Abstract
The reductive tricarboxylic acid (rTCA) cycle was reconstructed in Escherichia coli by introducing pGETS118KAFS, where kor (encodes α-ketoglutarate:ferredoxin oxidoreductase), acl (encodes ATP-dependent citrate lyase), frd (encodes fumarate reductase), and sdh (encodes succinate dehydrogenase) were tandemly conjugated by the ordered gene assembly in Bacillus subtilis (OGAB). E. coli MZLF (E. coli BL21(DE3) Δzwf, Δldh, Δfrd) was employed so that the C-2/C-1 [(ethanol + acetate)/(formate + CO2)] ratio can be used to investigate the effectiveness of the recombinant rTCA for in situ CO2 recycling. It has been shown that supplying ATP through the energy pump (the EP), where formate donates electron to nitrate to form ATP, elevates the C-2/C-1 ratio from 1.03 ± 0.00 to 1.49 ± 0.02. Similarly, when ATP production is increased by the introduction of the heterologous ethanol production pathway (pLOI295), the C-2/C-1 ratio further increased to 1.79 ± 0.02. In summary, the ATP supply is a rate-limiting step for in situ CO2 recycling by the recombinant rTCA cycle. The decrease in C-1 is significant, but the destination of those recycled C-1 is yet to be determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The impact of greenhouse gases on the eco-system has been a subject of concern in modern times and, therefore, the fixation of carbon dioxide (CO2) becomes an important objective, while it is a low-cost carbon source (Bogorad et al. 2013; Bang and Lee 2018; François et al. 2020). The Calvin cycle is one of the major routes in our eco-system for conversion of CO2 to sugars. A partial Calvin cycle has been constructed in Escherichia coli (E. coli) where phosphoribulokinase (Prk) and ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) were heterologously expressed for directed evolution of Rubisco (Parikh et al. 2006; Mueller-Cajar et al. 2007). Meanwhile, the partial Calvin cycle has also been used to construct Rubisco-based engineered E. coli for in situ CO2 recycling during fermentation. This Rubisco-based engineered E. coli is able to recycle CO2 in situ during fermentation of pentoses (Zhuang and Li 2013) and hexoses (Li et al. 2015; Tseng et al. 2018).The partial Calvin cycle has been constructed in yeast for CO2 fixation (Li et al. 2017; Guadalupe-Medina et al. 2013; Xia et al. 2017). Recently, the Calvin cycle has been engineered in E. coli to convert CO2 to sugars where the energy source is externally provided by supplemented pyruvate (Antonovsky et al. 2016) and formate (Gleizer et al. 2019). Recently, yeast has been engineered into autotroph by introducing Rubisco and more (Gassler et al. 2019).
The reductive tricarboxylic acid (rTCA) cycle was first found in green sulfur bacteria (Evans et al. 1966). The rTCA cycle is basically a reverse version of the TCA cycle where many enzyme activities are shared. Two enzymes, ATP-dependent citrate lyase (ACL) and α-ketoglutarate:ferredoxin oxidoreductase (KOR) (Tang and Blankenship 2010; Evans et al. 1966), are unique to rTCA cycle since these two enzymes individually catalyzed irreversible reactions in the opposite direction to the TCA cycle. Meanwhile, according to genomic data of Chlorobium tepidum LST (Eisen et al. 2002), fumarate reductase (FR) activity may be crucial for rTCA pathway, since there are two sets of genes that encode this enzyme (CT2040-CT2041-CT2042 and CT2266-CT2267-CT2268) (Eisen et al. 2002). It has been calculated that isocitrate dehydrogenase, the enzyme that is responsible for carbon fixation, has a high specific activity and energy efficiency (Bar-Even et al. 2012).
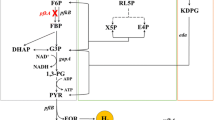
In this study, the rTCA cycle was constructed in E. coli (Fig. 1) by introducing a previously constructed plasmid pGETSKAFS (see Table S1 and Fig. S1 for detail information). The gene fragments encoding KOR, ACL, FR, and succinate dehydrogenase (SDH) were assembled to form pGETSKAFS by the OGAB method (Tsuge et al. 2003; Chen et al. 2013; Kaneko et al. 2005). With the confirmation of the enzymatic activities of each gene in pGETSKAFS (data not shown), the four genes korBA (CT0162 and CT0163 in GenBank, total 2.9 kb), aclAB (CT1088 and CT1089, total 3.1 kb), frdCBA (CT2040, CT2041, and CT2042, total 3.6 kb), and sdhBAC (CT2266, CT2267, and CT2268, total 3.4 kb) in pGETSKAFS shows a promising combination for constructing the recombinant rTCA cycle in E. coli (Fig. 1). The performance of the recombinant rTCA cycle was investigated by introducing pGETSKAFS in E. coli strain MZLF [E. coli BL21(DE3) Δzwf ΔldhA Δfrd (Yang et al. 2016)]. With glucose as the sole carbon source, the CO2 and energy released from the glucose oxidation can be in situ recycled by the recombinant rTCA cycle. Overall, the recombinant rTCA cycle converts 2 CO2 into 1 acetyl-CoA (see Theory below), and the C-2/C-1 ratio is an index for evaluating the activity of the recombinant rTCA cycle (see Theory below).
Also studied was the effect of ATP supply on in situ CO2 recycling by the recombinant rTCA cycle. Two methods of ATP supply were employed. The first method is called the energy pump (EP), where surplus NADH was converted to ATP by inducing heterologous formate dehydrogenase and nitrate reductase activities. The second method is to increase glucose consumption by introducing heterologous ethanol production (pLOI295), which increases ATP production. The pLOI295 allows ethanol overproduction and is a recombination plasmid that contains pdc and adhB genes from Zyommonas mobilis, which encode for pyruvate decarboxylase (PDC) and alcohol dehydrogenase II (ADH), respectively (Ingram et al. 1987). Overproduction of ethanol means deprivation of acetate production; consequently, one source of ATP productions is deprived as well. This creates an ATP demand, and it has been shown that this demand can be fulfilled by increasing glucose consumption.
Escherichia coli is able to utilize nitrate as a terminal electron acceptor for respiratory chain by nitrate reductase, which can be induced by the presence of nitrate (Lester and DeMoss 1971; Ingledew and Poole 1984). We have, therefore, developed a procedure called the energy pump (EP) in E. coli to promote anaerobic respiration. The EP consisted of heterologous formate dehydrogenase (FDH) and three supplements (sodium molybdate, sodium nitrate, and ferrous sulfate heptahydrate) that play essential roles in the electron transport chain and nitrate reductase. The FDH, originating in Candida boidinii (Sakai et al. 1997), first oxidizes formate to CO2 and then generates NADH. The electrons of NADH were then released and transferred through the electron transport chain (ETC) and finally transferred to the nitrate by nitrate reductase. The nitrate reductase spans the membrane, and it has been proposed that it creates a proton motive force (pmf, ∆p) via a Q loop across the cell membrane. The pmf is finally used to create ATP by ATP synthase (Kaim and Dimroth 1998). The molybdenum is a cofactor of the nitrate reductase, and the point where electrons are donated and accepted in nitrate reductase (Schwarz et al. 2009; Jagow and Walter 1980). The recipe of 1 mM sodium molybdate, 10 mM sodium nitrate, and 5 mM ferrous sulfate is called MNF. Throughout the process, the net generated ATP, from the conversion of NADH, can be used to elevate the efficiency of the recombinant rTCA cycle for CO2 fixation. Note that nitrate has a high standard reduction potential (0.421 V), second only to oxygen among common electron acceptors. The oxidation of one mole of NADH can be aerobically converted to 2.3 mol of ATP (Rich 2003).
Materials and methods
Bacterial strains and plasmids
Table 1 lists the bacterial strains used in this study.
Anaerobic batch fermentation
A 250 mL serum bottle containing 200 mL of M9 medium (20 g/L glucose, 2 mM MgSO4, 0.1 mM CaCl2, 12.8 g/L Na2HPO4·7H2O, 3 g/L KH2SO4, 0.5 g/L NaCl, 1 g/L NH4Cl) was prepared where initial pH was adjusted to 8.0. Rubber stopper was used to cap the bottle and then sealed with aluminum seal. The sealed serum bottle was flushed with nitrogen for 10 min (Zhuang and Li 2013). The pre-culture of E. coli strains were cultivated in LB medium, inoculated to obtain an initial OD600 of 0.05 for the main culture, and incubated on a shaker overnight (37 °C, 200 rpm). The pH of the culture solutions was adjusted to 8.0 at 8 and 24 h with 2 N NaOH. 0.02 mM of Isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to overexpress Rubisco (Table 2).
Analytical methods
Cell density was measured at 600 nm using a UV–Vis spectrophotometer (Thermo Scientific, US). Samples of extra-cellular metabolites were collected from the culture media followed by centrifugation for 5 min at 17,000×g. Before sample injection, the supernatant was filtered through a 0.2 μm PVDF filter. The quantification of residual glucose and extra-cellular formate, acetate, ethanol, lactate, pyruvate, and nitrate was determined by the Thermo Scientific DionexTM UltiMate 3000 LC Systems. The separation of the mixture was achieved with the HPLC column ORH-801 (Transgenomic, US) where the measurement was performed with a refractive index (RI, for glucose, acetate, ethanol) and an Ultraviolet–Visible detector (UV–Vis, for pyruvate (210 nm), formate (210 nm), lactate (210 nm), and nitrate (205 nm)). The mobile phase was 5 mM H2SO4. The temperature was maintained at 45 °C, while the flow rate was maintained at 0.6 mL per minute. The sample injection was done by an autosampler and the injection volume was 10 μL. The measurement of gaseous carbon dioxide was achieved by IR-based diffusive spectrometer (Sentry, Taiwan) where the dissolved CO2 and hydrated CO2 was calculated based on the equilibrium constants as described earlier (Zhuang and Li 2013). The elementary biomass composition of CH1.77O0.49N0.24 was used for calculating carbon recovery (Grosz and Stephanopoulos 1983). The formula to calculate carbon recovery is as follows:
The metabolites’ unit is mole.
Theory
To evaluate the efficacy of in situ CO2 recycling, E. coli MZLF was used to monitor the profile of fermentation products (Yang et al. 2016). As shown in Eqs. (1) and (2), the fermentation products of MZLF are limited to pyruvate, ethanol, acetate, and formate (and CO2) since ldh and frd (responsible for the lactate and succinate production, respectively) have been knocked-out in MZLF (Yang et al. 2016). On the other hand, the in situ CO2 recycling by the rTCA cycle can be described by Eq. (3) where two molecules of CO2 will be converted to 1 mol of acetyl-CoA-derived products, including ethanol and acetate.
The ratio of C-2/C-1 [i.e., (ethanol + acetate)/(formate + CO2)] for the bacterial strains can be evaluated by the performance of in situ CO2 recycling. While a typical fermentation behavior, as shown in Eqs. (1) and (2), provides a C-2/C-1 ratio of 1, the implement of Eq. (3) changes the C-2/C-1 ratio of conventional fermentation by utilizing C-1 produced from Eqs. (1) and (2). Note that Eq. (3) is the overall reaction as shown below:
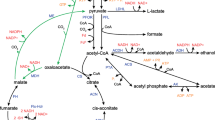
- (I)
Oxaloacetate + NADH → Malate + NAD+
- (II)
Malate → Fumarate + H2O
- (III)
Fumarate + NADH → Succinate + NAD+
- (IV)
Succinate + CoA + ATP → Succinyl-CoA + Pi + ADP
- (V)
Succinyl-CoA + CO2 + NADH → α-Ketoglutarate + NAD+ + CoA
- (VI)
α-Ketoglutarate + NADPH + CO2 → Isocicrate + NADP+
- (VII)
Isocitrate → Citrate
- (VIII)
Citrate + ATP + CoA + H2O → Oxaloacetate + Acetyl-CoA + ADP + Pi
Results
The performance of in situ CO2 recycling in MZLF3/pGETSKAFS was not significant
Figure 2 shows that pGETSKAFS has no significant effect on the growth of E. coli MZLF/pGETSKAFS and MZLF3/pGETSKAFS. Nevertheless, we demonstrated that the co-expression of Rubisco in MZLF3/pGETSKAFS significantly increased both the biomass yields of MZLF and MZLF/pGETSKAFS (Fig. 2). Rubisco has been found to behave as an epitope to stimulate physiological responses. One significant response is enhanced bacterial growth (from 0.39 ± 0.03 to 0.63 ± 0.11 mole/mole-glucose) which may be attributed to the enhanced transcription of genes that are responsible for the glyoxylate shunt (Yang et al. 2016). The increase in bacterial growth was not accompanied by an increase in C-2/C-1 ratio (Fig. S2). In Fig. 3, the biomass yields were derived from OD600 and the glucose consumption.
The energy pump (EP) increased the performance of in situ CO2 recycling in MZLF3/pGETSKAFS
In Fig. 3b, the formate yield has decreased significantly from 1.29 ± 0.02 to 1.16 ± 0.08 mol/mole-glucose, while the CO2 yield increased from 0.02 ± 0.00 to 0.05 ± 0.00 mol/mole-glucose after expressing heterologous FDH in MZLF3/pGETSKAFS + FDH. This demonstrated the proper function of FDH in E. coli where CO2 and NADH were provided. However, the C-2/C-1 ratio did not improve accordingly (Fig. 3c). When the energy pump was implemented, the product of C-1 for MZLF3 rTCA + EP reduced, where the formate yield was decreased from 1.29 ± 0.02 (MZLF3/pGETSKAFS) down to 0.66 ± 0.04 (MZLF3/pGETSKAFS + EP) mole/mole-glucose, and the CO2 yield was also reduced to below the detection limit. Therefore, the C-2/C-1 ratio effectively improved from 1.03 ± 0.01 to 1.49 ± 0.12 (Fig. 3c). The proper functioning of the EP can also be supported by the consumption of nitrate (Fig. 3d). The energy pump can only be brought about by combining FDH and MNF (Fig. 3). It should be noted that the bacterial growth and glucose consumption were significantly reduced when the EP or MNF was applied (Table 3). More specifically, the inhibitory effect on the bacterial growth was due to the supplemented molybdate (data not shown).
The ATP supply created by the heterologous ethanol production pathway dramatically increases the performance of in situ CO2 recycling in MZLF3/pGETSKAFS
First of all, pLOI295 significantly enhances the sugar consumption of MZLF3/pGETSKAFS + pLOI295 from 43 ± 8 to 113 ± 1 mM. (Fig. 4a). This increase in glucose consumption leads to a significant supply of ATP. The proper function of pLOI295 in MZLF3/pGETSKAFS + pLOI295 can be demonstrated by the increases in ethanol and CO2 yields compared to MZLF3/pGETSKAFS (Fig. 4b). The ethanol yield of MZLF/pGETSKAFS + pLOI295 increased from 0.92 ± 0.02 to 1.46 ± 0.11mole/mole-glucose. The total C-1 yield of MZLF/pGETSKAFS + pLOI295 decreased by 0.4 mol/mole-glucose (compared to MZLF3/pGETSKAFS, Fig. 4b); and, therefore, the final C-2/C-1 ratio of MZLF/pGETSKAFS + pLOI295 increased to 1.79 ± 0.02 (Fig. 4c). In summary, the introduction of pLOI295 elevated the performance of in situ CO2 recycling by rTCA cycle, which may be attributed to the ATP supply.
Discussion
The reconstruction of the rTCA cycle for in situ CO2 recycling in E. coli is an alternative way to use CO2 as feedstock. While gene elements of the rTCA cycle were put together in active form, the performance of in situ CO2 recycling was not demonstrated (Fig. 3). The performance of in situ CO2 recycling can be greatly enhanced by the employment of the EP or the heterologous ethanol production pathway (pLOI295), where these two methods increase the ATP supply.
The EP presumably converts NADH to ATP by actively extracting the reducing equivalent from formate and then converting it into ATP. The results show that the EP reduces the production of C-1 products in E. coli (Fig. 3). This was only achieved when both FDH (for formate oxidation) and MNF (for nitrate reduction through the electron transport chain) were also present (Fig. 3b). We can conclude that both the nitrate reductase and the electron transport chain play essential roles in in situ CO2 recycling. It is suggested that the reducing power released from the oxidation of formate is converted to ATP, and this ATP supply is important for directing carbon flow to the rTCA cycle. The appropriate MNF concentrations for in situ CO2 recycling in the rTCA cycle are 1 mM sodium molybdate, 10 mM nitrate, and 5 mM Ferrous sulfate heptahydrate (data not shown). In summary, anaerobic respiration can be deliberately activated only under the appropriate conditions, by means of the energy pump. The activation of anaerobic respiration facilitates in situ CO2 recycling.
Similarly, when ATP production is increased by the introduction of the heterologous ethanol production pathway (pLOI295), the level of C-1 products in E. coli significantly decreases. In other words, the strategy of additional ATP supply has been shown to be effective and allows in situ CO2 recycling via the rTCA cycle. It can be concluded that ATP is a rate-limiting step for Eq. (3). On the other hand, simply providing NADH may not be sufficient to drive the rTCA cycle activity, which can be exemplified by providing heterologous FDH (Fig. 3). The introduction of heterologous ethanol production pathway (pLOI295) is a better approach compared to the EP. We argued that the introduction of the heterologous ethanol production pathway (pLOI295) is a more efficient way for ATP production compared to the EP.
One thing that should be noted is that the decrease in C-1 is significant, but the destination of those recycled C-1 is yet to be determined. This is consistent with the result that MZLF3/pGETSKAFS + pLOI295 has the highest C-2/C-1 ratio among all but is accompanied with a low carbon recovery (less than 80%) (Table 3). The recombinant rTCA cycle has the potential for the production of butanol (Chen et al. 2013; Saini et al. 2016) and polyhydroxybutyrate (Lin et al. 2017) from CO2. It is suggested that the cell would need to carefully deliberate its intra-cellular energy balance to actually control the carbon flow.
Data availability
All data generated during this study are included in this article and its additional files.
References
Antonovsky N, Gleizer S, Noor E, Zohar Y, Herz E, Barenholz U, Zelcbuch L, Amram S, Wides A, Tepper N, Davidi D, Bar-On Y, Bareia T, Wernick-David G, Shani I, Malitsky S, Jona G, Bar-Even A, Milo R (2016) Sugar synthesis from CO2 in Escherichia coli. Cell 166(1):115–125. https://doi.org/10.1016/j.cell.2016.05.064
Bang J, Lee SY (2018) Assimilation of formic acid and CO2; by engineered Escherichia coli equipped with reconstructed one-carbon assimilation pathways. Proc Natl Acad Sci 115(40):E9271. https://doi.org/10.1073/pnas.1810386115
Bar-Even A, Noor E, Milo R (2012) A survey of carbon fixation pathways through a quantitative lens. J Exp Bot 63(6):2325–2342. https://doi.org/10.1093/jxb/err417
Bogorad IW, Lin T-S, Liao JC (2013) Synthetic non-oxidative glycolysis enables complete carbon conservation. Nature 502(7473):693–697. https://doi.org/10.1038/nature12575
Chen S-K, Chin W-C, Tsuge K, Huang C-C, Li S-Y (2013) Fermentation approach for enhancing 1-butanol production using engineered butanologenic Escherichia coli. Bioresour Technol 145:204–209. https://doi.org/10.1016/j.biortech.2013.01.115
Eisen JA, Nelson KE, Paulsen IT, Heidelberg JF, Wu M, Dodson RJ, Deboy R, Gwinn ML, Nelson WC, Haft DH, Hickey EK, Peterson JD, Durkin AS, Kolonay JL, Yang F, Holt I, Umayam LA, Mason T, Brenner M, Shea TP, Parksey D, Nierman WC, Feldblyum TV, Hansen CL, Craven MB, Radune D, Vamathevan J, Khouri H, White O, Gruber TM, Ketchum KA, Venter JC, Tettelin H, Bryant DA, Fraser CM (2002) The complete genome sequence of Chlorobium tepidum TLS, a photosynthetic, anaerobic, green-sulfur bacterium. Proc Natl Acad Sci 99(14):9509–9514. https://doi.org/10.1073/pnas.132181499
Evans MC, Buchanan BB, Arnon DI (1966) A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc Natl Acad Sci USA 55(4):928–934
François JM, Lachaux C, Morin N (2020) Synthetic biology applied to carbon conservative and carbon dioxide recycling pathways. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2019.00446
Gassler T, Sauer M, Gasser B, Egermeier M, Troyer C, Causon T, Hann S, Mattanovich D, Steiger MG (2019) The industrial yeast Pichia pastoris is converted from a heterotroph into an autotroph capable of growth on CO2. Nat Biotechnol. https://doi.org/10.1038/s41587-019-0363-0
Gleizer S, Ben-Nissan R, Bar-On YM, Antonovsky N, Noor E, Zohar Y, Jona G, Krieger E, Shamshoum M, Bar-Even A, Milo R (2019) Conversion of Escherichia coli to generate all biomass carbon from CO2. Cell 179(6):1255–1263.e1212. https://doi.org/10.1016/j.cell.2019.11.009
Grosz R, Stephanopoulos G (1983) Statistical mechanical estimation of the free energy of formation of E. coli biomass for use with macroscopic bioreactor balances. Biotechnol Bioeng 25:2149–2163
Guadalupe-Medina V, Wisselink H, Luttik M, de Hulster E, Daran J-M, Pronk J, van Maris A (2013) Carbon dioxide fixation by Calvin-Cycle enzymes improves ethanol yield in yeast. Biotechnol Biofuel 6(1):125
Ingledew WJ, Poole RK (1984) The respiratory chains of Escherichia coli. Microbiol Rev 48(3):222–271
Ingram LO, Conway T, Clark DP, Sewell GW, Preston JF (1987) Genetic engineering of ethanol production in Escherichia coli. Appl Environ Microbiol 53(0099–2240):2420–2425
Jagow G, Walter S (1980) b-Type cytochromes. Ann Rev Biochem 49(1):281–314. https://doi.org/10.1146/annurev.bi.49.070180.001433
Kaim G, Dimroth P (1998) ATP synthesis by the F1Fo ATP synthase of Escherichia coli is obligatorily dependent on the electric potential. FEBS Lett 434(1):57–60. https://doi.org/10.1016/S0014-5793(98)00969-7
Kaneko S, Akioka M, Tsuge K, Itaya M (2005) DNA shuttling between plasmid vectors and a genome vector: systematic conversion and preservation of DNA libraries using the bacillus subtilis genome (BGM) vector. J Mol Biol 349(5):1036–1044. https://doi.org/10.1016/j.jmb.2005.04.041
Lester RL, DeMoss JA (1971) Effects of molybdate and selenite on formate and nitrate metabolism in Escherichia coli. J Bacteriol 105(3):1006–1014
Li Y-H, Ou-Yang F-Y, Yang C-H, Li S-Y (2015) The coupling of glycolysis and the Rubisco-based pathway through the non-oxidative pentose phosphate pathway to achieve low carbon dioxide emission fermentation. Bioresour Technol 187:189–197. https://doi.org/10.1016/j.biortech.2015.03.090
Li Y-J, Wang M-M, Chen Y-W, Wang M, Fan L-H, Tan T-W (2017) Engineered yeast with a CO2-fixation pathway to improve the bio-ethanol production from xylose-mixed sugars. Sci Rep 7:43875. https://doi.org/10.1038/srep43875
Lin J-H, Lee M-C, Sue Y-S, Liu Y-C, Li S-Y (2017) Cloning of phaCAB genes from thermophilic Caldimonas manganoxidans in Escherichia coli for poly(3-hydroxybutyrate) (PHB) production. Appl Microbiol Biotechnol 101(16):6419–6430. https://doi.org/10.1007/s00253-017-8386-2
Mueller-Cajar O, Morell M, Whitney SM (2007) Directed evolution of rubisco in Escherichia coli reveals a specificity-determining hydrogen bond in the form II enzyme. Biochemistry 46(49):14067–14074. https://doi.org/10.1021/bi700820a
Parikh MR, Greene DN, Woods KK, Matsumura I (2006) Directed evolution of RuBisCO hypermorphs through genetic selection in engineered E. coli. Protein Eng Des Sel 19(3):113–119. https://doi.org/10.1093/protein/gzj010
Rich PR (2003) The molecular machinery of Keilin's respiratory chain. Biochem Soc Trans 31(6):1095
Saini M, Li S-Y, Wang ZW, Chiang C-J, Chao Y-P (2016) Systematic engineering of the central metabolism in Escherichia coli for effective production of n-butanol. Biotechnol Biofuels 9(1):1–10. https://doi.org/10.1186/s13068-016-0467-4
Sakai Y, Murdanoto AP, Konishi T, Iwamatsu A, Kato N (1997) Regulation of the formate dehydrogenase gene, FDH1, in the methylotrophic yeast Candida boidinii and growth characteristics of an FDH1-disrupted strain on methanol, methylamine, and choline. J Bacteriol 179(14):4480–4485
Schwarz G, Mendel RR, Ribbe MW (2009) Molybdenum cofactors, enzymes and pathways. Nature 460:839–847. https://doi.org/10.1038/nature08302
Tang KH, Blankenship RE (2010) Both forward and reverse TCA cycles operate in green sulfur bacteria. J Biol Chem 285(46):35848–35854. https://doi.org/10.1074/jbc.M110.157834
Tseng IT, Chen Y-L, Chen C-H, Shen Z-X, Yang C-H, Li S-Y (2018) Exceeding the theoretical fermentation yield in mixotrophic Rubisco-based engineered Escherichia coli. Metab Eng 47:445–452. https://doi.org/10.1016/j.ymben.2018.04.018
Tsuge K, Matsui K, Itaya M (2003) One step assembly of multiple DNA fragments with a designed order and orientation in Bacillus subtilis plasmid. Nucleic Acids Res 31(21):e133–e133. https://doi.org/10.1093/nar/gng133
Xia P-F, Zhang G-C, Walker B, Seo S-O, Kwak S, Liu J-J, Kim H, Ort DR, Wang S-G, Jin Y-S (2017) Recycling carbon dioxide during xylose fermentation by engineered Saccharomyces cerevisiae. ACS Synthetic Biol 6(2):276–283. https://doi.org/10.1021/acssynbio.6b00167
Yang C-H, Liu E-J, Chen Y-L, Ou-Yang F-Y, Li S-Y (2016) The comprehensive profile of fermentation products during in situ CO2 recycling by Rubisco-based engineered Escherichia coli. Microbial Cell Factor 15(1):1–10. https://doi.org/10.1186/s12934-016-0530-7
Zhuang Z-Y, Li S-Y (2013) Rubisco-based engineered Escherichia coli for in situ carbon dioxide recycling. Bioresour Technol 150:79–88. https://doi.org/10.1016/j.biortech.2013.09.116
Funding
This work was funded by the Ministry of Science and Technology Taiwan, MOST-106-2221-E-005-058-MY3 and the Ministry of Education, Taiwan, R.O.C. under the Higher Education Sprout Project.
Author information
Authors and Affiliations
Contributions
CH and IT carried out most of experiments. CH, IT, SC, ZR, JJ, YH, CC, and SY carried out data analysis and manuscript discussion. CH, IT, CC and SY designed the study. CH, IT, and SY drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, CH., Tseng, IT., Lo, SC. et al. Manipulating ATP supply improves in situ CO2 recycling by reductive TCA cycle in engineered Escherichia coli. 3 Biotech 10, 125 (2020). https://doi.org/10.1007/s13205-020-2116-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-2116-7