) Limited elimination of two viruses by cryotherapy of pelargonium apices related to virus distribution. Cryo Lett 32:111–122
Gautam K, Kaur C, Raj R, Kumar S, Jaidi M, Raj SK, Purshottam D, Roy R (2017) Elimination of cucumber mosaic virus from gerbera (Gerbera jamesonii) cv. Zingaro through in vitro chemotherapy of capitulum explants. Indian J Biotechnol 16:641–647
Gibbs A, Mackenzie A (1997) A primer pair for amplifying part of the genome of all potyvirids by RT-PCR. J Virol Methods 63:9–16. https://doi.org/10.1016/S0166-0934(96)02103-9
Hoagland DR, Arnon DS (1950) The water culture method for growing plants without soil. Calif Agric Exp Stn Circ 347:32
Hosoki T, Sasaki H (1991) In vitro propagation of Canna edulis Ker. by longitudinal shoot-split method. Plant Tissue Cult Lett 8:175–178. https://doi.org/10.5511/plantbiotechnology1984.8.175
Hu G, Dong Y, Zhang Z, Fan X, Ren F, Zhou J (2015) Virus elimination from in vitro apple by thermotherapy combined with chemotherapy. Plant Cell Tissue Organ Cult 121(2):435–443. https://doi.org/10.1007/s11240-015-0714-6
Joshi SC, Pant SC (2010) Effect of H2SO4 on seed germination and viability of C. indica, a medicinal plant. Am J Sci 6:24–26
Kumar S, Khan MS, Raj SK, Sharma AK (2009) Elimination of mixed infection of cucumber mosaic and tomato aspermy virus from Chrysanthemum morifolium Ramat. cv. Pooja by shoot meristem culture. Sci Hortic 119:108–112. https://doi.org/10.1016/j.scienta.2008.07.017
Kumari A, Kaur C, Kumar S, Raj SK, Roy RK, Nautiyal CS (2015) First report of bean yellow mosaic virus causing a mosaic disease of Canna sp. in India. Plant Dis 99:897. https://doi.org/10.1094/PDIS-01-15-0001-PDN
Kumari A, Kumar S, Raj SK (2014) First report of canna yellow mottle virus on canna from India. New Dis Rep 29:9. https://doi.org/10.5197/j.2044-0588.2014.029.009
Kumari A, Raj R, Kumar S, Chauhan PS, Raj SK (2016) Coexistence of three virus genera (Badnavirus, Potyvirus and Cucumovirus) in Canna species in India. Ann Virol Res 2:1008
Laimer M, Barba M (2011) Elimination of systemic pathogens by thermotherapy, tissue culture, or in vitro micrografting. In: Hadidi A, Barba M, Candresse T, Jelkmann M (eds) Virus and virus-like diseases of pome and stone fruits. American Phytopathological Society, St. Paul, pp 389–393. https://doi.org/10.1094/9780890545010.065
Lassois L, Lepoivre P, Swennen R, Houwe IVD, Panis B (2013) Thermotherapy, chemotherapy, and meristem culture in banana. Methods Mol Biol 13:419–433. https://doi.org/10.1007/978-1-62703-074-8_32
Loi JS, Lee SM, Lam Chan LT, Fan S, Yiang WH (1991) Eradication of orchid viruses in Dendrobium Sonia using virazole. Singap J Primary Ind 19:16–22
Martino TJ (1985) The occurrence and possible function of hydroxycinnamoyl acid amides in plants. Plant Growth Regul 3:381–399. https://doi.org/10.1007/BF00117595
Mellor FC, Stace-Smith R (1977) Virus free potatoes by tissue culture. In: Reinert J, Bajaj YPS (eds) Applied and fundamental aspects of plant cell, tissue and organ culture. Springer, Berlin, pp 616–635
Momol MT, Lockhart BEL, Ankers HD, Adkins S (2004) Canna yellow mottle virus detected in canna in Florida. Plant Health Prog. https://doi.org/10.1094/PHP-2004-0809-01-HN
Monger WA, Harju V, Skelton A, Seal SE, Mumford RA (2007) Canna yellow streak virus: a new potyvirus associated with severe streaking symptoms in canna. Arch Virol 152:1527–1530. https://doi.org/10.1007/s00705-007-0977-2
Murashige T, Skoog F (1962) A revised medium for rapid growth bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Panattoni A, Luvisi A, Triolo E (2013) Review Elimination of viruses in plants: twenty years of progress. Span J Agric Res 11:173–188. https://doi.org/10.5424/sjar/2013111-3201
Pandey S, Bhandari M (2021) Hidden potential of Canna indica-an amazing ornamental herb. IJTRS. https://doi.org/10.30780/specialissue-ICAASET021/020
Patil V (2021) Phytochemical analysis of Canna indica Linn. of root. Int J Appl Sci Eng 9:57–61. https://doi.org/10.1016/j.bbrep.2018.09.002
Pornsiriprasert D, Picha P, Preechanukool K (1986) Studies on the antitumor activity of a Thai folkloric remedy: traditional medicinal plants. Asian J Pharm Sci 6:124. https://doi.org/10.13040/IJPSR.0975-8232.6(10).4103-12
Prestowitz CM (2007) Virus elimination for in vitro Canna generalis cultivars. University of Delaware, Thesis, Publication number:1446833
Purshottam DK, Srivastava RK, Misra P (2019) Low-cost shoot multiplication and improved growth in different cultivars of Canna indica. 3 Biotech 9:67. https://doi.org/10.1007/s13205-019-1583-1
Rajakaruna P, Shafiekhani M, Kim T, Payton M, Chauhan R, Verchot J (2014) Production of discernible disease phenotypes in canna by five plant viruses belonging to the genera Potyvirus, Cucumovirus and Badnavirus. Plant Pathol J 63:821–830. https://doi.org/10.1111/ppa.12169
Ram R, Verma N, Singh AK, Singh L, Hallan V, Zaidi AA (2005) Indexing and production of virus-free chrysanthemums. Biol Plant 49:149. https://doi.org/10.1007/s10535-005-0152-0
Rani S, Dubey AK, Sanyal I (2019) Optimization of adventitious shoot regeneration and agrobacterium-mediated transformation in Canna generalis (Canna Lily). Hortic Plant J 8:42–49. https://doi.org/10.1016/j.hpj.2018.11.002
Roy RK (2007) Plant new Canna varieties in your garden. Indian J Hortic 52:17
Roy RK (2009) Cute Cannas for landscaping. Indian J Hortic 54:27–28
Silva JM, Carnelossi PR, Bijora T, Facco CU, Picoli MH, Souto ER, Oliveira AJB, Almeida ÁM (2011) Immunocapture-RT-PCR detection of Cassava common mosaic virus in cassava obtained from meristem-tip culture in Paraná state. Trop Plant Pathol 36:271–275. https://doi.org/10.1590/S1982-56762011000500001
Simpkins I, Walkey DGA, Neely HA (1981) Chemical suppression of virus in cultured plant tissues. Ann Appl Biol 99:161–169. https://doi.org/10.1111/j.1744-7348.1981.tb05143.x
Singh S, Roy RK, Prasad R, Kumar S (2014) Guidelines for the conduct of test for Distinctiveness, Uniformity and Stability. Technical Report. Protection of Plant Varieties and Farmers' Rights Authority (PPV & FRA) Government of India. https://plantauthority.gov.in/sites/default/files/canna.pdf
Taiba AM, Arkan AT (2020) A Chemical study compared by using GC-MS analysis of the active substances from the ethanolic extracts of leaves and flowers of Canna indica L. plant. Plant Arch 20:6383–6386
Tanaka N (2008) A new species of the genus Canna (Cannaceae) from eastern Honduras. J Jap Bot 83:7–10
Wafa SN, Taha RM, Mahmad SMN, Abdul BAA (2016) Organogenesis and ultrastructural features of in vitro grown Canna indica L. Bio Med Res Int. https://doi.org/10.1155/2016/2820454
Woradulayapinij W, Soonthornchareonnon N, Wiwat C (2005) In vitro HIV type 1 reverse transcriptase inhibitory activities of Thai medicinal plants and Canna indica L. rhizomes. J Ethnopharmacol 101:84–89. https://doi.org/10.1016/j.jep.2005.03.030
Xu PS, Niimi Y, Araki H (2000) Production of virus free bulblets from callus induced from scale culture of Lilium longiflorum Georgia. J Jpn Soc Hortic Sci 69:97–102. https://doi.org/10.2503/jjshs.69.97
Acknowledgements
The authors are thankful to the Director, CSIR-NBRI, Lucknow for the necessary laboratory facilities. Thanks are due to Dr. R. K. Roy, the Head, Floriculture Department, CSIR-NBRI for allowing the collection of canna samples from the repository. Aarti Kumari is thankful to University Grants Commission, India for her fellowship.
Author information
Authors and Affiliations
Contributions
A.K. has done all the laboratory experiments and collected data. P.S.C. and S.K. have analyzed the data and wrote the manuscript. S.K.R. has conceived the idea of this research work and wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumari, A., Kumar, S., Chauhan, P.S. et al. Elimination of coexisting canna yellow mottle virus, bean yellow mosaic virus and cucumber mosaic virus from Canna generalis cv. black knight through in vitro chemotherapy of rhizome explants. 3 Biotech 12, 267 (2022). https://doi.org/10.1007/s13205-022-03330-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-022-03330-z