Abstract
In this study, the exact contribution of T. versicolor fungal biomass and laccase in the removal of the Orange II dye from liquid culture was determined. Biomass and laccase were produced with three different carbon sources [bran flakes (BF), wheat bran (WB) and wheat flour (WF)]. The contribution of the biomass and the laccase enzyme in the removal of the Orange II dye was assessed as follows: (A) in vivo treatment with fungal biomass; in vivo treatment with fungal biomass and inhibited laccase (using 0.6 mM sodium azide); and (B) in vitro treatment with crude laccase. The results of fungal biomass production were similar for all the carbon sources evaluated, while laccase volumetric activities were different. The highest enzyme production was obtained with WB, followed by BF and WF. In the in vivo treatment with fungal biomass–laccase, dye removal was over 84% for all the carbon sources. Dye adsorption by fungal biomass varied from 1.5–2%, presenting enzymatic activities ranging from 62 to 163 U L−1. In the in vivo treatment with fungal biomass-inhibited laccase, the removal of the dye varied from 30 to 72%. In this case, the percentage of dye adsorption by fungal biomass was significantly increased and ranged from 18 to 53%. In the in vitro treatment with laccase, the removal ranged from 80 to 84%. The best treatment for dye removal involved the use of both fungal biomass and laccase. The carbon source for biomass and laccase production had an impact on dye removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Effluents generated by textile industries cause serious water contamination problems, due to the fact that they contain elevated concentrations of different synthetic dyes used in the processing and dyeing (Kirby et al. 1995; McMulan et al. 2001; Forgacs and Cserháti 2004; Daneshvar et al. 2017). Industrial textile effluents are released into the environment without adequate treatment, causing adverse effects on ecosystems (Goncalves et al. 2000; Kumari and Naraian 2016). Azo-type dyes are among the most used dyes in this industry due to their high staining efficiency and tonal dyeing that present high chemical stabilities and resistance to abiotic removal such as light, temperature, and alkalinity (Robinson et al. 2001; Gajera et al. 2015). The main problems caused by the presence of a mixture of diverse synthetic dyes in water bodies are that they directly affect photosynthetic processes and form aromatic amines that can be generated by anaerobic removal processes (Jin et al. 2007; Singh et al. 2015). Physicochemical and biological treatments can be used to reduce pollution by these dyes. Physicochemical treatments are efficient, but lead to the formation of toxic by-products and these processes are generally expensive (Zablocka-Godlewska et al. 2014). Biological treatment of the pollution is considered to be environmentally friendly and cost-effective. Biological treatment involves the degradative capacity of bacteria, fungi and plants. Fungi, mainly basidiomycetes, are considered adequate to treat water polluted with dyes, because their metabolic versatility has been associated with the presence of a multi-enzymatic system, capable of degrading a diversity of xenobiotic compounds (Rodríguez et al. 1999; Wesenberg et al. 2003; Vanhulle et al. 2007). Different studies have demonstrated that basidiomycetes such as Phanerochaete chrysosporium, Pleurotus ostreatus, Trametes villosa and Trametes versicolor are capable of decolorizing effluents polluted with azo dyes (Cripps et al. 1990; Yesilada et al. 2003; Chagas and Durrant 2001; McMullan et al. 2001; Zille et al. 2005). However, even with the number of reports related to dye degradation by fungi, the mechanisms by which enzymatic transformation and/or metabolic degradation take place is not well understood (Rodríguez et al. 2004; Blánquez et al. 2004; Mielgo et al. 2001).
The purpose of this study was to evaluate the contribution of the fungal biomass T. versicolor and/or laccase in the removal of the Orange II dye in liquid culture. The production of the enzyme with three different carbon sources (BF, WB and WF) was evaluated. Subsequently, the contribution of the fungal biomass and the laccase enzyme in the removal of the Orange II dye was assessed.
Materials and methods
Azo dye
Orange II (sodium 4-[(E)-(2-hydroxy-1-naphthyl) diazenyl] benzenesulfonate (C16H11N2NaO4S) and ABTS (2, 2′-azino-bis (3-ethyl-benzo-thiazoline-sulfonate) were purchased from SIGMA.
Organism
The white-rot fungus T. versicolor HEMIM-9 was isolated from decayed oak (Quercus sp.) in Morelos, central Mexico, and kept in the culture collection of the Mycology Laboratory at the Centro de Investigaciones Biológicas, UAEM, México. A sample was kindly provided by M.C. M.L. Acosta-Urdapilleta.
Culture media
Potato dextrose agar (PDA) 39 gL−1 was used as the solid culture media for the growth and conservation of T. versicolor. The PDA media was sterilized at 121 °C at 100 kPa for 20 min. Subsequently, Petri dishes prepared with PDA were inoculated using approximately 0.5 cm2 of the fungus grown on the solid media and incubated at 25 °C in darkness for 8 days.
Laccase production
Laccase production was carried out in 1 L Erlenmeyer flasks, with a final volume of 500 mL. The inoculum was prepared with the biomass grown on the solid PDA for 8 days, then the mycelium was separated from the solid medium and resuspended in 50 mL of 100 mM phosphate buffer (pH 6), and then homogenized in a Ultra-Turrax handheld blender at 30,000 rpm for 10 s; 10 mL was then inoculated from the fungal suspension. The liquid cultures with different carbon sources (BF, WB and WF) were added at 1% in phosphate buffer (100 mM, pH 6.0), inoculated and incubated at 29 °C and 150 rpm for 10 days. Samples were taken from the cultures every 24 h. The samples were centrifuged at 10,000 rpm for 10 min for quantification of enzyme activity.
Dye removal experiments
To investigate whether Orange II removal by T. versicolor was associated with laccase extracellular activity and/or growth mechanisms, the following experiments were performed: in vivo treatment with fungal biomass of T. versicolor obtained after 5 days cultivation (fungal biomass and extracellular laccase); in vitro treatment with laccase obtained from culture filtrates after 5 days cultivation with the different carbon sources and in vivo treatment with fungal biomass of T. versicolor obtained after 5 days cultivation with 0.6 sodium azide (NaN3) to inhibit laccase activity. The NaN3 concentration did not inhibit the fungal growth.
In vivo dye treatment with fungal biomass (fungal biomass–laccase)
Dye biodegradation was carried out in 50 mL, containing a phosphate buffer solution, and the Orange II dye (25 mg L−1), and inoculated with 10% of wet biomass. Biodegradation was carried out at 29 ºC and maintained with constant shaking (150 rpm) for 24 h. Samples were taken every 4 h during a 24 h period and dye removal was quantified by spectrophotometry.
In vivo dye treatment with fungal biomass and laccase inhibited (fungal biomass–laccase inhibited)
Dye removal was carried out in 50 mL flasks containing phosphate buffer solution, Orange II at 25 mg L−1, NaN3 0.6 mM and 10% of the inoculum. Biodegradation was carried out at 29 ºC and constant shaking maintained (150 rpm) for 24 h. Samples were taken every 4 h during a 24 h period and dye removal was quantified by spectrophotometry.
In vitro dye treatment with enzymatic extracts (laccase)
Azo dye removal was performed in 50 mL flasks, in phosphate buffer with 25 mg L−1 of Orange II. 120 UL−1 of laccase (obtained using the different carbon sources) was added to the reaction mixture. Samples were taken every 4 h during a 24 h period and dye removal was quantified by spectrophotometry.
Enzymatic activity assay
Laccase activity was determined at 25 ºC by oxidation of ABTS (1 mM) in 100 mM sodium acetate buffer (pH 3.6) mixed with 100 µl aliquots of liquid culture in a total volume of 1 mL. The oxidation of ABTS was determined at 436 nm (e = 29,300 M−1 cm−1) using a UV/Vis spectrophotometer (DU 640 Beckman). One activity unit was defined as the amount of enzyme that oxidized 1 µmol of ABTS per min. Laccase activity was expressed in UL−1. Experiments were carried out in triplicate.
Orange II quantification
Dye concentration was determined by spectrophotometry at wavelength of 480 nm using a Beckman (DU 640) spectrophotometer. A standard curve ranging from 1 to 30 ppm Orange II dye was used.
Dye extraction from fungal biomass
The biomass content of each replicate, previously washed, was homogenized in 20 mL of methanol for 20 s in Ultra-Turrax handheld blender at 13,500 rpm. Subsequently, the suspension obtained was centrifuged for 5 min to eliminate solid particles. The dye was recovered and quantified by spectrophotometry.
Effect of enzymatic and metabolic activities on Orange II dye degradation
The effect of the factors on Orange II dye degradation was assessed using a 22 factorial experimental design (Table 1). Laccase and biomass were produced in three different culture media (BF, WB or WF) and were independently evaluated. The effect of the metabolic activity (active biomass) was assessed at 0 and 5 gL−1. The effect of laccase activity was assessed at 0 and 120 UL−1. The main effects for each of the factors evaluated on the response were as follows:
where i is the effect of the ith factor on the response, and yi+ and yi− are the mean responses for the upper ( +) and the lower (−) levels of the ith factor. The interactions of two factors were also calculated by this equation. The general equation is a first-degree polynomial:
where Y is the estimated response, β0 the general mean, ΣβiXi the sum of main effects of the factors, ΣβijXij the sum of two-factor interaction effects, ΣβijkXijk the sum of three-factor interaction effects, and is the lack of fit of the model (error).
ANOVA analysis was made by using the SAS software and the Design Expert 5-Stat-Ease 1996 software.
Results and discussion
T. versicolor laccase production using different carbon sources
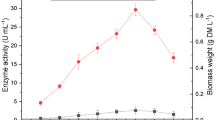
Laccase production by T. versicolor grown in different carbon sources (BF, WB and WF) are presented in Fig. 1. It should be pointed out that only laccase production curves are presented due to the fact that no other phenol oxidase (MnP and LiP) activity was detected (data not shown).
Laccase production of T. versicolor on three different carbon sources. Enzyme production was carried out in 1 L Erlenmeyer flasks, with a final volume of 500 mL. Bran flakes (BF), wheat bran (WB) and wheat flour (WF) were added at 1% into phosphate buffer, inoculated and incubated at 29 °C and 150 rpm for 10 days
The production of laccase in carbon sources WB and BF began on the 5th day, while in the presence of WF enzymatic activity was not detected until the 7th day. The highest laccase production was achieved with WB (2010 UL−1) on the 9th day, while with BF and WF enzyme production was less (800 and 303 UL−1, respectively) on the 10th day. These results demonstrate that the source of non-lignocellulosic carbon (WF) leads to low enzyme production, while in lignocellulosic media (BF and WB) the production was higher. Between the two lignocellulosic sources, the one with the highest enzyme production was that of WB. This may be a consequence of the difference in fiber content, since the BF contains 34% fiber while the WB contains more than twice the fiber (78%). This indicates that the synthesis and secretion of the enzyme are regulated by the composition of the culture medium (Pereira et al. 2003; Patel et al. 2009). In other studies with the strain of T. versicolor, it has been shown that the bands in zymograms correspond not only to one isoform, but that up to five isoforms can be found. The above has been demonstrated when isoelectric point gels are made when phenolic extracts from wood residues were used as inducers (Bertrand et al. 2014).
In vivo Orange II treatment with fungal biomass (fungal biomass–laccase)
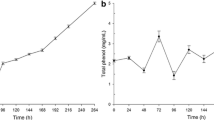
A synthetic Orange II dye effluent (pH 6.5) was used for biodegradation experiments. Twenty-five mgL−1 of the azo dye was added to T. versicolor submerged culture and grown in three different media (BF, WB and WF). The evolution of the removal percentage and the extracellular enzymatic activity is shown in Fig. 2. In Fig. 2a, a rapid decrease in dye concentration was observed, and 65–80% removal was achieved in the first 24 h. Differences in removal by the biomass grown in the carbon sources were observed, the lowest being with WF. Only 50–200 UL−1 volumetric activity of laccase was detected with respect to extracellular enzyme activity (Fig. 2b). It seemed that removal could be related to extracellular enzyme activity. During biotic removal experiments, the dye was adsorbed into the biomass in the first half hour, after 24 h complete dye removal was observed. While under abiotic conditions, with the inactivated biomass, the remaining dye was adsorbed into the fungal biomass.
In vivo Orange II treatment by T. versicolor biomass grown on three different carbon sources at 24 h. a Removal: 25 mg L−1 of Orange II was added to the fungal culture and for (b) laccase production. Laccase production was carried out as described in Fig. 1
With the aim of demonstrating whether the dye removal was as a result of biological or enzymatic degradation, mass balances were performed; the remaining dye in the supernatant and biomass quantified previous extraction with ethyl acetate. The results obtained are shown in Fig. 3. In all three cases, the dye remaining in the biomass was less than 2%. Biotic and abiotic processes were observed to affect dye removal from the supernatant. Biotic processes involve the metabolic degradation/transformation of the dye. In the case of abiotic processes, the phenomenon of dye adsorption by fungal biomass was observed.
The dye removal balances showed that for the three carbon sources, the biotic factors were responsible for the 65–83% removal of dye concentration, while less than 2% was attributed to an abiotic process in the fungal biomass–laccase treatment. It should be mentioned that a lower enzymatic transformation/metabolic degradation was obtained when the biomass was grown in WF. The results of dye adsorption by the fungal biomass were low with respect to studies realized by Zhao and Hardin (2007), since they reported dye adsorption levels higher than 10% in the fungal biomass.
Orange II dye treatment by T. versicolor and laccase inhibited (fungal biomass–laccase inhibited)
To investigate the exact contribution of the fungal biomass in the removal and/or biodegradation process, experiments using one inhibitor of extracellular enzymatic activity in in vivo T. versicolor cultures were performed. A concentration of 0.6 mM of NaN3 was determined as the lowest concentration that completely inhibited laccase activity without affecting fungal growth (data not shown).
The results obtained in the Orange II dye removal process, with the fungal biomass and the inhibited laccase can be observed in Fig. 4. In the dye removal treatment (fungal biomass- laccase inhibited) obtained with the three carbon sources BF, WB and WF, a rapid decrease in dye concentration was observed in the first 12 h. The removal percentage in this period was 60, 12 and 76% for BF, WB and WF, respectively. After 24 h, a decrease in dye concentration of 72, 30 and 80.7% of BF, WB and WF was observed, respectively.
After 4 h of treatment, the WF biomass presented a higher decrease in dye concentration. Dye removal velocity constants for BF, WB and WF were 0.0738, 0.11104 and 0.040 h−1, respectively. Statistical analysis of the velocity constants showed no significant differences between BF and WB fungal biomass; however, WF fungal biomass was statistically different.
The contribution of biotic and abiotic processes in the removal of the dye using the fungal biomass–laccase inhibited treatment was also evaluated. A mass balance for the dye was performed for all conditions; the remaining dye in the supernatant and biomass quantified previous extraction with ethyl acetate (Fig. 5).
Contribution of biotic and abiotic factors on dye removal by T. versicolor biomass grown on three different carbon sources, in the presence of NaN3 (0.6 mM) for laccase inhibition. Control without fungal biomass. For mass balances, the remaining dye in the supernatant and biomass was extracted with ethyl acetate and quantified
Differences in dye biosorption percentages and biodegradation were observed after comparing the balances obtained in this (fungal biomass–laccase inhibited) treatment with the biomass obtained from different carbon sources. In the first place, rapid adsorption of the dye in the biomass was observed (Fig. 4) indicating the participation of the abiotic process (18–53%). While for the biotic process, metabolic degradation varied from 18 to 29.5%. This indicates the contribution of the fungal metabolism in the dye removal process. It is important to note that the greatest effect was observed with the BF fungal biomass, where less adsorption and less dye removal was observed. Meanwhile with WF fungal biomass treatment, high adsorption and less removal levels of dye were detected.
The results obtained demonstrate that the fungal biomass contributes to the decrease of the dye, since it was not involved in the laccase enzymatic transformation process and the adsorption process was evident this favored the biotic degradation process. Hence. dye adsorption by the biomass is directly related to dye degradation by fungal metabolism; high adsorption resulted in a higher degradation percentage.
Enzymatic treatment of Orange II dye (laccase)
The crude laccase that was used was obtained after 10 days of fungal growth utilizing different carbon sources (BF, WB and WF). Orange II dye transformation was carried out with 120 UL−1 of laccase activity. The results obtained in the Orange II dye removal process with the crude laccase extract are presented in Fig. 6. Dye removal with the crude laccase treatment from T. versicolor revealed a rapid decrease in dye concentration in the first 4 h. The removal percentages in this period were 41.6, 40 and 40% for BF, WB and WF, respectively. After 24 h, the dye removal with BF and WF was 80%, while 84% was obtained with WB. The dye removal rates for BF, WB and WF were 5.62, 6.08 and 5.72 µM h−1, respectively. Statistical analysis demonstrated that no significant differences were detected between treatments with crude laccase extracts obtained with BF and WF, while the laccase extract that was obtained with WB was statistically different from the other carbon sources.
Studies realized by Stoilova et al (2010) on Orange II removal with a 100 mgL−1 showed 100% removal in 48 h and used 2000 UmL−1of laccase extracts from T. versicolor. Decreasing enzyme concentration (1000 UmL−1) resulted in 75.3% removal after 312 h. In this study, the volumetric laccase activity used was ten times lower and 80% removal was achieved in 24 h. These differences could be attributed to the production of the most active laccase isoforms.
This suggests that enzymatic activity could be related to dye degradation; however, the contribution of the fungal biomass in in vivo removal was not evident.
Effects of fungal biomass and laccase on removal treatments
The contribution of all the three factors (fungal biomass–laccase (AB interaction); crude laccase (B-laccase); fungal biomass–laccase + inhibitor (A-fungal biomass)) on Orange II dye removal was evaluated by using a 22 factorial design. Due to the fact that the biomass and enzyme production was different for the different carbon sources (BF, WB and WF), the study of each of the factors earlier mentioned were performed. The statistical analyses of the factors obtained with each carbon source are presented in the following results.
The effect of laccase and metabolic activities produced in the three different carbon sources on the Orange II dye degradation was evaluated through a 22 factorial experimental design and the results are summarized in Table 1.
Effects of laccase and fungal biomass produced in BF on Orange II dye removal
The Orange II dye removal was significantly influenced by the laccase and metabolic activities. The ANOVA indicated that there were significant factor effects (F test, p < 0.0001), but this was not the case with the lack of fit of the model (F test, p = 0.05). Analysis of the most significant effects demonstrated that Eq. 2could be reduced to main effects as follows:
Orange II dye removal was enhanced up to 21.06 + 0.45 mgL−1 when the reaction was conducted with a high level (+ 1) of both fungal biomass and laccase (Fig. 7a).
Effects of laccase and biomass produced in WB on Orange II dye removal
The Orange II dye removal was significantly influenced by the laccase and metabolic activities. ANOVA indicated that there were significant factor effects (F test, p < 0.0001), but this was not the case with the lack of fit of the model (F test, p = 0.05). Analysis of the most significant effects demonstrated that Eq. 2 could be reduced to main effects as follows:
Orange II dye removal was enhanced up to 21.6 + 0.55 mgL−1 when the reaction was conducted with a high level (+ 1) of both fungal biomass and laccase (Fig. 7b).
Effects of laccase and fungal biomass produced in WF on Orange II dye removal
Similarly, as in the case of the effects on dye removal of laccase and fungal biomass produced in BF, the ANOVA indicated that there were significant factor effects (F test, p < 0.0001). Equally, this was not the case with the lack of fit of the model (F test, p = 0.05). Equation 2 was also reduced to main effects and represented as follows:
Orange II dye removal was enhanced up to 17.1 + 0.46 mgL−1 when the reaction was conducted with a high level (+ 1) of both fungal biomass and laccase (Fig. 7c).
The results obtained from the statistical analysis indicate that all three factors participate in the dye removal process. The laccase mad the most important contribution to the dye removal process (66–78%), while the biomass contributed to a lesser extent (8.4–16.44%). However, the fungal biomass–laccase interaction presented a negative effect (− 12 to 20%). In the case of this interaction, a synergistic effect would be expected due to the significant influence of each individual factor on dye removal. With respect to the condition (fungal biomass and laccase inhibited), the abiotic process significantly influences the metabolic degradation of the dye, because one part is found in the fungal biomass (biosorption), limiting its biodegradation. In other studies on dye degradation using T. hisurta, the effect of biotic processes (metabolic degradation and enzymatic transformation) and the abiotic process (adsorption) have also been demonstrated (Rodriguez et al. 2004; Casas et al. 2010). To investigate whether dye decolorization (150 mgL−1 of Lanaset Blue) could be attributed to laccase only and/or growth associated mechanisms, Rodriguez et al. (2004) performed experiments with whole culture of T. hisurta, laccase extracts from 6-day-old cultures and 6-day-old cultures inhibited with antibiotic solution (to inhibit fungal growth). After 24 h, they reported 96% of dye removal with whole culture, whereas in the culture filtrate, removal was lower (70%). When the growth of T. hisurta was inhibited by addition of antibiotics, more than 80% of removal was observed, but associated with adsorption into the mycelium (substantially colored). However, the adsorbed dye was not quantified. The authors concluded that both the enzyme and the fungal biomass participate in the dye removal process. Casas et al. (2010), demonstrated that the fungal biomass treatment underwent further removal, as no residual color appeared at any wavelength after the process, compared with enzymatic treatment where residual color was detected. In our experiments, the absorption spectra profiles obtained from the treatments show differences between materials used for laccase production also with treatment (Fig. 8). According to our results, we concluded that metabolic biodegradation and enzymatic transformation take place together (fungal biomass–laccase) and that in the absence of the enzyme the biomass realizes metabolic degradation of the dye, but less efficiently and under that condition the dye adsorption was higher. The crude laccase was equally efficient in dye removal with fungal biomass–laccase together, i.e., there was no statistical difference.
Absorption spectra profiles of the Orange II dye before (red dotted lines) and after the three biological treatments (black continuous lines) obtained with the different carbon sources. a Fungal biomass and laccase treatment; (b) treatment with fungal biomass and inhibited laccase and (c) only laccase treatment
It is convenient to highlight that, in dye removal, the biotic and abiotic processes that are involved must be considered for the evaluation of the efficiency of these processes and also the evaluation of the toxicity of the generated by-products.
Conclusions
According to the results obtained, it can be concluded that metabolic biodegradation and enzymatic transformation take place together (fungal biomass + laccase), and that in the absence of the enzyme, the fungal biomass realizes dye metabolic biodegradation in a less efficient way. The crude laccase was equally efficient as the fungal biomass–laccase treatment. But more importantly, metabolite formation was less in the fungal biomass–laccase together. The participation of the fungal biomass and the enzyme in the Orange II dye removal process was statistically significant. Additionally, the abiotic processes are important in the dye removal, since they limit the biodegradation of the dye by the extracellular enzymes as a result of being adsorbed by the fungal biomass.
References
Bertrand B, Martínez-Morales F, Tinoco R, Rojas-Trejo S, Serrano-Carreón L, Trejo Hernández MR (2014) Induction of laccases in Trametes versicolor by aqueous wood extracts. World J Microbiol Biotechnol 30(1):135–142
Blánquez P, Casas N, Font X, Gabarrell X, Sarrà M, Caminal G, Vicent T (2004) Mechanism of textile metal dye biotransformation by Trametes versicolor. Water Res 38(8):2166–2172
Casas N, Blánquez P, Gabarrell X, Vicent T, Caminal G, Sarrá M (2010) Degradation of orange G by laccase: fungal versus enzymatic process. Environ Technol 28(10):1103–1110
Chagas E, Durrant L (2001) Decolorization of azo dyes by Phanerochaete chrysosporium and Pleurotus sajor caju. Enzyme Microb Technol 29(8–9):473–477
Cripps C, Bumpus A, Aust D (1990) Biodegradation of azo and heterocyclic dyes by Phanerochaete chrysosporium. Appl Environ Microbiol 56(4):1114–1118
Daneshvar E, Vazirzadeh A, Niazi A, Sillanpää M, Bhatnagar A (2017) A comparative study of methylene blue biosorption using different modified brown, red and green macroalgae- effect of pretreatment. Chem Eng J 307:435–446
Forgacs E, Cserháti T (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971
Gajera HP, Bambharolia RP, Hirpara DG, Patel SV, Golakiya BA (2015) Molecular identification and characterization of novel Hypocrea koningii associated with azo dyes decolorization and biodegradation of textile dye effluents. Process Saf Environ 98:406–416
Goncalves I, Gomes A, Brás R, Ferra M, Amorim MTP, Porter S (2000) Biological treatment of effluent containing textile dyes. J Soc Dyers Colour 6(12):393–397
Jin XC, Liu GQ, Xu ZH, Tao YW (2007) Decolorization of a dye industry effluent by Aspergillus fumigatus XC6l. Appl Microbiol Biotechnol 74(1):239–243
Kirby N, McMullan G, Marchant R (1995) Decolorization of an artificial textile effluent by Phanerochaete chrysosporium. Biotechnol Lett 17(7):761–764
Kumari S, Naraian R (2016) Decolorization of synthetic brilliant green carpet industry dye through fungal co-culture technology. J Environ Manage 180:172–179
McMullan G, Meehan C, Conneely A, Kirby N, Robinson T, Nigam P, Banat I, Marchant R, Smyth F (2001) Microbial decolorization and degradation of textile dyes. Appl Microbiol Biotechnol 56(1–2):81–87
Mielgo I, Moreira G, Feijoo I, Lema M (2001) A packed-bed fungal bioreactor for continuous decolorization of azo-dyes (orange II). J Biotechnol 89(2–3):99–106
Patel H, Gupte A, Gupte S (2009) Effect of different culture conditions and inducers on production of laccase by a basidiomycete fungal isolate Pleurotus Ostreatus Hp-1 under solid state fermentation. BioResources 4(1):268–284
Pereira H, Graça J, Rodrigues JC (2003) Wood chemistry in relation to quality. In: Barnett JR, Jeronimidis G (eds) Wood quality and its biological basis. Blackwell Publishing Ltd, UK, pp 53–83
Robinson T, McMullan R, Marchant S, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresource Technol 77:247–255
Rodríguez C, Sanromán A, Hofer D, Guibitz G (2004) Stainless steel sponge: a novel carrier for immobilization of the white-rot fungus Trametes hirsuta for decolorization of textile dyes. Bioresource Technol 95(1):67–72
Rodríguez E, Pickard M, Vazquez-Duhalt R (1999) Industrial dye decolorization by laccases from lignolytic fungi. Curr Microbiol 38:27–31
Singh RL, Singh PK, Singh RP (2015) Enzymatic decolorization and degradation of azo dyes-a review. Int Biodeterior Biodegradation 104:21–31
Stoilova I, Krastanov A, Stanchev V (2010) Properties of crude laccase from Trametes versicolor produced by solid-substrate fermentation. Adv Biosci Biotechnol 1(3):208–215
Vanhulle S, Enaud E, Trovaslet M, Nouaimeh N, Bols M, Keshavarz T (2007) Overlap of laccases/cellobiose dehydrogenase activities during the decolorization of anthraquinone dyes with close chemical structures by Pycnoporus strain. Enzyme Microbiol Technol 40(7):1723–1731
Wesenberg D, Kyriakides I, Agathos N (2003) White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Adv 22:161–187
Yesilada O, Asma D, Cing S (2003) Decolorization of textile dyes by fungal pellets. Process Biochem 38(6):933–938
Zablocka-Godlewska E, Przystaś W, Grabińska-Sota E (2014) Decolourisation of different dyes by two Pseudomonas strains under various growth conditions. Water Air Soil Pollut 225(2):1846
Zhao X, Hardin I (2007) HPLC and spectrophotometric analysis of biodegradation of azo dyes by Pleurotus ostreatus. Dyes and Pigments 73:322–325
Zille A, Górrnacka B, Rehorek A (2005) Degradation of azo dyes by Trametes villosa laccase over long periods of oxidative conditions. ASM 71(11):66711–66718
Acknowledgements
A. Riegas-Villalobos acknowledges the fellowship of CONACYT (México). This study was partially financed by PROMEP-UAEM/11/182. We thank D. Morales for the technical assistance.
Author information
Authors and Affiliations
Contributions
AR-V: main project contributor, planning and development. FM-M: supervision in all steps of project development. RT-V: experimental and technical assistance and supervision in all steps of project development. LS-C: supervision in all steps of project development. BB: preparation of the manuscript from thesis to manuscript format, Spanish to English translation, supervision on data analysis and presentation. MdRT-H: contributed with project proposal and is director, physical space (laboratory), supervision on all aspects of the project data analysis and discussion and manuscript revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Riegas-Villalobos, A., Martínez-Morales, F., Tinoco-Valencia, R. et al. Efficient removal of azo-dye Orange II by fungal biomass absorption and laccase enzymatic treatment. 3 Biotech 10, 146 (2020). https://doi.org/10.1007/s13205-020-2150-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-2150-5