Abstract
This study is aimed to investigate the protective effect against type 2 diabetes mellitus (T2DM) and Alzheimer’s disease (AD) of Berberine (BBR), and the underlying mechanism of action is explored. We established a rat model of combined AD and T2DM and used it to investigate the effect of BBR (150 mg/kg) on the course of these pathologies. The Morris water maze, biochemical analysis, hematoxylin–eosin staining, immunohistochemical study, immunofluorescent staining, TUNEL assay, RT-qPCR and western blot were used to reveal the effect of BBR on blood glucose, lipid changes, hippocampal injuries and cognitive impairment. The results showed that BBR could alleviate memory deficits, restore the disordered arrangement of nerve cells, the damage of neurons, improve TUNEL-positive cells and decrease the elevated levels of fasting blood glucose, triglyceride, total cholesterol and glycosylated serum protein levels in Alzheimer diabetic rats. Moreover, BBR treatment reduces the transcription of mRNAs and expression of proteins related to endoplasmic reticulum (ER) stress. These findings conclude that BBR can protect neurons by inhibiting the pathway of ER stress and thereby play an essential role in the preventive and therapeutic of AD and T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Berberine (BBR) is an isoquinoline alkaloid, isolated from C. chinensis. Many researches on clinical and preclinical have demonstrated that BBR is beneficial for diabetes (Bhutada et al. 2011; Cok et al. 2011; Gu et al. 2010; Lee et al. 2006). The increase of peripheral and central cholinergic nervous system activity is related to the antiamnesic effect of BBR (Peng et al. 1997). It has been reported that the neurobiological mechanism of BBR may contribute to clinical effects for Alzheimer’s disease (AD) as a result of decreasing the generation of amyloid-β peptide (Aβ) (Ji and Shen 2011; Haghani et al. 2015). However, whether BBR can ameliorate cognitive dysfunction and hyperglycemia or hyperlipidemia in Alzheimer diabetic rats remains unclear.
Type 2 Diabetes Melllitus (T2DM) is the most common disorder of glucose metabolism (Chen et al. 2011), mainly due to relative insulin resistance (Kahn 2003). Both AD and T2DM can cause cognitive dysfunction 41 (Luchsinger 2012). A multitude of studies have shown that diabetes mellitus affects the nervous system (Román-Pintos et al. 2016; Verdile et al. 2015; Surguchov 2020). Chronic hyperglycemia and hyperlipidemia are the main trigger factors for diabetic cognitive dysfunction (Cameron et al. 2001; Biessels et al. 2002).
AD is the most common form of dementia, with the accumulation of Aβ peptide as the main major pathological hallmark. Aβ-mediated imbalance of various signal cascades, causing damage to hippocampal function and structure is one of the earliest detectable features of AD (Boeve 2012; Cavallucci et al. 2012. In addition, cerebral insulin resistance is an important factor in the development of AD, which can be manifested as cognitive dysfunction and cerebral neurodegeneration (Arnold et al. 2018). Elevated Aβ induces the removal of insulin receptors on the cell surface, thereby further increasing insulin resistance. In turn, brain insulin resistance can affect Aβ clearance (Dineley et al. 2014). Up to 80% of AD patients may have diabetes or impaired fasting blood glucose. This coexistence of diabetes and cognitive dysfunction has led some researchers to propose the concept of “AD is Type 3 Diabetes Melllitus” (Monte et al. 2006; Kravitz et al. 2013). Disorder in biochemical indexes, abnormal morphology and function of the hippocampus, and Aβ deposition are the common pathological basis of AD and T2DM, which are also our exploration of this experiment.
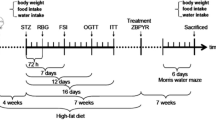
Endoplasmic reticulum (ER) stress-initiated apoptosis has a key function in the cognitive dysfunction secondary to T2DM (Wang et al. 2016a), and which is also considered to be an important aspect of the etiology of AD. In this study, we used the classical Morris water maze test to assess behavior, related biochemical analysis to assess blood glucose and lipid level, hematoxylin–eosin staining, immunohistochemical study, TUNEL assay to assess neuron pathology and cell apoptosis. Moreover, RT-qPCR and western blot experiments assessed protein and mRNA changes, to investigate the influence of BBR on the prevention and treatment of AD and T2DM, and the possible mechanisms of its effects by alleviating cognitive dysfunction through the regulation of pathology changes (Fig. 1).
Results
BBR treatment enhanced the cognitive ability in Alzheimer diabetic rats
As shown in Fig. 2, compared with the normal group, Alzheimer diabetic rats showed severe learning dysfunction in the Morris water maze test, which is manifested as prolonged escape latency (P < 0.01). However, contrast to the Alzheimer diabetic group, the escape latency of the BBR and DMBG groups were reduced (P < 0.05). The results suggested that BBR was beneficial in reducing cognitive dysfunction in Alzheimer diabetic rats.
BBR improved cognitive deficits in Alzheimer diabetic rats. a Swimming trajectory of rats in Normal group. b Swimming trajectory of rats in the Model group. c Swimming trajectory of rats in the BBR group. d Swimming trajectory of rats in DMBG group. e The time spent in the removed escape platform quadrant. Data are expressed as mean ± SD, n = 12. **P < 0.01 vs. normal group; #P < 0.05 vs. model group
BBR attenuated the disturbance of glucose in Alzheimer diabetic rats
As shown in Fig. 3, first, we detected the FBG before STZ injection. Compared with 72 h later of STZ injection, diabetic rats underwent high glucose (> 11.1 mmol/L) after modeling. After treatment of 28 days, BBR reversed elevated FBG. The results indicated that BBR has specific beneficial effects on FBG. Similar hypoglycemic effects were observed in DMBG group, considered administration of metformin as a positive control.
BBR attenuated the pathological changes of brain tissue in Alzheimer diabetic rats
As shown in Fig. 4, compared with the normal group (Fig. 4a), histological evaluation of brain tissue in Alzheimer diabetic rats showed significant hippocampal neuron injury and loss. Furthermore, the intercellular space became larger, and some cells were dead as characterized by cell shrinkage, pyknosis and dark red staining after lysis (Fig. 4b). However, the damage and loss of hippocampal neurons were significantly reduced after administration of BBR or metformin (Fig. 4c, d). After BBR treatment, the nerve cells were also arranged in an orderly manner (Fig. 4c). These results showed that BBR has an important inhibitory effect on neuron injury and loss in Alzheimer diabetic rats, which contributed to its improvement on cognitive dysfunction.
BBR reduces the damage of hippocampal neurons in Alzheimer diabetic rats [hematoxylin–eosin staining, ×400 (hippocampus) and × 6 (CA1, CA3, DG)]. a–d The representative Hematoxylin–eosin staining images of the hippocampus and enlarged CA1, CA3 and DG region in Normal, Model, BBR and DMBG group, respectively
BBR improved synaptophysin immunoreactivity in Alzheimer diabetic rats
Synaptophysin immunoreactivity is its punctate-like distribution pattern in all the subfields of the hippocampus. In the stratum pyramidale, the staining was visible on and around the neuronal perikarya. As shown in Fig. 5, in the normal group, the bouton-like structures in this region not only appeared to be more in number but also bigger in size as compared to other layers (Fig. 5a). In stratum radiatum and oriens, some of the neuronal perikarya showed punctate staining around or on them. There was a significant reduction in the synaptophysin immunoreactivity in all of the three layers in Alzheimer diabetic rats. The number of bouton-like structures also decreased considerably in the apical region of the principal neuron layer (Fig. 5b). After treatment with BBR or metformin, a reversal of the staining pattern was observed, which resembled more like that of normal group, but evidently there was an increase in the number of bouton-like structures (Fig. 5c, d).
BBR reduced Aβ deposition in Alzheimer diabetic rats
To further confirm the relationship between Aβ pathology and cognitive decline, we observed the expression characteristic of Aβ in the brains of model rats which exhibit remarkable Aβ deposits in the brain. As shown in Fig. 6, it was found that Aβ was remarkable detected in Alzheimer diabetic rats (Fig. 6b). Conversely, the immunoreactivity of Aβ was significantly decreased with BBR or metformin treatment versus model group. Above results suggested that Aβ induced in the cognitive dysfunction of diabetes rats and BBR could reduce Aβ deposition in Alzheimer diabetic rats.
BBR improved the glucose and lipid metabolism disorder in Alzheimer diabetic rats
To test the effect of BBR on glucose and lipid metabolism disorder, Alzheimer diabetic rats model were established by STZ and high-fat diet, which caused the disorders of blood glucose and lipid metabolism. As shown in Fig. 7, compared with the normal group, GSP level in model group was significantly increased (P < 0.01), While BBR and metformin ameliorated hyperglycemia, which manifested as decreased GSP level (P < 0.01, P < 0.01) (Fig. 7a). Moreover, a significant increase in TG and T-CHO contents in serum were observed in the model group, compared with the normal group (P < 0.01). However, after BBR or metformin treatment, the expression levels of TG and T-CHO were significantly decreased (P < 0.01) (Fig. 7b, c). These results suggested that BBR could improve glucose and lipid metabolism disorder in Alzheimer diabetic rats.
BBR rescued apoptosis of Alzheimer diabetic rats
TUNEL reaction is commonly used to detect DNA fragmentation caused by the cascade of apoptosis signals as late-stage apoptosis indicator. As shown in Fig. 8, the apoptotic cells in the model group were shown as brown, and the apoptotic cells significantly increased contrast to the normal group (Fig. 8b). However, BBR significantly decreased the apoptosis rate comparison of model group which inducing neurotoxicity (Fig. 8c). Similar neuroprotection effects were observed in DMBG group (Fig. 8d). The above findings led us to conclude that BBR played a key role in preventing apoptosis of Alzheimer diabetic rats.
BBR regulated mRNA expression levels of GRP78, CHOP, procaspase-12, procaspase-9 and procaspase-3 in the hippocampus of Alzheimer diabetic rats
GRP78, CHOP, procaspase-12, procaspase-9 and procaspase-3 are the main components of ER stress pathway involved in AD and T2DM, thus, we investigated whether BBR altered the ER stress pathway in Alzheimer diabetic rats. As shown in Fig. 9, compared with the normal group, GRP78, CHOP, procaspase-12, procaspase-9 and procaspase-3 expression were obviously increased in Alzheimer diabetic rats, respectively, indicating that the ER stress was activated in the hippocampus of Alzheimer diabetic rats (P < 0.05, P < 0.01). After BBR and metformin treatment, the expression of GRP78, CHOP, procaspase-12, procaspase-9 and procaspase-3 expression levels were significantly decreased (P < 0.01, P < 0.05). Therefore, our results indicated that BBR could reduce the levels of GRP78, CHOP, procaspase-12, procaspase-9 and procaspase-3 in the Alzheimer diabetic rats’ hippocampus.
BBR regulated main protein expression levels of ER stress in the hippocampus of Alzheimer diabetic rats
To investigate whether BBR could alter the ER stress pathway in Alzheimer diabetic rats, we detected the expression levels of GRP78, CHOP, Caspase-12, Caspase-9 and Caspase-3, the main proteins of ER stress pathway in Alzheimer diabetic rats. As shown in Fig. 10, compared with the normal group, the main proteins expression were obviously increased in Alzheimer diabetic rats, which indicated that the ER stress was activated in the hippocampus in Alzheimer diabetic rats (P < 0.01). After BBR and metformin treatment, the expression levels of related proteins were significantly decreased (P < 0.05, P < 0.01). Our results indicated that BBR could reduce the protein expression of GRP78, CHOP, Caspase-12, Caspase-9 and Caspase-3 in the hippocampus of Alzheimer diabetic rats which related to ER stress.
BBR inhibited the protein expression of GRP78, CHOP, Caspase-12, Caspase-9 and Caspase-3 levels in the hippocampus of Alzheimer diabetic rats. Western blot (a–e) assay was used to measure the effects of BBR on the levels of GRP78, CHOP, Caspase-12, Caspase-9 and Caspase-3 in the hippocampus of Alzheimer diabetic rats. The protein level was quantified using β-actin as the loading controls. Data are expressed as mean ± SD, n = 3. **P < 0.01 vs. normal group; ##P < 0.01 vs. Model group
Discussion
Currently neurodegenerative diseases and T2DM are the most devastating disorders with a considerable overlap in risk factors, complications and pathophysiological mechanisms (Surguchov 2016). Increasing evidences showed that hyperglycemia and hyperlipidemia are considered as a determinant reason of cognitive decline in diabetes, indicating that control of blood glucose and blood lipid is necessary to improve the cognitive deficit caused by diabetes. Epidemiological investigations have found that 70–80% of AD patients have T2DM or abnormal blood glucose or insulin levels (Janson et al. 2004; Mwamburi and Qiu 2016). Long-term hyperglycemia not only causes neuropathy of the peripheral nervous system but also causes neuropathy of the central nervous system. High glucose plays an important role in metabolic disorders in diabetic patients which is also a major risk factor for nerve damage (Zhang et al. 2016).
Aβ is a complex biological molecule which interacts with many types of receptors forms insoluble assemblies, and its non-physiological depositions alternate with the normal neuronal conditions. In this situation, AD signs appear and patients experience marked cognitional disabilities (Sadigh et al. 2015). Chronic hyperglycemia promotes Aβ deposition (Huang et al. 2007) and increases the sensitivity of nerve cells, which producing Aβ neurotoxicity in brain microvascular endothelial cells (Carvalho et al. 2014). Based on the common pathological, we established Alzheimer diabetic rats model with STZ and injection of Aβ25–35. Our findings showed that Alzheimer diabetic rats had hyperglycemia, hyperlipidemia, memory declined, neuron cells damage and Aβ deposition in hippocampus. These behavioral and pathological results further confirmed the reliability of this animal model. However, BBR treatment could improve these abnormalities.
ER pathway is a newly discovered apoptosis pathway, which core is ER stress. In pathological conditions, long-term excessive stress leads to the failure of ER function to return to normal, and ER stress can activate the apoptosis pathway and lead to the injury or death of nerve cells (Cameron 2013). High glucose increases ER stress levels, and ER stress-dependent lipid metabolism disorders may cause dyslipidemia (Escribano-Lopez et al. 2019). In addition, activation of ER stress pathway could induce neuronal injuries and following damage of synaptic structure and function of learning and memory (Kong et al. 2018). Glucose-regulated protein 78 (GRP78) is a member of the heat shock protein family localized in the ER lumen (Lee 2005). When cells are subjected to ER stress, GRP78 is titrated by binding to the folded protein, thus activating the PERK signaling pathway. PERK dimerizes and self-phosphorylates, thereby phosphorylating its two main substrates eIF2a and Nrf2. Phosphorylation of eIF2a activates C/EBP-homologous protein (CHOP), which lead to apoptosis. GRP78 and CHOP drive the cell destiny after ER stress (Zhao et al. 2015). After releasing from GRP78, Cysteinyl aspartate specific proteinase-12 (Caspase-12) is activated, followed by activation of Cysteinyl aspartate specific proteinase-9 (Caspase-9). Then, this in turn activates the downstream Cysteinyl aspartate specific proteinase-3 (Caspase-3) and initiates the classical apoptosis pathway of the caspase-family, eventually leading to apoptosis (Luo and Lee 2013). GRP78, Chop, Caspase-12, Caspase-9 and Caspase-3 in the ER stress pathway were selected to explore the common pathogenesis of AD and T2DM, so as to further explore the neuroprotective effect of BBR.
BBR has a dual effect in declining the elevated contents of hyperlipidemia and hyperglycemia in diabetic rats (Brusq et al. 2006; Kong et al. 2004; Zhou et al. 2008). Meanwhile, BBR also contributes to clear and inhibit Aβ deposition in the brain (Huang et al. 2017). BBR can regulate hyperglycemia and alleviate the oxidative stress in diabetes (Liu et al. 2015; Li et al. 2019). In addition, recent studies have revealed that BBR can significantly improve synaptic pathology, alleviate Aβ pathology and gliosis, as well as improve cognitive and memory dysfunction in AD (Huang et al. 2016; Durairajan et al. 2012). Altogether, the above findings led us to explore the neuroprotective effect of BBR associated with T2DM and AD and its underlying mechanism. Our findings showed that the mRNA and protein expression of GRP78, CHOP, Caspase-12, Caspase-9 and Caspase-3 were increased in Alzheimer diabetic rats while the mRNA and protein expression of GRP78, CHOP, Caspase-12, Caspase-9 and Caspase-3 were significantly decreased with BBR treatment, which indicating that the neuroprotective of BBR on AD and T2DM may be related to inhibition of ER stress.
Conclusion
In conclusion, our findings demonstrated the protective effect of BBR on cognitive dysfunction in STZ and Aβ25–35 induced Alzheimer diabetic rats, which may be attributed to its ability of reversing FBG and lipids, alleviating neuron cells and synapsis injury, clearance of Aβ deposition in the brain and suppressing ER stress. These data suggested that BBR could be beneficial for the improvement of T2DM and AD. Future research should explore the potential common mechanism between T2DM and AD, and investigate more beneficial prevention and treatment methods of other Chinese herbs in T2DM and AD.
Materials and methods
Experimental animals
Male Sprague–Dawley rats aged 9 weeks (220–250 g) were obtained from Zhejiang Experimental Animal Center (Zhejiang, China). All procedures were approved by the Center of Scientifc Research of Anhui University of Chinese Medicine. The animal use protocol was in accordance with the Regulations for the Use of Laboratory Animals issued by the State Science and Technology Commission. All experimental subjects were allowed to acclimatize to the laboratory environment with food and water available for 7 days before the experiment.
Chemicals and drugs
Streptozotocin (STZ) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Amyloid-β peptide (25–35) (Aβ25–35) was obtained from Sigma-Aldrich (St. Louis, MO, USA), steamed with sterile double configuration into 2 μmol·L−1 for 5 days incubation solution in 37 °C constant temperature box before use. BBR was provided by Northeast Pharmaceutical Group Shenyang NO.1 Pharmaceutical CO., LTD (Shenyang, China). Metformin (DMBG) was provided by Merck Serono(Shanghai, China). Portable glucometer and test paper were obtained from Yicheng (Beijing, China).
Establishment of rat models and treatment
After the adaptive feeding of 48 rats for 7 days, 12 rats were fed with basic feed without any treatment as normal control (Normal), other 36 rats were organized into three groups, each of 12 individuals, the details are as below: Alzheimer diabetic rats without any drug treatment (Model); Alzheimer diabetic rats treated with BBR in a dose of 150 mg/kg (BBR, equivalent to three times the clinical dose of adults) (Wang et al. 2011; Wang et al. 2016b); Alzheimer diabetic rats treated with metformin in dose of 540 mg/kg (DMBG, equivalent to three times the clinical dose of adults, as a positive control).
Alzheimer diabetic rats were established by intraperitoneal injection of 35 mg/kg STZ in combination with a bilateral hippocampal injection of 10 μg Aβ25–35. Firstly, the diabetic rat model was established. Rats of model group, BBR group and DMBG group were fed with high-fat diet (70% standard diet, 10% lard, 5% yolk powder, 0.5% cholesterol, 10% sucrose) for 4 weeks. At the last day of the 4th week, rats in each group began to fast besides water at 5:00 PM 1 day before injection of STZ. At 9:00 am the next day, rats were injected with STZ (35 mg/kg b.w.) which dissolved in freshly prepared 0.1 mol/L sodium citrate buffer (pH 4.4). 72 h later, fasting blood glucose (FBG) was measured by portable glucometer from the tail vein. Rats with FBG levels of 11.1 mmol/L or higher were considered as a successful diabetic model and were used in subsequent experiments. Secondly, Alzheimer diabetic rats model were established. 7 days later, all diabetic rats were anesthetized and fixed rats on brain stereotaxic instrument. Two holes were drilled by dental auger after defined points stereotaxically. Two points were in bilateral hippocampal (behind the bregma 4.4 mm, meta 2.2 mm, deep 3.0 mm). The left hippocampus was localized first, then the right hippocampus. Rats were injected with Aβ25–35 with 5 μL (10 μg) each side. Increased escape latency in Morris water maze results indicated that Alzheimer diabetic rats model were established successfully compared with the normal group.
Model rats were treated with BBR and metformin for 28 days, respectively. At the same period, Model group rats were fed with high-fat food without drug treatment, and normal group rats were fed basic feed without any intervention, totally for 28 days (Ghumatkar et al. 2019; Wang et al. 2018) (Fig. 1).
Sample preparation
After drug treatment, six rats selected from each group were executed. Brain tissues were quickly removed on saline ice. Hippocampal tissues were isolated from both sides of the brain and stored at − 80 °C for western blot experiments.
The remaining rats in each group underwent cardiac perfusion and fixation. After anesthesia as shown above, the chest of rats were opened and exposed their hearts. The infusion needle was inserted from the apex and the right atrial appendage was cut. After perfusion with 100–200 mL normal saline through the heart, 250–300 mL 4% paraformaldehyde was perfused until the body was rigid. The brain was removed carefully and fixed in 4% paraformaldehyde, three rats for hematoxylin–eosin staining and other three rats for immunohistochemical study, TUNEL assay and immunofluorescent staining.
Morris water maze test
Escape latency was measured by the Morris water maze test, which measures learning and memory ability. The test was conducted in a black circular pool (diameter: 120 cm) which temperature of the water was kept at 25 °C ± 2 °C. Place the black circular platform (diameter: 9 cm) 1 cm below the surface of the water in a fixed location in the middle of the pool. There are four equally spaced quadrants in the pool. Rats were subjected to daily training trials in each quadrant for four days (once a day), each limited to 90 s.
Each experiment ends when rats swam reached the submerged platform and stay on the platform for 5 s. On the fifth day, the rats were placed in the pool with their backs to the platform and record their escape latency as final results. If the rat did not find the platform in 90 s, the time was recorded as 90 s. Data collection and processing was completed by Morris water maze image automatic monitoring and processing system (ZH, Huaibei, Anhui Province, China).
Metabolic changes studies
To test the blood glucose changes and assess the model establishment, before injection of STZ, all rats performed FBG (overnight fasting for 12 h) with a portable glucometer (Yicheng, Beijing, China) using tail vein blood. After injection of STZ, FBG was determined at 72 h post-injection. Before sacrifice, all rats were fasted for 12 h and detected FBG levels.
Hematoxylin–eosin staining
Hematoxylin–eosin (H&E) staining detect morphological changes in the rat hippocampus. After the entire brain was stored overnight in 10% formalin, the brain was embedded in paraffin for 4 h. Preparing paraffin blocks were prepared and 5 μm coronal sections of hippocampus were cutting with a microtome. Sections were mounted on silane-coated slides, washed in xylene to dewax, rehydrated in graded ethanol, and finally stained with H&E (Servicebio, Wuhan, China). Using light microscopy (Nikon Eclipse E100, Japan) to observed and captured hippocampal neurons morphology at 400× magnification.
Immunohistochemical study
Paraffin sections were dewaxed and repaired in a repair box filled with citrate antigen repair buffer (PH6.0). After washing three times with PBS, incubated in 3% hydrogen peroxide solution. After washing with PBS, the serum was blocked and incubated overnight with Synaptophysin antibody. After washing with PBS, add secondary antibody to incubate, added DAB chromogenic solution, re-stained the cell nucleus, dehydrated and sealed the slide, observed and captured hippocampal neurons morphology at 400× magnification.
Immunofluorescent staining
Immunofluorescent staining was used to detect Aβ deposition in the brain of Alzheimer diabetic rats. The slides were washed with PBS 5 min three times, incubated with BSA for 30 min and Aβ antibodies for 24 h. The sections were incubated with anti-rat secondary antibodies for 50 min after washing. Then, the slides were washed with PBS and add DAPI incubated for 50 min. Finally, slides were rinsed and covered with anti-fluorescence quenching sealing tablets. Images were captured by fluorescent microscopy (Nikon Eclipse C1, Japan), and analyzed by IPP 6.0 (Image-pro plus 6.0, USA).
Biochemical analysis
To analyze the effects of BBR on blood glucose and lipid metabolism disturbances, at the end of the experiment period, Glycated Serum Protein (GSP), Total Glyceride (TG) and Total Cholesterol (T-CHO) were tested. After fasting for 12 h, rats were anesthetized and blood samples were taken from abdominal aorta without EDTA for serum separation (3000 r/min,15 min). The biochemical indexes including GS, TG and T-CHO were tested using commercial diagnostic kits (Jiancheng, Nanjing, China).
TUNEL assay
TUNEL assay was used to observe apoptosis in hippocampus. The paraffin sections were dewaxed, rehydrated, and treated with proteinase K for 25 min and rinsing three times with PBS (5 min each). TUNEL assay kit (Roche, Basel, Switzerland) was used to stain according to the manufacturer protocol. Removing PBS and colorized with DAPI. Slides were rinsed three times (5 min each) in PBS and mounted by anti-fluorescence quenching and blocking tablets reagent. Using light microscope at 400× magnification (CIC XSP-C204, Chongqing, China) to counted TUNEL-positive cells with three random regions of each sample. The percentage of apoptotic cells were calculated by IPP 6.0 (Image-pro plus 6.0, USA).
RT-qPCR
Fluorescent quantitative polymerase chain reaction assays were used to detect the mRNA expression of GRP78, CHOP, procaspase-12, procaspase-9 and procaspase-3 in the rat hippocampus. Total RNA was isolated from hippocampus tissues using Trizol reagent (Ambion, USA), according to manufacturer’s instructions. The complementary cDNA was synthesized from the total RNA sample using RevertAid First Strand cDNA Synthesis Kit (Thermo scientific, Massachusetts, USA). Quantitative PCR was performed on 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) using SYBR green kit (TOYOBO, Japan) with β-actin as control, and a mixed cDNA sample control was incorporated into each PCR cycle, each sample repeat three holes. All target gene primers were designed using the comparative Ct (2−ΔΔCt) (cycle threshold) method. The specific primers used for the current study are listed in Table 1.
Western blot
Western blot assays were used to detect the protein expression of GRP78, CHOP, Caspase-12, Caspase-9 and Caspase-3 in the rat hippocampus. The total protein of the tissue was extracted with RIPA lysate containing 1% protease inhibitor and was denaturated by boiling water and stored at – 20 °C. Protein concentration was calculated by BCA protein concentration assay kit (Beyotime, Jiangsu, China). Afterward, the protein was separated by 10% SDS-PAGE electrophoresis and transferred to PVDF membrane (120 V, 2 h). Subsequently, the membrane was blocked in 5% skimmed milk on a shaker for 2 h and washed with TBST for three times (each time for 10 min). Then, the rabbit anti-rat monoclonal primary antibody GRP78, CHOP (1:1300, Affinity Bioscience, USA), Caspase-12, Caspase-9, Caspase-3 (1:1000, Bioss, Beijing, China)were added. After overnight incubation at 4 °C, primary antibody was removed by washing with TBST for three times (each time for 10 min), followed by adding the goat anti-rabbit IgG (1:20,000; ZSGB-BIO, Beijing, China) to combine with the primary antibody. And then, placing it on a shaker at room temperature and slowly shaken for 2 h. After washing with TBST for three times (each time for 10 min), we detected the protein levels by ECL chemiluminescence, automatic exposure in the system of the gel imaging device (ProteinSimple, CA, USA). The ratio of the target protein band to the β-actin band was used to determine the relative protein expression. The bands were visualized and the grayscale values were analyzed by the Image J. All the experiments were repeated three times.
Statistical analysis
Data were expressed as mean values ± SD. SPSS 24.0 software (SPSS, Chicago, IL, USA) were used for statistical analysis. The data were compared by one-way ANOVA followed by the least significant difference test (homogeneity of variance) or Tamhane post hoc test (heterogeneity of variance). The differences were considered statistically significant at P < 0.05.
References
Arnold SE, Arvanitakis Z, Macauley Rambach SL, Koenig AM, Wang HY, Ahima RS, Craft S, Gandy S, Buettner C, Stoeckel LE, Holtzman DM, Nathan DM (2018) Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol 14:168–181
Bhutada P, Mundhada Y, Bansod K, Tawari S, Patil S, Dixit P, Umathe S, Mundhada D (2011) Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav Brain Res 220:30–41
Biessels GJ, van der Heide LP, Kamal A, Bleys RLA, Gispen WH (2002) Ageing and diabetes: implications for brain function. Eur J Pharmacol 441:1–14
Boeve BF (2012) Mild cognitive impairment associated with underlying Alzheimer’s disease versus Lewy body disease. Parkinsonism Relat Disord 18:S41–44
Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, Issandou M (2006) Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res 47:1281–1288
Cameron NE (2013) Role of endoplasmic reticulum stress in diabetic neuropathy. Diabetes 62:696–697
Cameron NE, Eaton SE, Cotter MA, Tesfaye S (2001) Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 44:1973–1988
Carvalho C, Katz PS, Dutta S, Katakam PV, Moreira PI, Busija DW (2014) Increased susceptibility to amyloid-β toxicity in rat brain microvascular endothelial cells under hyperglycemic conditions. J Alzheimers Dis 38:75–83
Cavallucci VD, Amelio M, Cecconi F (2012) A beta toxicity in Alzheimer%26apos;s disease. Mol Neurobiol 45:366–378
Chen L, Magliano DJ, Zimmet PZ (2011) The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nat Rev Endocrinol 8:228–236
Cok A, Plaisier C, Salie MJ, Oram DS, Chenge J, Louters LL (2011) Berberine acutely activates the glucose transport activity of GLUT1. Biochimie 93:1187–1192
de la Monte SM, Tong M, Lester-Coll N, Plater M Jr, Wands JR (2006) Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer’s disease. J Alzheimers Dis 10:89–109
Dineley KT, Jahrling JB, Denner L (2014) Insulin resistance in Alzheimer’s disease. Neurobiol Dis 72:92–103
Durairajan SS, Liu LF, Lu JH, Chen LL, Yuan Q, Chung SK, Huang L, Li XS, Huang JD, Li M (2012) Berberine ameliorates β-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer’s disease transgenic mouse model. Neurobiol Aging 33:2903–2919
Escribano-Lopez I, Bañuls C, Diaz-Morales N, Iannantuoni F, Rovira-Llopis S, Gomis R, Rocha M, Hernandez-Mijares A, Murphy MP, Victor VM (2019) The Mitochondria-targeted antioxidant MitoQ modulates mitochondrial function and endoplasmic reticulum stress in pancreatic β cells exposed to hyperglycaemia. Cell Physiol Biochem 52:186–197
Ghumatkar PJ, Patil SP, Peshattiwar V, Vijaykumar T, Dighe V, Vanage G, Sathaye S (2019) The modulatory role of phloretin in Aβ25-35 induced sporadic Alzheimer’s disease in rat model. Naunyn Schmiedebergs Arch Pharmacol 392:327–339
Gu Y, Zhang Y, Shi X, Li X, Hong J, Chen J, Gu W, Lu X, Xu G, Ning G (2010) Effect of traditional Chinese medicine berberine on type 2 diabetes based on comprehensive metabonomics. Talanta 81:766–772
Haghani M, Shabani M, Tondar M (2015) The therapeutic potential of berberine against the altered intrinsic properties of the CA1 neurons induced by Abeta neurotoxicity. Eur J Pharmacol 758:82–88
Huang HJ, Liang KC, Chen CP, Chen CM, Hsieh-Li HM (2007) Intrahippocampal administration of Aβ1-40 impairs spatial learning and memory in hyperglycemic mice. Neurobiol Learn Mem 87:483–494
Huang M, Chen S, Liang Y, Guo Y (2016) The role of berberine in the multi-target treatment of senile dementia. Curr Top Med Chem 16:867–873
Huang M, Jiang X, Liang Y, Liu Q, Chen S, Guo Y (2017) Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp Gerontol 91:25–33
Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC (2004) Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 53:474–481
Ji HF, Shen L (2011) Berberine: a potential multipotent natural product to combat Alzheimer’s disease. Molecules 16:6732–6740
Kahn SE (2003) The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46:3–19
Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, Wang S, Wu J, Wang Y, Li Z, Liu J, Jiang JD (2004) Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med 10:1344–1351
Kong FJ, Ma LL, Guo JJ, Xu LH, Li Y, Qu S (2018) Endoplasmic reticulum stress/autophagy pathway is involved in diabetes-induced neuronal apoptosis and cognitive decline in mice. Clin Sci 132:111–125
Kravitz E, Schmeidler J, Schnaider Beeri M (2013) Type 2 diabetes and cognitive compromise: potential roles of diabetes-related therapies. Endocrinol Metab Clin North Am 42:489–501
Lee AS (2005) The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 35:373–381
Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB (2006) Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 55:2256–2264
Li M, Dang Y, Li Q, Zhou W, Zuo J, Yao Z, Zhang L, Ji G (2019) Berberine alleviates hyperglycemia by targeting hepatic glucokinase in diabetic db/db mice. Sci Rep 9:8003
Liu C, Wang Z, Song Y, Wu D, Zheng X, Li P, Jin J, Xu N, Li L (2015) Effects of berberine on amelioration of hyperglycemia and oxidative stress in high glucose and high fat diet-induced diabetic hamsters in vivo. Biomed Res Int 2015:313808
Luchsinger JA (2012) Type 2 diabetes and cognitive impairment: linking mechanisms. J Alzheimers Dis 30(Suppl 2):S185–198
Luo B, Lee AS (2013) The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene 32:805–818
Mwamburi M, Qiu WQ (2016) Different associations of premorbid intelligence vs. current cognition with BMI, insulin and diabetes in the homebound elderly. Integr Mol Med 3:547–552
Peng WH, Hsieh MT, Wu CR (1997) Effect of long-term administration of berberine on scopolamine-induced amnesia in rats. Jpn J Pharmacol 74:261–266
Román-Pintos LM, Villegas-Rivera G, Rodríguez-Carrizalez AD, Miranda-Díaz AG, Cardona-Muñoz EG (2016) Diabetic polyneuropathy in type 2 diabetes mellitus: inflammation, oxidative stress, and mitochondrial function. J Diabetes Res 2016:3425617
Sadigh-Eteghad S, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, Mahmoudi J (2015) Amyloid-beta: a crucial factor in Alzheimer’s disease. Med Princ Pract 24:1–10
Surguchov A (2016) Association between type-2 diabetes and parkinson’s disease: a cross-talk between amylin and α-synuclein. Diabetes Metab Syndr Clin Res Rev 1:1–7
Surguchov A (2020) Caveolin: a new link between diabetes and Alzheimer’s disease. Mol Cell Neurobiol. https://doi.org/10.1007/s10571-020-00796-4
Verdile G, Fuller SJ, Martins RN (2015) The role of type 2 diabetes in neurodegeneration. Neurobiol Dis 84:22–38
Wang Y, Campbell T, Perry B, Beaurepaire C, Qin L (2011) Hypoglycemic and insulin-sensitizin geffects of berberine in high-fat diet- and streptozotocin-induced diabetic rats. Metabolism 60:298–305
Wang B, Xu X, He X, Wang Z, Yang M (2016a) Berberine improved aldo-induced podocyte injury via inhibiting oxidative stress and endoplasmic reticulum stress pathways both in vivo and in vitro. Cell Physiol Biochem 39:217–228
Wang Z, Huang Y, Cheng Y, Tan Y, Wu F, Wu J, Shi H, Zhang H, Yu X, Gao H, Lin L, Cai J, Zhang J, Li X, Cai L, Xiao J (2016b) Endoplasmic reticulum stress-induced neuronal inflammatory response and apaptosis likely plays a key role in the development of diabetic encephalopathy. Oncot arget 7:78455–78472
Wang Y, Shen J, Yang X, Jin Y, Yang Z, Wang R, Zhang F, Linhardt RJ (2018) Akebia saponin D reverses corticosterone hypersecretion in an Alzheimer’s disease rat model. Biomed Pharmacother 107:219–225
Zhang Z, Li B, Meng X, Yao S, Jin L, Yang J, Wang J, Zhang H, Zhang Z, Cai D, Zhang Y, Ning G (2016) Berberine prevents progression from hepatic steatosis to steatohepatitis and fibrosis by reducing endoplasmic reticulum stress. Sci Rep 6:20848
Zhao Y, Yan Y, Zhao Z, Li S, Yin J (2015) The dynamic changes of endoplasmic reticulum stress pathway markers GRP78 and CHOP in the hippocampus of diabetic mice. Brain Res Bull 111:27–35
Zhou JY, Zhou SW, Zhang KB, Tang JL, Guang LX, Ying Y, Xu Y, Zhang L, Li DD (2008) Chronic effects of berberine on blood, liver glucolipid metabolism and liver PPARs expression in diabetic hyperlipidemic rats. Biol Pharm Bull 31:1169–1176
Funding
This study was supported by the National Natural Science Foundation of China (81574040 and 81873351) and the Key Project Foundation of Support Program for the Excellent Young Faculties in Universities of Anhui Province in China (Grant no. gxyq ZD2018053).
Author information
Authors and Affiliations
Contributions
Conceptualization, YW and BC; data curation, HW, PZ and JW; formal analysis, WX; Investigation, WX; Methodology, YW and BC; project administration, WX, HW, TY, HG, SY and MC; supervision, YW and BC; validation, YW and BC; writing-original draft, WX and HW; writing-review and editing, PZ, YW, BC and HS.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures were approved by the Animal Management Center of Anhui University of Chinese Medicine, China. The protocol of animal use consists with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
Rights and permissions
About this article
Cite this article
Xuan, Wt., Wang, H., Zhou, P. et al. Berberine ameliorates rats model of combined Alzheimer’s disease and type 2 diabetes mellitus via the suppression of endoplasmic reticulum stress. 3 Biotech 10, 359 (2020). https://doi.org/10.1007/s13205-020-02354-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02354-7