Abstract
In this study, a bacterial consortium ASDF was developed, capable of degrading fluoranthene (a non-alternant poly-aromatic hydrocarbon). It comprised of three bacterial strains: Pseudomonas sp. ASDF1, Burkholderia sp. ASDF2 and Mycobacterium sp. ASDF3 capable of degrading 100 mg/L of fluoranthene under experimentally defined and optimum conditions (37 °C, pH 7.0, 150 rpm) within 7 days. Consortium had metabolized fluoranthene as sole source of carbon and energy with maximum degradation rate of 0.52 mg/L/h and growth rate of 0.054/h. Fluoranthene degradation is an aerobic process, therefore with increasing the gyratory shaking from 50 to 150 rpm, degradation was concurrently enhanced by 7.1-fold. The synthetic surfactants SDS and CTAB had antagonistic effect on fluoranthene degradation (decreased up to 2.8-fold). The proficiency of consortium was assessed for its inherent ability to degrade seven other hydrocarbons both individually as well as in mixture. The degradation profile was studied using HPLC and the detection of two degraded intermediates (salicylic acid and derivatives of phthalic acid) suggested that fluoranthene degradation might have occurred via ortho- and meta-cleavage pathways. The competency of consortium was further validated through simulated microcosm studies, which showed 96% degradation of fluoranthene in soil ecosystem under the ambient conditions. Hence, the study suggested that the consortium ASDF has an inherent potential for its wide applicability in bioremediation of hydrocarbon-contaminated sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous pollutants, which are of either anthropogenic origin (industries, combustion of fossil fuels, etc.) or produced through natural phenomena (natural fires, volcanic eruptions, etc.) (Juhasz and Naidu 2000; Kumari et al. 2018). They are thermodynamically very stable and comprised of two or more aromatic rings in linear, angular or cluster arrangements (Nwinyi et al. 2016). Fluoranthene is a high molecular weight (HMW) PAH, which is highly persistent in environment because of its sparingly aqueous solubility, i.e., 0.25 mg/L (Haritash and Kaushik 2009). Fluoranthene is the product of pyrolysis of raw materials such as coal and petroleum at high temperature and incomplete combustion of fossil fuels, organic matter, main stream smoke of cigarette and in char boiled food (Kahla et al. 2020). It is thereby considered as a priority pollutant by the European Union and the United States Environmental Protection Agency (Lawal 2017; Storey et al. 2014). Fluoranthene is an experimental carcinogen; however, its carcinogenic potency is approximately 20 times less than that of benzo[a]pyrene and, therefore, it can be used as complementary indicator to benzo[a]pyrene (Boström et al. 2002).
Bioremediation is an eco-friendly and economically viable approach for the removal of pollutants from the environment with help of biological potential. It has an advantage of complete mineralization of pollutants or conversion to less toxic compounds over physico-chemical methods which suffers from inherent inefficiency and produce secondary toxic compounds (Ukiwe et al. 2013). Fluoranthene degradation has been reported by various bacterial species including Mycobacterium spp., Pseudomonas spp., Burkholderia spp., Rhodococcus spp., Sphingomonas spp. and many more (Morya et al. 2020; Ahmed and Ahmed 2016; Hickey et al. 2007; López et al. 2005; Somtrakoon et al. 2008; Sangkharak et al. 2020). Previous studies have reported that the efficiency of PAHs degradation was enhanced by consortia as compared to pure cultures, due to effective synergism and coordinated metabolic activities amongst the different microorganisms/bacteria of the consortia (Kumari et al. 2018; Patel et al. 2012, 2013; Vaidya et al. 2017, 2018).
Due to the persistent exposure to pollutants at contaminated environment, the indigenous microorganisms might have evolved the essential catabolic mechanisms for utilizing them as carbon and energy sources. Therefore, development of consortia from the polluted site itself had proved to be an efficient approach for degradation of various PAHs (Patel et al. 2018; Vaidya et al. 2018). On-field application of bioremediation of PAHs is still limited, because of various field conditions, such as different environmental parameters (pH, temperature, dissolved oxygen, and nutrient availability) and presence of multiple pollutants (heavy metals, other aromatic and non-aromatic hydrocarbons, pesticides, and inorganic ions). Thus, developing microbial consortia from polluted environments and optimizing the various environmental parameters may influence the degradation rate of PAHs, particularly in field conditions (Kumari et al. 2018).
Therefore, in present study, we have developed the fluoranthene-degrading consortium ASDF consisting of three bacterial species belonging to three different genera from long-term polluted Amlakhadi canal, Ankleshwar, Gujarat, India, using enrichment culture technology. Amlakhadi canal is a stretch of polluted water flowing through the Ankleshwar industrial estate which is one of the largest industrial estates of the Asia consisting of nearly 3000 industrial units manufacturing dyes, chemicals, pharmaceuticals and petrochemical products (Patel et al. 2012). The optimization of fluoranthene degradation by consortium ASDF was performed under various environmental parameters. The degradation was explored in the presence of co-supplements such as different surfactants, heavy metals, degradation pathway intermediates, nutrients, mono-aromatic hydrocarbons and other poly-aromatic hydrocarbons. Further, the degradation efficiency of consortium ASDF was investigated in the presence and absence of indigenous microflora, along with the co-supplementation of other PAHs during soil microcosm studies.
Materials and methods
Development and taxonomic identification of consortium ASDF
Bacterial consortium ASDF was developed from the polluted soil samples collected from Amlakhadi canal, Amlakhadi, Ankleshwar, Gujarat, India. Ten grams of these polluted samples were inoculated in minimal salt Bushnell Haas medium (BHM; 100 mL) amended with fluoranthene (100 mg/L) and incubated for 15 d, at 37 °C under shaking conditions (150 rpm). The fluoranthene-degrading bacteria were enriched by successive transfer from this master inoculum at every 15th day into fresh BHM containing fluoranthene. After 25th transfer, the enriched community was spread on BHM agar spiked with fluoranthene and incubated at 37 °C. Post-incubation several of bacterial colonies was observed on agar medium and on the basis of largest zone of clearance; three colonies with distinct morphological characters were further examined for fluoranthene degradation. These isolates were separately grown in BHM amended with fluoranthene (50 mg/L) and incubated under shaking conditions (150 rpm) at 37 °C for 15 days. To develop the consortium ASDF, all three isolates were mixed in equal concentration (1.7 × 107 cells of each isolate) and inoculated in fresh BHM amended with fluoranthene (100 mg/L). The fluoranthene degradation by the three individual isolates and by constructed consortium ASDF was compared.
To identify the bacterial isolates, genomic DNA from all three bacterial strains was extracted. The 16S rRNA gene from each bacterium was amplified using universal primers 8F and 1492R and was sequenced by the automated ABI 3500 genetic analyzer (Thermo Scientific, ABI, USA) (Desai and Madamwar 2007).
Inoculum preparation
The inoculum was developed by harvesting the pre-grown consortium ASDF (from late exponential phase of growth) at 5000 × g for 10 min at 4 °C. The cell pellets were washed twice with sterile distilled water and resuspended in fresh BHM to obtain the cell suspension with OD of 1.00 at A600. Ten percent of inoculum was used for all the experiments in the study.
Characterizing the nutritional requirement of the consortium
To enhance the degradation potential of the consortium ASDF, salicylic acid (144.8 mM), phthalic acid (120.4 mM) and glucose (111.0 mM) (i.e., 2%; (w/v) each), ammonium nitrate (12.49 nm), peptone, yeast extract and sodium succinate (6.17 mM) (i.e., 0.1%; (w/v) each) were supplemented in BHM amended with fluoranthene (100 mg/L). Similarly, surfactants were found to have significant effect on PAH degradation. Therefore, synthetic surfactants such as cetyl tri-methyl ammonium bromide (CTAB), sodium dodecyl sulfate (SDS), Tween 80 and Triton X-100 (0.02%; (w/v) or (v/v)) were supplemented in the degradation medium. The effect of initial concentration of fluoranthene (25–4000 mg/L) on its degradation was also studied.
Likewise, the effect of other hydrocarbons on fluoranthene degradation was investigated by supplementing pyrene (100 mg/L), phenanthrene (250 mg/L), naphthalene (500 mg/L), chrysene (5 mg/L), benzene, toluene and xylene (0.1%; (v/v) each) separately and in a mixture along with fluoranthene (100 mg/L) in BHM. Moreover, the degradation potential of consortium ASDF was also examined for multiple hydrocarbons degradation when mixture of poly- and mono-aromatic hydrocarbons such as fluoranthene (100 mg/L), pyrene (100 mg/L), phenanthrene (250 mg/L), naphthalene (500 mg/L), chrysene (5 mg/L), benzene, toluene and xylene (0.1%; (v/v) each) were provided together in BHM.
The flasks were inoculated with consortium ASDF and incubated at 37 °C under shaking conditions (150 rpm). The biotic control without any surfactants, pathway intermediates, nutrients and other hydrocarbons with consortium ASDF were kept under similar conditions. To determine abiotic loss of fluoranthene, abiotic controls without consortium ASDF were also kept under similar conditions. Fluoranthene degradation was analyzed at regular time intervals from 1 to 7 days.
Impact of physico-chemical environment on fluoranthene degradation
Besides nutritional requirement, various environmental parameters also influence fluoranthene degradation. The degradation experiments were performed at different temperatures (30–50 °C), pH (5–9; the pH of the medium was adjusted using either 1N NaOH or HCl), static and shaking (50–150 rpm) conditions, heavy metals (lead (Pb2+), mercury (Hg2+), chromium (CrO42−), zinc (Zn2+) and cadmium (Cd2+)) at various concentrations of 1, 5 and 10 mM.
Abiotic controls without inoculum and biotic control without any heavy metals were kept under similar conditions in all the experiments. Fluoranthene degradation was analyzed at regular time intervals from 1 to 7 days.
Extraction, analysis and quantification of fluoranthene and its degraded metabolites
Fluoranthene degradation was analyzed using High-Performance Liquid Chromatography (HPLC) (LC-20AD, Shimadzu, Japan) equipped with photodiode array detector (PDA) and Pursuit 3 PAH C18 reverse phase column (100 mm length, 4.6 mm inner diameter, 3 μm particle size) (Agilent, USA). To study the degradation profile of fluoranthene along with its degraded metabolites and its quantification, the entire content of the flask (i.e., 100 mL) was utilized for the extraction in dichloromethane (20 mL) as described in Vaidya et al. (2018). The 70% acetonitrile was used as the mobile phase with 1 mL/min isocratic flow rate. Each extracted samples for HPLC analysis were appropriately diluted and the solvent was evaporated under vacuum using SpeedVac (Thermo Electron Corporation, Waltham, MA). The dried content was resuspended in 1 mL of mobile phase. Percentage degradation was calculated using equations as described in Patel et al. (2018).
Microcosm studies for fluoranthene degradation
The microcosm studies for fluoranthene degradation were performed using polluted soil of Amlakhadi canal. The pristine soil was collected from the site near the Amlakhadi canal, where there was no visible pollution. Total 12 different experimental sets were prepared, 6 with pristine soil and 6 with polluted soil as described in Table 1. The sets with sterile soil were kept to determine the actual degradation potential of consortium ASDF in soil ecosystem. The soil samples were sterilized at 121 °C and 15 psi for 1 h at least for three cycles to remove spores and spore-forming organisms. The stimulated microcosms were prepared with 50 g of soil along with 100 mL BHM in 250 mL flasks. Fluoranthene, pyrene, chrysene, phenanthrene and naphthalene were dissolved in acetone and sprayed on soil to achieve their final concentration. The inoculum of consortium ASDF was prepared as mentioned above. After inoculum addition, soil systems were thoroughly homogenized and incubated at 37 °C under static conditions. Fluoranthene degradation was monitored after 20 days.
The microcosm was extracted using 20 mL of n-Hexane to determine the fluoranthene degradation. The whole content after adding n-Hexane was kept under shaking conditions (150 rpm) at room temperature for 30 min and allowed to settle down for 20 min. The anhydrous sodium sulfate was used to remove remaining aqueous content and samples were diluted appropriately. Degradation was measured spectrophotometrically against n-hexane blank using Double Beam Specord® 210 BU UV–Vis spectrophotometer (Analytica Jena AG, Germany) in spectral range of 190–500 nm (Vaidya et al. 2017). All PAHs possess characteristic absorption maxima proportional to their concentration in ultraviolet (UV) range of light spectra due to their planer cyclic structure and overlapping pie electron clouds (Saranya et al. 2011). The standards of naphthalene, phenanthrene, pyrene, fluoranthene and chrysene (10 mg/L each) were measured separately for their λmax in UV region. The λmax for naphthalene, phenanthrene, pyrene, fluoranthene and chrysene were 220, 250, 340, 286 and 268 nm, respectively, in n-hexane.
Statistical and linear regression analysis
All the above experiments were performed in triplicate. The mean percentage degradation was considered and the variation within same experiments was represented by standard deviation with the error bar in the figures. The obtained results were analyzed with ANOVA and the significance was considered at p ≤ 0.05. Linear regression as described in Vaidya et al. (2018) was used to assess the fitness for linearity between fluoranthene degradation and environmental parameters. The maximum growth rate (µmax), maximum degradation rate (qmax) and half substrate saturation constant (Ks) were obtained from exponential growth phase applying Monod equation (Ghosh et al. 2014; Okpokwasili and Nweke 2006).
Results and discussion
Enhanced fluoranthene degradation by consortium as compared to pure cultures
The consortium ASDF consisting of three bacterial cultures, which were taxonomically identified as Pseudomonas sp. ASDF1 (GenBank Accession number, MW149116), Burkholderia sp. ASDF2 (MW149117) and Mycobacterium sp. ASDF3 (MW149118), based on 16S rRNA gene sequencing. Phylogenetically, the former two belongs to Gammaproteobacteria, and Betaprotepbacteria class respectively, of phylum Proteobacteria, while later one is from Actinobacteria phylum. All three genera are previously well studied for fluoranthene degradation (Alemayehu et al. 2004; He et al. 2018; López et al. 2005; Somtrakoon et al. 2008; Zhong et al. 2011).
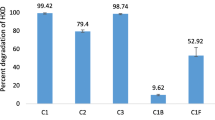
The initial study with individual cultures of the consortium showed the incompetence of all three isolates, as 61, 70 and 23% of fluoranthene (100 mg/L) was degraded by Pseudomonas sp. ASDF1, Burkholderia sp. ASDF2 and Mycobacterium sp. ASDF3, respectively (Fig. 1a). On crafting the various combinations of all three bacterial cultures, degradation rate was not increased as essentially required, until all three cultures were mixed together as a consortium ASDF (Fig. 1a). In consortium, complete metabolism (degradation) of 100 mg/L of fluoranthene within 7 days was observed, which was significant (p ≤ 0.05) as compared to individual and combinations of isolates. The enhanced degradation by consortium was due to synergistic and co-metabolic metabolic activities and the presence of complementary degradation pathways among all three cultures (Kumari et al. 2018; Wanapaisan et al. 2018; Zhong et al. 2011).
a Fluoranthene degradation by consortium ASDF (1 + 2 + 3), individual and paired bacterial isolates of consortium ASDF (Pseudomonas sp. ASDF1, Burkholderia sp. ASDF2 and Mycobacterium sp. ASDF3), b influence of initial concentration of fluoranthene on specific degradation rate (q) and specific growth rate (µ) of consortium ASDF at 37 °C and pH 7 under shaking conditions (150 rpm) in BHM
Fluoranthene degradation is dependent on its (initial) concentration
Poornachander et al. (2016) suggested that microbial degradation of xenobiotic compounds is concentration dependent. In a polluted environment, the xenobiotic compound concentration is always dynamic and their sensitivities towards microbial degradation are also specific for specific microorganisms. Moreover, there always exists a threshold on both sides for pollutant concentrations as well as for microorganisms. Therefore, it is imperative to determine a threshold concentration for fluoranthene, which can be effectively degraded by consortium ASDF. It can be determined by analyzing few relevant kinetic parameters such as specific growth rate (µ), specific degradation rate (q) and half substrate saturation constant (Ks) (Patel et al. 2012).
The observed results evidently shows the linear increase in specific fluoranthene degradation rate (q) and specific growth rate of consortium ASDF (μ) in concentration range of 1–100 ppm (Fig. 1b). At 1000 ppm both the parameters declined sharply and the higher concentration of 4000 ppm, proved to be more toxic for the consortial bacteria, with the obvious decline in degradation as well as growth rate. Fluoranthene degradation rate was maximum at 100 mg/L. The specific degradation rate (q) decreased nearly 58 fold (0.52–0.009 mg/L/d) and specific growth rate (µ) decreased by 11 fold (0.054–0.005/h) when fluoranthene concentration increased to 1000 ppm from 100 ppm (Fig. 1b). Further, the maximum fluoranthene degradation rate (qmax), maximum growth rate of consortium ASDF (µmax) and half substrate saturation constant (Ks) were estimated to be 0.52 mg/L/h, 0.054/h and 37 mg/L, respectively (obviously at 100 ppm fluoranthene concentration). Similar observations were found in the previous studies for pyrene and chrysene degradation (Vaidya et al. 2018, 2017). The observed results, therefore, indicated that the fluoranthene degradation was growth associated (i.e., a biologically mediated degradation) process, rather than an abiotic loss.
Fluoranthene degradation is an aerobic process
It is a proven fact that oxygen is a prime requirement in an aerobic degradation. Enzymes such as monooxygenases and dioxygenases utilize molecular oxygen for catalysis and play a significant role in PAHs degradation (Pandey et al. 2016). In nature, there also exists an anaerobic degradation mechanisms for 2–3 ring PAHs, but at an exceedingly slower rates and PAHs with more than 3 rings are rarely degraded under anaerobic environment (Haritash and Kaushik 2009). Since fluoranthene is a high molecular weight compound, possessing four aromatic rings, it is expected to require high oxygen concentration during its metabolism by consortium ASDF. The results from Figure S1A evidently supported the above notion, where fluoranthene degradation was significantly less (merely 14%) (p ≤ 0.05) even after 7 days of incubation, under static conditions. When degradation study was performed under oxygen-rich environment (i.e., under shaking conditions), degradation potential of consortium was steeply increased by 4.9, 6.4 and 7.1 folds (p ≤ 0.05) by increasing shaking speed from 50 to 150 rpm and achieving complete degradation of fluoranthene at 7th day.
Surfactants acts antagonistically during fluoranthene degradation
Due to the hydrophobic nature of PAHs, their bioavailability is one of the major limitations during biological degradation. Nevertheless, this can be minimized by providing external surfactants during bioremediation studies or if bacteria inherently produce surface-active compounds which may enhance PAHs degradation potential of the bacteria (Li and Chen 2009). But, in this study, the extraneous surfactants have decreased the fluoranthene degradation by consortium ASDF (Fig. 2a). It was decreased significantly (p ≤ 0.05) by 2.8 fold, when supplemented with SDS (anionic) or CTAB (cationic) surfactants. The non-ionic surfactants, Tween-80 and Triton X-100 have also decreased fluoranthene degradation by 2.3 and 1.9 folds, respectively. The decline in the degradation rate might be due to the disruption of cellular structure and dissolving the cell membrane or their preferential use as carbon and energy source before fluoranthene (PAHs) (Szczepaniak et al. 2016; Vaidya et al. 2018). The surfactants also might have proved toxic if their concentrations had reached beyond the tolerance capability of the consortium, indicating that constituent bacteria might be producing bio-surfactants (Pathak et al. 2009). These may also lead to the decrease in growth rate of the consortium.
Impact of various chemical factors on fluoranthene degradation by consortium ASDF a external surfactants, b nitrogen nutrients, c carbon nutrient and pathway intermediates under optimized conditions (37 °C, pH 7 and 150 rpm shaking conditions). Control is BHM + fluoranthene without any co-supplements
Impact of organic nutrients and pathway intermediates
Studies suggested that organic nutrients and PAH degradation pathway intermediates might play an active role in aromatic pollutant degradation (Patel et al. 2018). During initial stages of fluoranthene degradation (for first 48 h), peptone, yeast extract, glucose and salicylic acid have enhanced the fluoranthene degradation rate; however, with prolong incubation the degradation rate was decreased. Ammonium nitrate, peptone, sodium succinate and yeast extract have decreased fluoranthene degradation by 1.8, 1.2, 1.7 and 1.4 folds, respectively (Fig. 2b). The degradation rate was also declined by 1.4, 2.0 and 1.5 folds in presence of glucose, phthalic and salicylic acids, respectively (Fig. 2c). The observed results unexpectedly indicated that maximum fluoranthene degradation was actually achieved without any supplementation of the nutrients. The consortium might have utilized these compounds as simple source of carbon and energy, which initially might have increased the fluoranthene degradation. With the prolong incubation, decline in degradation rate could be attributed to the depletion in nutrients and feedback inhibition of enzymes involved in fluoranthene degradation (Seo et al. 2009).
Co-metabolism of different hydrocarbons and their impact on fluoranthene degradation
The co-metabolism (simultaneous degradation) of multiple hydrocarbons is one of the most significant and positive trait of the consortium ASDF. Since PAHs commonly co-exist in the polluted environment, however, in many studies, much attention has been given to degradation of single PAH only. Moreover, during scaling-up process at bioreactor level or in field studies using bioremediation, consortium needs to grow in the presence of mixture of compounds (Jiang et al. 2018). Figure 3a depicts the degradation potential of consortium for co-metabolism of multiple hydrocarbons. The consortium was able to degrade eight different hydrocarbons simultaneously and nearly 40% of all hydrocarbons used in the study were degraded within 7 days. The order of rate of degradation (from higher to lower) was naphthalene > phenanthrene > benzene > toluene > pyrene > xylene > chrysene > fluoranthene.
The polluted sites invariably consist of different xenobiotic compounds at varying concentrations which may impede the degradation of target pollutant during onsite remediation (Singh and Tiwary 2017). Thus, efficiency of fluoranthene degradation by consortium ASDF in the presence of various mono- and poly-aromatic hydrocarbons individually as well in mixture was studied. The observed results indicated that fluoranthene degradation was deceased by 30, 42 and 58% (p ≤ 0.05), in the presence of benzene, toluene and xylene, respectively. Phenanthrene, pyrene and chrysene have decreased the fluoranthene degradation by 42, 49 and 65% (p ≤ 0.05), respectively, while naphthalene had negligible effect (Fig. 3b). The mixture of all seven hydrocarbons (benzene, toluene, xylene, naphthalene, phenanthrene, pyrene and chrysene) further decreased the fluoranthene degradation by 2.5 fold (i.e., upto 40%) after 7th day (Fig. 3b). Patel et al. (2019) also observed similar decrease in degradation of phenanthrene and fluoranthene by mixed bacterial cultures upon addition of lubricant oil and diesel fuel. This is the evidence for co-metabolism and competitive degradation of structurally similar pollutants due to the broad substrate specificity of degrading enzymes (Patel et al. 2013; Poornachander et al. 2016).
Impact of heavy metals
Besides organic pollutants, heavy metals are also co-contaminants at many sites polluted with industrial and other anthropogenic activities. The presence of heavy metals might impede the key enzymatic activities during metabolism of xenobiotic compounds (Chen et al. 2008). Therefore, impact of heavy metals on fluoranthene degradation was assessed to imitate the complexity of polluted sites. Results from Fig. 4a revealed that with increasing concentrations of heavy metals from 1 to 10 mM, fluoranthene degradation as well as growth of the consortium ASDF were declined significantly (p ≤ 0.05). Mercury, chromium, zinc and cadmium (at 1 mM concentrations each) have decreased fluoranthene degradation, respectively, by 2.0, 3.3, 1.8 and 2.0 folds after 7 d, while lead did not affect its degradation (Fig. 4a).
Results further revealed that consortium ASDF was relatively more resistant towards lead and cadmium as compared to mercury, chromium and zinc at the higher concentrations (Fig. 4b). In a similar study, Patel et al. (2018) have reported that degradation of naphthalene, phenanthrene, fluoranthene and pyrene by mixed bacterial cultures DAK11 was reduced by 1.5, 2.0, 19.0 and 12.0 folds in presence of 1 mM lead, respectively. Moreover, consortium ASDF showed higher tolerance towards lead as compared to mixed bacterial cultures DAK11 (Patel et al. 2018). The EC50 denotes effective concentration of particular heavy metal at which 50% growth of consortium ASDF was inhibited (Pepi et al. 2009). The impact of heavy metals on consortial growth was further evaluated in terms of EC50. The EC50 values for consortium ASDF were 2.7, 0.9, 1.5 and 1.7 mM in the presence of lead, mercury, zinc and cadmium, respectively. The heavy metal tolerance properties of consortium ASDF would definitely help during its field applications in PAH bioremediation.
Stoichiometry profile of fluoranthene degradation
The degradation profile of fluoranthene was analyzed using HPLC. The single major peak was observed at retention time of 6.1 min corresponding to intact fluoranthene compound. During fluoranthene metabolism by the consortium, the first visible observation was the color of the medium gradually changes and becomes faded with increase in incubation period. This might be due to the production of few water soluble fluoranthene degraded intermediates, which we could not extract (Juhasz et al. 1997). Mueller et al. (1989) in their study have reported the accumulation of catechol-like compounds along with their meta-ring compounds which might be associated with the color changes as observed in this study.
However, couple of peaks were observed in HPLC chromatogram, at retention time of 1.8 and 2.7 min after 7th day, corresponding to a salicylic acid and a derivative of phthalic acid, respectively. Salicylic acid is a product of meta-cleavage pathway which is further transformed into catechol leading to the formation of acetyl-CoA or pyruvate and enters into TCA cycle (Somnath et al. 2007). Likewise, phthalic acid is a product of ortho-cleavage pathway and transformed into proto-catechuic acid followed by catechol and enters into TCA cycle (Seo et al. 2009).
The study further revealed that the rate of fluoranthene degradation was nearly 1.5–2.0 times higher when all three bacterial strains are in form of consortium, rather than as individual strains (or in combinations of two) (Fig. 1a). In consortium, the synergistic interactions and metabolic exchanges found to enhance the catabolic range of the bacterium and may result in complete degradation xenobiotic recalcitrant compounds, which as an individual strain could not complete the mineralization of pollutants.
The degradation rate sharply decreases when studied with individual strains of the consortium (e.g., the efficiency of Mycobacterium sp., for fluoranthene degradation was very low, that might be due to its slower growth rate than Pseudomonas sp. and Burkholderia sp.). The fluoranthene degradation by consortium ASDF was, therefore, a combined process of both primary utilization and co-metabolism.
The fluoranthene at 0.4 mM (100 ppm) of initial concentration was metabolized completely and had produced 0.02 mM (2.7 ppm) of derivative of phthalic acid and 0.01 mM (2.9 ppm) of salicylic acid after 7 days (Table S1). These results imply that there was no accumulation of degraded intermediates in the medium upon complete mineralization of fluoranthene. Further, HPLC profiles also reveal a few minor peaks, besides for salicylic acids and derivatives of phthalic acids. Their peak areas do not increase with increase in incubation period, which was an indication of continuous degradation of all intermediates too. These observations can be further validated through stoichiometric analysis from Table S1, where total utilized concentration of fluoranthene was not equal with total concentration of intermediates formed and there was no significant increase of intermediate concentrations also. The obtained results are concomitant with the Lopez et al. (2005), where mass balancing of fluoranthene utilized and intermediates generated showed non-equality between fluoranthene and intermediate concentrations. Therefore, obtained results indicate the complete mineralization of pollutant compound.
Thus, considering the above observations, the consortium ASDF might have degraded fluoranthene through ortho/meta-cleavage pathway as postulated in Figure S2. The constituent bacteria of the consortium have been reported to possess the genes encoding the enzymes capable of degrading fluoranthene and other PAHs. The type of intermediates detected during fluoranthene degradation suggests that both ortho- and meta-cleavage pathways were active in the consortium (and postulated in Figure S2), which might be due to the presence of catechol dioxygenases genes in all the three strains. The previous studies have reported the presence of catechol dioxygenases genes in Mycobacterium, Pseudomonas and Burkholderia (Kang et al. 2016; Xie et al. 2014; Suzuki et al. 2002; Lopez et al. 2005; Cenci et al. 1999; Juhasz et al. 1997).
Linear regression analysis
The observed results were further analyzed and validated to have statistically significant influence of various factors on fluoranthene degradation by consortium ASDF. After initial analysis using ANOVA, abiotic factors, viz., temperature, oxygen concentration (i.e., static and shaking), initial fluoranthene concentration, biotic factors such as nitrogen and organic nutrient sources were found to have significant impact on fluoranthene degradation. The Pearson’s Correlation Coefficient (i.e., Coefficient of Correlation, r) were 0.916, 0.914, and 0.998 for temperature, oxygen concentration and initial fluoranthene concentration, respectively, indicating a positive correlation (Table 2). However, as observed from the Table 2, the Pearson’s Regression suggested that few of the studied parameters such as sodium succinate, 45 and 50 °C temperature, and higher initial fluoranthene concentration (> 100 mg/L) were negatively correlated and have decreased the fluoranthene degradation rate. The half-life for fluoranthene degradation at 37 °C was 0.82 days which gradually increased to 1.79 and 1.91 days at 45 and 50 °C, respectively (Table S2). Similar results were observed in the previous study, where influence of temperature, pH, oxygen concentration and organic nutrients were positively correlated with chrysene degradation (Vaidya et al. 2018).
Microcosm studies with polluted and pristine soil
The flask scale observations were validated and the competence of consortium ASDF for PAHs degradation was assessed in soil microcosms, using polluted and pristine soils distributed in 12 sets of experiments.
Consortium ASDF in the presence of indigenous microflora of pristine and polluted soil (non-sterile) exhibited 99 and 96% fluoranthene degradation within 20 days (Table 1), respectively. While, 100 and 98% fluoranthene degradation was observed when sterile pristine and polluted soil were augmented with consortium ASDF, respectively. Augmented non-sterile, polluted and pristine soils when supplemented with four other PAHs supported 75 and 90% fluoranthene degradation, respectively, which was significantly higher as compared to sterile sets (68 and 69%) under similar conditions. Microcosms with consortium ASDF showed higher percentage degradation as compared to microcosms without consortium ASDF (Table 1). Similar observation was found by Hickey et al. (2007), where significantly higher fluoranthene removal was observed from soil microcosms inoculated with Pseudomonas alcaligenes PA-10 after 28 days as compared to an uninoculated control. Consortium ASDF was quite competent since, it can also degrade fluoranthene in the presence of multiple PAHs. Declination in fluoranthene degradation in the presence of multiple PAHs might occur due to enzymatic inhibition for similarly structured substrates (Zhong et al. 2010). The abiotic loss was negligible since fluoranthene is a high molecular weight PAH and hence its tendency is to persist in the organic part of the soil. Somtrakoon et al. (2008) reported 25.8 and 12.1% of pyrene and fluoranthene degradation in soil by Burkholderia sp. VUN10013 after 60 days of incubation. Muckian et al. (2009) found 45% of fluoranthene degradation within 28 days by indigenous microorganisms of agricultural soil when soil was amended with 200 mg/kg of fluoranthene. Overall, fluoranthene degradation in microcosm study was quite high as compared to previous studies.
Conclusion
The above study has developed an effective bacterial consortium ASDF capable of metabolizing of fluoranthene (100 mg/L) as a sole source of carbon and energy within 7 days. The synthetic surfactants have exerted antagonistic effect on fluoranthene degradation. The consortium through synergistic co-metabolic interactions has degraded multiple hydrocarbons and also shown an equal proficiency in degrading fluoranthene under soil (microcosms) ecosystem. Taken together, the developed consortium has all the potential for its large-scale applications for bioremediation of sites contaminated with PAHs.
Accession numbers
Pseudomonas sp. ASDF1 (GenBank accession number MW149116),
Burkholderia sp. ASDF2 (MW149117).
Mycobacterium sp. ASDF3 (MW149118).
References
Ahmed RZ, Ahmed N (2016) Isolation of Rhodococcus sp. CMGCZ capable to degrade high concentration of fluoranthene. Water Air Soil Pollut 227:162–175. https://doi.org/10.1007/s11270-016-2857-4
Alemayehu D, Gordon L, O’Mahony M, O’Leary N, Dobson A (2004) Cloning and functional analysis by gene disruption of a novel gene involved in indigo production and fluoranthene metabolism in Pseudomonas alcaligenes PA-10. FEMS Microbiol Lett 239:285–293. https://doi.org/10.1016/j.femsle.2004.08.046
Boström CE, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T, Rannug A, Törnqvist M, Victorin K, Westerholm R (2002) Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect 110:451–488. https://doi.org/10.1289/ehp.110-1241197
Cenci G, Caldini G, Boari L (1999) Dioxygenase activity and relative behaviour of Pseudomonas strains from soil in the presence of different aromatic compounds. World J Microbiol Biotechnol 15:41–46. https://doi.org/10.1023/A:1008868124715
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Bioresour Technol 99:4044–4064. https://doi.org/10.1016/j.biortech.2007.01.057
Desai C, Madamwar D (2007) Extraction of inhibitor-free metagenomic DNA from polluted sediments, compatible with molecular diversity analysis using adsorption and ion-exchange treatments. Bioresour Technol 98:761–768. https://doi.org/10.1016/j.biortech.2006.04.004
Ghosh I, Jasmine J, Mukherji S (2014) Biodegradation of pyrene by a Pseudomonas aeruginosa strain RS1 isolated from refinery sludge. Bioresour Technol 166:548–558. https://doi.org/10.1016/j.biortech.2014.05.074
Haritash A, Kaushik C (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169:1–15. https://doi.org/10.1016/j.jhazmat.2009.03.137
He C, Li Y, Huang C, Chen F, Ma Y (2018) Genome sequence and metabolic analysis of a fluoranthene-degrading strain Pseudomonas aeruginosa DN1. Front Microbiol 9:2595. https://doi.org/10.3389/fmicb.2018.02595
Hickey AM, Gordon L, Dobson AD, Kelly CT, Doyle EM (2007) Effect of surfactants on fluoranthene degradation by Pseudomonas alcaligenes PA-10. Appl Microbiol Biotechnol 74:851–856. https://doi.org/10.1007/s00253-006-0719-5
Jiang Y, Zhang Z, Zhang X (2018) Co-biodegradation of pyrene and other PAHs by the bacterium Acinetobacter johnsonii. Ecotoxicol Environ Saf 163:465–470. https://doi.org/10.1016/j.ecoenv.2018.07.065
Juhasz AL, Naidu R (2000) Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: a review of the microbial degradation of benzo[a]pyrene. Int Biodeterior Biodegrad 45:57–88. https://doi.org/10.1016/S0964-8305(00)00052-4
Juhasz AL, StanleyGA BML (1997) Degradation of fluoranthene, pyrene, benz[a]anthracene and dibenz[a, h]anthracene by Burkholderia cepacia. J Appl Microbiol 83:189–198. https://doi.org/10.1046/j.1365-2672.1997.00220.x
Kahla O, Garali SMB, Karray F, Abdallah MB, Kallel N, Mhiri N, Zaghden H, Barhoumi B, Pringault O, Quemeneur M, Tedetti M, Sayadi S, Hlaili AS (2020) Efficiency of benthic diatom-associated bacteria in the removal of benzo(a)pyrene and fluoranthene. Sci Total Environ 751:141399. https://doi.org/10.1016/j.scitotenv.2020.141399
Kang JK, Lia ZM, Aia GM, Deng Y, Liu SJ, Jiang CY (2016) Reconstruction of metabolic networks in a fluoranthene-degrading enrichments from polycyclic aromatic hydrocarbon polluted soil. J Hazard Mater 318:90–98. https://doi.org/10.1016/j.jhazmat.2016.06.055
Kumari S, Regar RK, Manickam N (2018) Improved polycyclic aromatic hydrocarbon degradation in a crude oil by individual and a consortium of bacteria. Bioresour Technol 254:174–179. https://doi.org/10.1016/j.biortech.2018.01.075
Lawal AT (2017) Polycyclic aromatic hydrocarbons. A review. Cogent Environ Sci 3:1339841. https://doi.org/10.1080/23311843.2017.1339841
Li JL, Chen BH (2009) Surfactant-mediated biodegradation of polycyclic aromatic hydrocarbons. Materials (Basel) 2:76–94. https://doi.org/10.3390/ma2010076
López Z, Vila J, Grifoll M (2005) Metabolism of fluoranthene by Mycobacterial strains isolated by their ability to grow in fluoranthene or pyrene. J Ind Microbiol Biotechnol 32:455–464. https://doi.org/10.1007/s10295-005-0022-y
Morya R, Salvachua D, Thakur IS (2020) Burkholderia: an untapped but promising bacterial genus for the conversion of aromatic compounds. Trends Biotechnol 38(9):963–975. https://doi.org/10.1016/j.tibtech.2020.02.008
Muckian LM, Grant RJ, Clipson NJ, Doyle EM (2009) Bacterial community dynamics during bioremediation of phenanthrene-and fluoranthene-amended soil. Int Biodeterior Biodegrad 63:52–56. https://doi.org/10.1016/j.ibiod.2008.04.005
Mueller JG, ChapmanPritchard PJPH (1989) Action of a fluoranthene-utilising bacterial community on polycyclic aromatic hydrocarbon components of creosote. Appl Environ Microbiol 55:3085–3090
Nwinyi OC, Ajayi OO, Amund OO (2016) Degradation of polynuclear aromatic hydrocarbons by two strains of Pseudomonas. Braz J Microbiol 47:551–562. https://doi.org/10.1016/j.bjm.2016.04.026
Okpokwasili G, Nweke C (2006) Microbial growth and substrate utilization kinetics. Afr J Biotechnol 5:305–317. http://www.academicjournals.org/AJB
Pandey P, Pathak H, Dave S (2016) Microbial ecology of hydrocarbon degradation in the soil: a review. Res J Environ Toxicol 10:1–15. https://doi.org/10.3923/rjet.2016.1.15
Patel V, Cheturvedula S, Madamwar D (2012) Phenanthrene degradation by Pseudoxanthomonas sp. DMVP2 isolated from hydrocarbon contaminated sediment of Amlakhadi canal, Gujarat, India. J Hazard Mater 201:43–51. https://doi.org/10.1016/j.jhazmat.2011.11.002
Patel V, Patel J, Madamwar D (2013) Biodegradation of phenanthrene in bioaugmented microcosm by consortium ASP developed from coastal sediment of Alang-Sosiya ship breaking yard. Mar Pollut Bull 74:199–207. https://doi.org/10.1016/j.marpolbul.2013.07.001
Patel AB, Mahala K, Jain K, Madamwar D (2018) Development of mixed bacterial cultures DAK11 capable for degrading mixture of polycyclic aromatic hydrocarbons (PAHs). Bioresour Technol 253:288–296. https://doi.org/10.1016/j.biortech.2018.01.049
Patel AB, Singh S, Patel A, Jain K, Amin S, Madamwar D (2019) Synergistic biodegradation of phenanthrene and fluoranthene by mixed bacterial cultures. Bioresour Technol 284:115–120. https://doi.org/10.1016/j.biortech.2019.03.097
Pathak H, Kantharia D, Malpani A, Madamwar D (2009) Naphthalene degradation by Pseudomonas sp. HOB1: in vitro studies and assessment of naphthalene degradation efficiency in simulated microcosms. J Hazard Mater 166:1466–1473. https://doi.org/10.1016/j.jhazmat.2008.12.074
Pepi M, Lobianco A, Renzi M, Perra G, Bernardini E, Marvasi M, Gasperini S, Volterrani M, Franchi E, Heipieper HJ (2009) Two naphthalene degrading bacteria belonging to the genera Paenibacillus and Pseudomonas isolated from a highly polluted lagoon perform different sensitivities to the organic and heavy metal contaminants. Extremophiles 13:839–848. https://doi.org/10.1007/s00792-009-0271-1
Poornachander R, Anitha Y, Satyaprasad K (2016) Abilities of Bacillus cereus CPOU13 in biodegradation of polycyclic aromatic hydrocarbons (PAHs). J Pharm Chem Biol Sci 4:54–64
Sangkharak K, Choonut A, Rakkan T, Prasertsan P (2020) The degradation of phenanthrene, pyrene, and fluoranthene and its conversion into medium-chain-length polyhydroxyalkanoate by novel polycyclic aromatic hydrocarbon-degrading bacteria. Curr Microbiol 77:897–909. https://doi.org/10.1007/s00284-020-01883-x
Saranya G, Kolandaivel P, Senthilkumar K (2011) Optical absorption and emission properties of fluoranthene, benzo[k]fluoranthene, and their derivatives. A DFT study. J Phys Chem A 115:14647–14656. https://doi.org/10.1021/jp208617s
Seo JS, Keum YS, Li Q (2009) Bacterial degradation of aromatic compounds. Int J Environ Res Public Health 6:278–309. https://doi.org/10.3390/ijerph6010278
Singh P, Tiwary BN (2017) Optimization of conditions for polycyclic aromatic hydrocarbons (PAHs) degradation by Pseudomonas stutzeri P2 isolated from Chirimiri coal mines. Biocatal Agric Biotechnol 10:20–29. https://doi.org/10.1016/j.bcab.2017.02.001
Somnath M, Subhankar C, Tapan K (2007) A novel degradation pathway in the assimilation of phenanthrene by Staphylococcus Sp. strain PN/Y via meta-cleavage of 2-hydroxy-1-naphthoic acid: formation of trans-2,3-dioxo-5-(2′-hydroxyphenyl)-pent-4-enoic acid. Microbiology 150:2104–2115. https://doi.org/10.1099/mic.0.2006/004218-0
Somtrakoon K, Suanjit S, Pokethitiyook P, Kruatrachue M, Lee H, Upatham S (2008) Phenanthrene stimulates the degradation of pyrene and fluoranthene by Burkholderia sp. VUN10013. World J Microbiol Biotechnol 24:523–531. https://doi.org/10.1007/s11274-007-9503-7
Storey S, Ashaari M, McCabe G, Harty M, Dempsey R, Doyle O, Clipson N, Doyle E (2014) Microbial community structure during fluoranthene degradation in the presence of plants. J Appl Microbiol 117:74–84. https://doi.org/10.1111/jam.12518
Suzuki K, Ichimura A, Ogawa N, Hasebe A, Miyashita K (2002) Differential expression of two catechol 1,2-dioxygenases in Burkholderia sp. strain TH2. J Bacteriol 184:5714–5722. https://doi.org/10.1128/JB.184.20.5714-5722.2002
Szczepaniak Z, Czarny J, Staninska-Pięta J, Lisiecki P, Zgoła-Grześkowiak A, Cyplik P, Chrzanowski Ł, Wolko Ł, Marecik R, Juzwa W (2016) Influence of soil contamination with PAH on microbial community dynamics and expression level of genes responsible for biodegradation of PAH and production of rhamnolipids. Environ Sci Pollut Res 23:23043–23056. https://doi.org/10.1007/s11356-016-7500-9
Ukiwe LN, Egereonu UU, Njoku PC, Nwoko CI, Allinor JI (2013) Polycyclic aromatic hydrocarbons degradation techniques: a review. Int J Chem 5:43–55. https://doi.org/10.5539/ijc.v5n4p43
Vaidya S, Jain K, Madamwar D (2017) Metabolism of pyrene through phthalic acid pathway by enriched bacterial consortium composed of Pseudomonas, Burkholderia and Rhodococcus (PBR). 3 Biotech 7:29. https://doi.org/10.1007/s13205-017-0598-8
Vaidya S, Devpura N, Jain K, Madamwar D (2018) Degradation of chrysene by enriched bacterial consortium. Front Microbiol 9:1333. https://doi.org/10.3389/fmicb.2018.01333
Wanapaisan P, Laothamteep N, Vejarano F, Chakraborty J, Shintani M, Muangchinda C, Morita T, Suzuki-Minakuchi C, Inoue K, Nojiri H (2018) Synergistic degradation of pyrene by five culturable bacteria in a mangrove sediment-derived bacterial consortium. J Hazard Mater 342:561–570. https://doi.org/10.1016/j.jhazmat.2017.08.062
Xie Y, Yu F, Wang Q, Gu X, Chen W (2014) Cloning of catechol 2,3-dioxygenase gene and construction of a stable genetically engineered strain for degrading crude oil. Indian J Microbiol 54:59–64. https://doi.org/10.1007/s12088-013-0411-2
Zhong Y, Zou S, Lin L, Luan T, Qiu R, Tam NF (2010) Effects of pyrene and fluoranthene on the degradation characteristics of phenanthrene in the co-metabolism process by Sphingomonas sp. strain PheB4 isolated from mangrove sediments. Mar Pollut Bull 60:2043–2049. https://doi.org/10.1016/j.marpolbul.2010.07.017
Zhong Y, Luan T, Lin L, Liu H, Tam NF (2011) Production of metabolites in the biodegradation of phenanthrene, fluoranthene and pyrene by the mixed culture of Mycobacterium sp. and Sphingomonas sp. Bioresour Technol 102:2965–2972. https://doi.org/10.1016/j.biortech.2010.09.113
Acknowledgements
The authors are thankful to Department of Biotechnology, Ministry of Science & Technology, Government of India, New Delhi (BT/01/CEIB/09/V/05) for financial support. Miss A. B. Patel gratefully acknowledged Council of Scientific and Industrial Research (CSIR), for providing Senior Research Fellowship (09/157(0054)2K18 EMR-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vaidya, S.S., Patel, A.B., Jain, K. et al. Characterizing the bacterial consortium ASDF capable of catabolic degradation of fluoranthene and other mono- and poly-aromatic hydrocarbons. 3 Biotech 10, 491 (2020). https://doi.org/10.1007/s13205-020-02478-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02478-w