Abstract
The present study aimed to verify the effect of methanolic extract, fractions, and phenolic compounds of Eugenia mattosii D. Legrand leaves on the aorta relaxation. Isometric tensions were measured on the aorta of normotensive (NTR) and spontaneously hypertensive rats (SHR). The results showed that both methanolic extracts of leaves and stems, as well as, fractions obtained from leaves were able to induce a concentration-dependent relaxation in both endothelium-intact and -denuded aortas. The methanolic extract of leaves (ME-leaves) was the most effective since the maximal relaxation (≈ 83%) obtained was at the concentration of 300 μg/mL. As the endothelium-dependent relaxation was more significant, we investigated the mechanisms by which ME-leaves induced this effect. After the pretreatment with LNAME, ME-leaves-induced relaxation was decreased in the aorta of NTR and SHR. However, the pretreatment with methylene blue only reduced the relaxation in the aorta of NTR. Furthermore, pretreatment with ME-leaves decreased phenylephrine-induced contraction in preparation Ca2+-free only in aortic rings from NTR. This study also reveals that both compounds, cryptostrobin isolated from chloroform fraction and catechin from the ethyl acetate fraction induced a marked relaxation in endotheliumintact aortic rings of NTR. In conclusion, ME-leaves induces relaxation in the rat aorta involves the modulation of NO/cGMP dependent signaling pathway, this mechanism may at least, in part, explain the endothelium-dependent relaxation. Furthermore, cryptostrobin and catechin also induced relaxation, which may contribute synergistically to the vasorelaxation effect of the ME-leaves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Eugenia genus is one of the largest within the Myrtaceae family, presenting about 5000 species (Limberger et al. 2004; Fiuza et al. 2008). In Brazil, there are 350 native species from this genus (Landrum and Kawasaki 1997). Eugenia species are appreciated for their fruits such as Eugenia uniflora (pitanga), Eugenia involucrate (cereja or cherry) and Eugenia caryophyllata (cravo-da-índia). Other is used in folk medicine as antidiarrheic (E. uniflora) and antidiabetic (Eugenia jambolana) (Limberger et al. 2004; Lopes et al. 2000). Previous studies demonstrate that the Eugenia genus is an important source of medicinal plants, that can present pharmacological promising activity for the treatment of several diseases, including cardiovascular diseases. Infusions or hot water extracts of E. uniflora are used as an antihypertensive and diuretic in traditional medicine (Amat and Yajiá 1991; Bandoni et al. 1972; Ratera and Ratera 1980). Indeed, Consolini et al. (1999) found that aqueous extract of leaves of E. uniflora has a hypotensive effect mediated by direct vasodilatation and diuretic activity. However, the mechanism has not been investigated yet. Moreover, hydroalcoholic extracts of leaves from E. uniflora had a relaxation endothelium-dependent effect on aortic rings pre-contracted with noradrenaline. This vasodilatation was mediated by nitric oxide (Wazlawik et al. 1997). Besides, in one study performed on isolated heart of rats, E. Jambolana aqueous extract from leaves was able to exert cardiovascular activity (Consolini and Sarubbio 2002; Shukla et al. 2014). Recently Santos et al. (2018) demonstrate that methanolic extracts from Eugenia mattossi D. Legrand fruits reduced the injured areas against ethanol and indomethacin-induced gastric ulcer. It has been demonstrated that endogenous or exogenous substances that can maintain or increase gastric blood flow, due to their vasodilatory properties prevented the acute gastric injury (Magierowski et al. 2017; Farrugia and Szurszewski 2014).
Eugenia mattosii is a bush popularly known as “cerejinha de mattos” and despite the large distribution of this species, especially in Southern Brazil, the pharmacological profiles of this plant have not been investigated yet. For that, the purpose of this study was to demonstrate the effect and the mechanism of vasodilatation of the methanolic extract (ME) of E. mattosii dried leaves and stems, as well as their fractions and isolated compounds on aortic rings from normotensive and spontaneously hypertensive rats.
Many studies describe the potential of natural products for the treatment of cardiovascular diseases. Since blood pressure depends on the arterial tone and considering the therapeutic potential of the compounds described for the genus, as well as of others already studied species, we highlight the potential of the species Eugenia mattosii D. Legrand.
Arterial hypertension is a multifactorial disease characterized by increased blood pressure, arising from a complex interaction between genes and the environment. Even with unknown etiology, the study in an animal model of hypertension has shown to be an important tool for understanding the mechanisms that control blood pressure and allowing the development of new drugs for its treatment or prevention. Biological studies with animals are developed to understand changes in blood pressure and that several vascular beds are used. Although the aortic artery does not effectively participate in blood pressure control, the study in this vessel contributes to the compression of the cellular mechanisms involved in these pathologies. Besides, the use of the aorta artery allows the use of a reduced number of animals since from an aorta artery it is possible to use approximately eight rings.
Thus, based on these data and the wide distribution of the Eugenia genus in the south of Brazil and the use of several species by the population with their already confirmed pharmacological effects, we hypothesized that E. mattosii D. Legrand could have a vasodilatory effect.
Materials and methods
Animals
The experiments were performed with normotensive male Wistar rats (NTR: 200–250 g) and male spontaneously hypertensive rats (SHR: 250–280 g). The animals were provided by The Central Animal Facility of Universidade do Vale do Itajaí (UNIVALI) and kept under conditions of constant temperature (22 ± 2 °C) with a 12 h light/12 h dark cycle with free accesses to water and food.
The studies and all methodologies used were approved by the Ethical Committee for the Care and the Use of animals of (UNIVALI) (Authorization No 055/17p), which adopted all the recommendations of the Guide for the Care and Use of Laboratory Animals and approved on November 10, 2017.
Plant material
The leaves and stems from E. mattosii were collected in the city of Itajaí-SC, Brazil.
The plant material was identified by Prof. Oscar Iza (Universidade do Vale do Itajaí, UNIVALI) and a voucher specimen was deposited at the Barbosa Rodrigues Herbarium under Number VCFilho 150, in Itajaí-SC, Brazil.
Preparation of extracts, fractions, isolation of compounds and phytochemical screening
Extracts, fractions, and compounds isolation and identification were provided by Vechi et al. (2019). Briefly, fresh leaves (560 g) and stems (700 g) of E. mattosii were minced and extracted separately by maceration with methanol at room temperature for 7 days. The solvent was filtered and concentrated in a rotary evaporator under reduced pressure and at a constant temperature (50 °C). After the elimination of methanol, the methanolic extract (ME-leaves 15.34% and ME-stems 2.75%), have been partitioned with solvents of increasing polarity (chloroform and ethyl acetate) to obtain the respective fractions and yields: chloroform fractions (CLF-leaves) (5.43%), CLF-stems (0.51%), ethyl acetate fractions (AEF-leaves) (2.77%) and AEF-stems (0.15%). Both fractions were submitted to a chromatographic column, separately, to isolate the major compounds. The chloroform leaf fraction (15 g) was submitted to open silica gel column chromatography (CC) eluted with hexane: acetone gradient, resulting in the collection of 183 subfractions (10 mL each). Similar subfractions were combined based on their thin layer chromatography (TLC) profiles, and re-chromatographed as before. Subfractions 75–89 (664 mg) furnished a pure solid, identified as pinostrobin (362 mg), while subfractions 153–177 (1056 mg) led to the isolation of 84 mg of cryptostrobin (Massaro et al. 2014; Solladié et al. 1999). The ethyl acetate fraction (12 g) was also submitted to chromatographic procedures as indicated before, using chloroform: methanol gradient. About 138 subfractions were collected and combined according to similarity. Sub fractions 48–56 (233 mg) was re-chromatographed given catechin (19 mg). The compounds were identified based on TLC and spectral data (NMR) in comparison with standard sample and literature, respectively (Fig. 1) (Rosandy et al. 2013; Moresco et al. 2016; Wishart et al. 2009). Qualitative phytochemical screening of the ME-leaves and stems was performed to evaluate the presence or absence of flavonoids, anthraquinones, alkaloids, saponins, tannins, and terpenoids according to the previously described method by Biavatti and Leite (2005).
Estimation of total phenolic and flavonoids content
The content of total phenols was determined using the Folin–Ciocalteau reagent. In brief, about 50, 100, 150 and 200 mg/mL of extracts of ME-leaves and stems were mixed with 5 mL of distilled water. After 2.5 mL of Folin-Ciocalteu reagent (1: 1 dilution) and 2.0 mL of sodium carbonate (7.5% w/v) were added to each sample. Besides, the Falcons’ tubes were incubated at 45 °C for 15 min. The absorbance was determined at a 760 nm spectrophotometer. The total polyphenol concentration was calculated from a calibration curve, using tannic acid as a standard. Results were expressed as tannic acid equivalents (TAE) in mg. The flavonoids content was evaluated using the method of Dowd. In this methodology, 500 µL of aluminum chloride (AlCl3), prepared in 2% of methanol were mixed with the same volume of extract solution (400–100 µg/mL) or distilled water (for white). In this protocol, white was used as a negative control to assure us of the positive results. It does not contain extract. Quercitrin was used as a standard for the calibration curve. The total flavonoids quantity was expressed in mg quercitrin equivalent per gram of ME-leaves and-stems.
In vitro 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assay
The reducing potential of the stable free radical DPPH was determined under absorbance at 517 nm. The reaction medium was composed of 750 μL of extract or the compound solution at different concentrations (1, 10 and 100 μg/mL) and 250 μL DPPH methanol solution (10 µg/mL). After 5 min, the decrease in absorbance was measured. Samples of ascorbic acid (AA: 50 µg/mL) and 10% dimethyl sulfoxide solution (vehicle) were used as a positive and negative control groups, respectively.
Drugs and reagents
Phenylephrine hydrochloride (PE), acetylcholine chloride (ACh), Nω-nitro-l-arginine methyl ester (L-NAME), indomethacin (Indo), atropine and methylene blue (MB) were purchased from Sigma (St. Louis, MO, USA). All drugs were freshly prepared in physiological saline solution (PSS). Methanolic extracts, chloroform, ethyl acetate fractions, and isolated compounds were prepared on the day of the experiment in regular Kreb’s solution.
Preparation of rat thoracic aorta rings
After anesthesia with ketamine/xylazine (100/20 mg/kg) the thoracic aorta was quickly removed and placed in recipients containing Kreb’s solution with the following composition (mM): pH 7.4; composition in mM: NaCl 115.3, KCl 4.9, CaCl2·2H2O 1.46, KH2PO4 1.2, MgSO4 1.2, d-glucose 11.1, NaHCO3 25, cleaned of connective tissue and cut into rings (3–4 mm in length). In some preparations, the endothelium was removed by gently rolling the intimal surface of vessel lumen with a thin wire. The isolated aorta rings were then kept in organ baths containing 3 mL of Kreb’s solution, under a resting tension of 1 g, maintained at 37 °C and continuously aerated with 95% O2 and 5% CO2. A 60 min equilibration period was applied before any experimental study, during which the bath was flushed with fresh Kreb’s solution every 15 min. Following equilibration (60 min), rings were activated with KCl (60 mM) for 10 min to test their contractility. To confirm the presence of functional-endothelium a further 30 min stabilization period is expected for the addition of PE (1 µM) and, on the maximal contraction, the preparation was exposed to ACh (an endothelium-dependent vasodilator; 1 µM). Rings that produced > 80% relaxation to PE contraction, were considered having functional endothelium.
Pharmacological studies
Investigation of the vascular effect of ME, fractions and isolated compounds from E. mattosii in aortic rings from normotensive and SHR
In order to study the vascular effect of ME-leaves or stems, by chloroform (CLF) and ethyl acetate fractions (AEF) and phenolic compounds isolated from leaves, cryptostrobin and pinostrobin from the chloroform fraction and catechin from the ethyl acetate fraction, the aortic rings with and without functional endothelium were pre-contracted by phenylephrine (an α1-adrenergic receptor agonistic; 1 µM). During the tonic phase obtained with PE-induced contraction, the relaxation was evaluated through the addition of cumulative concentration of extracts, fractions (0.1–1000 µg/mL) and isolated compounds (0.1–300 µg/mL).
Evaluation of endothelium mediators and membrane receptors on the vascular effect of ME-leaves in aortic rings from normotensive rats
Comparing the results obtained in this study we found that ME-leaves were the most efficient to relax PE-contracted aortic rings. Therefore, the mechanisms that induce the vasodilatation were conducted using the ME from leaves. To investigate if NO/cGMP, prostacyclin (PGI2) or muscarinic receptor were involved in the vasorelaxant effect, the endothelium-intact aortic rings were incubated with L-NAME (a nitric oxide synthase inhibitor; 100 µM), MB (a soluble guanylyl cyclase inhibitor; 100 µM), indomethacin (a non-selective inhibitor of cyclooxygenase; 10 µM) or atropine (a muscarinic receptor antagonist; 1 µM) for 30 min prior the addition of PE. ME-leaves were then cumulatively added to the baths.
Effects of ME-leaves on extracellular calcium influx and intracellular calcium store
To verify the involvement of calcium uptake or its intracellular release in the relaxation induced by ME-leaves, two protocols were carried out in aortic rings with functional endothelium. In the first protocol to investigate the effects of ME-leaves on the contraction induced by intracellular calcium release, Ca2+-free Kreb’s solution, was replaced the regular Kreb’s solution. The rings were allowed to stabilize for 15 min, followed by incubation of ME-leaves (300 µg/mL) and subsequent addition of PE (1 µM). In the control group PE (1 µM)-induced contraction was assessed in the absence of ME-leaves. To study if extracellular Ca2+ influx was involved in the ME-leaves induced relaxation, the regular Kreb’s solution was replaced by depolarizing Ca2+-free Kreb’s solution. The aortic rings were allowed to stabilize for 30 min, and under this condition were incubated with ME-leaves (300 µg/mL) for 30 min. A new contraction was induced by PE (1 µM) and cumulative concentration–response curves for calcium chloride (CaCl2, 10 µM–100 mM) were constructed. In the control group, the addition of CaCl2 was made in the absence of ME-leaves.
Changes in vascular reactivity responses of the aorta from SHR
Aortic rings from SHR were exposed to PE (1 µM) and the cumulative concentration of ME-leaves (0.1–300 µg/mL) was added to the baths. Involvement of NO/cGMP and calcium movement were also studied using the same protocols as described in Sects. 3.2 and 3.3.
Statistical analysis
The results were expressed as mean ± SEM, of six or eight experiments. The data were analyzed by ordinary one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test, 2-way ANOVA followed by Bonferroni’s multiple comparisons, or multiple t test, when applicable. A value of p < 0.05 was accepted as statistically significant. Graphs were drawn and statistical analyses were performed using GraphPad Prism version 6.0 g (GraphPad Software, La Jolla, CA, USA).
Results
Phytochemical screening
The qualitative phytochemical screening demonstrated the presence of flavonoids and tannins as summarized in Table 1. Among the phytochemicals, tannins were detected in high concentrations in both extracts.
Total phenolic and flavonoids content
ME-leaves and-stems displayed a significant quantitative phenolic and flavonoids compounds profile. However, ME-leaves presented a higher concentration of flavonoids than ME-stems, evidencing high levels of these constituents in E. mattosii preparations (Table 2).
Antioxidant properties of ME-leaves and stems
As shown in Table 3, ME-leaves and stems also possess the ability to scavenge free-radical DPPH. Ascorbic acid used as a positive control (AA; 50 µg/mL), decreases the absorption of DPPH solution around 62%. ME-leaves and stems, at 1, 10 and 100 µg/mL, also significantly reduced the absorption of DPPH solution when compared to the control group (≈ 80–90%).
Concentration-dependent relaxation of ME and fractions of E.mattosii on isolated rat aorta rings in the presence and the absence of functional endothelium
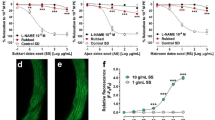
The ME of stems and leaves relaxed PE-pre-contracted aortic rings in a concentration-dependent manner. The maximal relaxant effect of ME-stems was 88.3 ± 8.1% at a concentration of 500 µg/mL, and 83.3 ± 4.8% to ME-leaves at a concentration of 500 µg/mL, which was reduced to 37.2 ± 8.7% and 42.6 ± 7.3%, respectively, in endothelium denuded-aortic rings (Fig. 2a). Both fractions obtained from leaves (CLF-leaves and AEF-leaves) were more effective in promoting relaxation than the fractions obtained from stems (CLF-stems and AEF-stems) (Fig. 2c and b, respectively). As shown in Fig. 2b, we can visualize only the relaxation induced by AEF-stems on aorta endothelium preserved, the addition of the other fractions did not promote any relaxation.
Relaxation of rat aortic rings previously contracted with phenylephrine induced by methanolic extracts, fractions and compounds of E. mattosii. a Methanolic extracts of stems and leaves; b chloroform (CLF) and ethyl acetate (AEF) fractions of stems and c CLF and AEF fractions of leaves of E. mattosii. b, e cryptostrobin and catechin induced endothelium-dependent relaxation. c Absence of vasorelaxation with pinostrotobin responses obtained in endothelium-intact, as indicated by E+ and endothelium-denuded, indicated by E−. Results are expressed as mean ± S.E.M. of 6–8 experiments. Statistical comparisons were performed using 2-way analysis of variance (ANOVA) followed by t test subjected to the Bonferroni’s correction. *Indicates p < 0.05, **indicates p < 0.01, ***indicates p < 0.001, when compared with the respective control E−, ##indicates p < 0.01 when compared with the respective control E−
Indeed, as we could verify, the relaxation elicited by ME-leaves at a concentration of 300 µg/mL, were quite more effective than the relaxation induced by both the fractions of leaves and stems (Table 4).
To investigate if the extract, its fractions or the isolated compounds have a potentially deleterious effect on vascular functionality, the baths were washed and allowed to rest for 60 min, and the contraction induced by PE and relaxation induced by ACh was measured again. In all experiments, Kreb’s solution was used as a vehicle group. Importantly, neither extracts nor compounds tested impaired the ability of PE-induced contraction and the relaxation induced by ACh (data not shown), thus reducing the possibility of the local toxic effect, inactivation of the site of action or irreversibility of the effects.
Relaxation of aorta rings from normotensive rats by compounds isolated of E. mattosii
Three phenolic compounds isolated from E. mattosii were studied. In preparations with preserved endothelium, cryptostrobin and catechin induced concentration–response relaxation. Both of them relaxed about 85% of the contraction induced by PE (1 µM), at a concentration of 300 µg/mL (Fig. 2d and e, respectively). On the other hand, pinostrobin did not induce relaxation (Fig. 2f).
Mechanisms underlying the vascular relaxation induced by ME-leaves in endothelium intact aortic rings from normotensive rats
As aforementioned the ME-leaves relaxed rat aortic rings more effectively (ME-leaves relaxed aortic rings ≈ 83%) when compared to the effect demonstrated with the other preparations at the concentration of 300 µg/mL. Taking it account, we investigated the mechanisms underlying the relaxation using the ME-leaves. To investigate whether NO/cGMP pathway was involved in the ME-leaves-induced aorta relaxation, L-NAME (100 µM, an inhibitor of NO synthase) and methylene blue (MB, 100 µM), an inhibitor of soluble guanylyl cyclase (sGC) for catalyzing the formation of cGMP were added to the baths 30 min before the addition of PE (1 µM) to induce contraction. In the L-NAME and MB pretreated aortic rings, the Emax values were reduced to 14.5 ± 2.4% and 40 ± 7.3%, respectively (Fig. 3a, b, respectively). The incubation of atropine (1 µM, a muscarinic receptor antagonist) and indomethacin (10 µM, an inhibitor of cyclooxygenase) did not change the ME-leaves induced relaxation (Fig. 3c, d, respectively).
Effect of enzyme blockers and antagonists on endothelium-dependent relaxation induced by ME-leaves in aortic rings with functional endothelium. Blockage of endothelium-dependent relaxation induced by ME-leaves by the non-selective nitric oxide synthase inhibitor L-NAME (a), and the soluble guanylate cyclase inhibitor methylene blue (b); c, d lack of influence of membrane muscarinic receptor antagonist atropine and the cyclooxygenase inhibitor indomethacin, against ME-leaves-induced rat aortic relaxation. Results are expressed as mean ± S.E.M. of 6–8 experiments. Statistical comparisons were performed using 2-way analysis of variance (ANOVA) followed by t-test subjected to the Bonferroni’s correction. ***Indicates p < 0.001, when compared with the respective vehicle
In SHR aortic rings pre-contracted with PE (1 µM) with preserved endothelium, ME-leaves also induced a concentration-dependent relaxation, just like in normotensive rats (64.2 ± 4.7% and 83.3 ± 4.8%, respectively), (Fig. 4a). As shown in Fig. 4b, pre-incubation with L-NAME significantly reduced the relaxing effect of ME-leaves (from 64.2 ± 4.7 to 14 ± 3.6% p < 0.0001). MB did not modify the maximal response, but the relaxation was reduced at 50 µg/mL (35.8 ± 7.2 to 15.4 ± 2.6%) and at 100 µg/mL (55.0 ± 8.9 to 28.1 ± 2.3%) (Fig. 4c).
Assessment of the vasodilator effect of ME-leaves in aortic rings of normotensive (NTR) and hypertensive (SHR) rats. Vascular relaxation induced by ME-leaves in aortic rings of NTR and SHR, responses obtained in endothelium-intact vessels (a). Blockage of endothelium-dependent relaxation induced by ME-leaves by the non-selective nitric oxide synthase inhibitor L-NAME in aortic rings of SHR (b) and, c reduction of the relaxation promoted by addiction of methylene blue in aortic rings of SHR. The results show the mean ± S.E.M. of six experiments. Statistical comparisons were performed using 2-way analysis of variance (ANOVA) followed by t-test subjected to the Bonferroni’s correction. **Indicates p < 0.01, ***indicates p < 0.001 when compared to vehicle
Involvement of ME-leaves (300 µg/mL) on extracellular Ca2+ influx and intracellular Ca2+ release
As shown in Fig. 5a, in the Ca2+ free-Kreb’s solution, PE (1 µM) induces a transient contraction due to the release of intracellular Ca2+. The second contraction induced by PE (1 µM) in the presence of ME-leaves (30 min), was significantly decreased (from 0.71 ± 0.21 to 0.44 ± 0.06 g, p < 0.05). To evaluate the effect of ME-leaves on calcium influx the aortic rings were exposed to PE (1 µM) in preparations kept in depolarizing Ca2+-free solution, which produced a small increase in the resting tone, and subsequent cumulative concentration–response curves for CaCl2 (10 µM–100 mM) was constructed. However, when the aortic rings were pre-incubated with ME-leaves (300 µg/mL) for 30 min before PE was applied, the maximal contraction induced by cumulative concentration–response curves for CaCl2 was reduced (from 1.53 ± 0.15 to 1.05 ± 0.11 g; p < 0.05) (Fig. 5b).
Effects of ME-leaves on contraction induced by phenylephrine and calcium in aortic rings with functional endothelium of NTR and SHR. NTR (a) and SHR (c), Phenylephrine-induced contraction of vessels maintained in calcium-free PSS in the absence or after incubation of ME-leaves. NTR (b) and SHR (d) Calcium-induced contraction in the absence or after incubation of ME-leaves in depolarizing Krebs solution. The results show the mean ± S.E.M. of 6–8 experiments. Statistical comparisons were performed using 2-way analysis of variance (ANOVA) followed by t test subjected to the Bonferroni’s correction, or Student’s t test when applicable (a, c and d). *Indicates p < 0.05, ***indicates p < 0.001 when compared to vehicle
Calcium movement was also studied in aortic rings from SHR, and the same protocols described above with NTR aortic rings were used. In Ca2+-free Kreb’s solution preparations, PE (1 µM) induced a reduced contraction in aortic rings from SHR (0.32 ± 0.09 g) (Fig. 5c), compared to those of NTR (0.71 ± 0.21 g) (Fig. 5a). However, pre-incubation with ME-leaves (300 µg/mL) in aortas from SHR, did not modify the maximal contraction induced by PE (Fig. 5c). Cumulative addition of CaCl2 (10 µM to 100 mM), in aorta from SHR, in the presence of ME-leaves, was significantly reduced (Fig. 5d).
Discussion
Our study provides that methanolic extracts of leaves and stems of E. mattosii induced a concentration-dependent relaxation on aortic rings pre-contracted with PE. ME-leaves and stems of E. mattosii were able to relax both endothelium-intact and denuded aortic rings. However, the relaxation in the aorta with endothelium was greater than in aorta without endothelium, suggesting that the relaxation effect was mainly mediated through an endothelium-dependent mechanism. Although additional experiments were not carried in our current study, ME-leaves may contain compounds that act synergistically inducing vasodilation, since the extract was more effective than the chloroform and ethyl acetate fractions in the concentration of 300 µg/mL. Therefore, the extract components could act at different targets enhancing the vasodilatation activity. Both phenolic compounds isolated from ME-leaves, cryptostrobin from the chloroform fraction, and catechin from the ethyl acetate fraction induced concentration-dependent relaxation in PE-contracted aortic rings from normotensive rats. Many studies showed that catechin has a vasodilatory effect on vascular and non-vascular smooth muscle.
It is well described that phenolic compounds, which were quantified in ME-leaves and stems, could show antioxidant activity (Oroian and Escriche 2015). Indeed, in our experiments both ME-leaves and stems showed a high profile of phenolic contents, as well as a significant DPPH radical scavenging-linked antioxidant activity. It is well described in the literature that a high intake of polyphenol rich-diet from natural products may improve cardiovascular health (Sofi et al. 2014; Basu et al. 2010). Several studies confirmed that polyphenol-rich sources such as extracts from red wines, green and black tea, and several plants caused endothelium-dependent relaxations in large arteries, arterioles, and veins that were prevented by competitive inhibitors of the enzymes endothelial nitric oxide synthase and guanylyl cyclase (Fitzpatrick et al. 1993; Schini-Kerth et al. 2010).
Considering the vasodilator effects obtained with ME-leaves, we herein studied its mechanism of relaxation in thoracic aorta strips with functional endothelium. The vascular endothelium plays a central role in the control of vascular tone. The monolayer of endothelial cells is located between the vascular lumen and the smooth muscle cells of the vessel wall and can release vasoactive substances from endogenous ligands such as acetylcholine, bradykinin, histamine, and by endogenous blood flow as, shear stress. Through these stimuli, the endothelium cells produce vasodilators such as endothelium-derived relaxing factor (EDRF), endothelium-derived hyperpolarizing factor (EDHF) and prostacyclin (Furchgott and Zawadzki 1980; Feletou and Vanhoutte 1988; Moncada and Vane 1978), with these findings, the vascular endothelium emerged as an important regulator of vascular tone and blood pressure. Among them, the most important and studied vasodilators seem to be the EDRF which has been identified as the nitric oxide (NO) radical (Ganz and Ganz 2001). NO is produced after activation of endothelium nitric oxide synthase (eNOS also called NOS III) by acetylcholine (ACh), and results in synthesis and release of NO, which in turn leads to guanylate cyclase activation and cyclic guanosine 3′,5-monophosphate (cGMP) elevation, leading to vascular smooth muscle relaxation, for review see Moncada and Higgs (2006). Therefore, based on the inhibition of ME-leaves-induced relaxation by l-NAME, it implies that ME-leaves induce the release of NO from endothelium cells.
Apart from NO, PGI2 is another important endothelium-derived relaxation factor, so the effect of ME-leaves on PGI2-mediated vascular dilation was also studied. Prostaglandins are synthesized by cyclooxygenase (COX) enzymes. The relaxing effect of ME-leaves was not inhibited by indomethacin (a non-selective inhibitor of COX), as well as was not affected by the pretreatment with atropine (a muscarinic receptor antagonist), suggesting ME-leaves-induced vasorelaxation neither involves the prostaglandin pathway nor activation of endothelial muscarinic receptor (type M3). However, as we could observe after the inhibition of COX with indomethacin, there was an intensification of the relaxation. These results suggest that endothelial COX when not inhibited should contribute to the production of a contractile factor and that this factor has been lost after its inhibition, or that COX after its inhibition contributes to the potentiating in the production of a relaxing factor.
The depletion of intracellular calcium significantly reduced the contraction induced by PE in preparations previously incubated with ME-leaves, as well as reduces the maximal contraction obtained in response to the cumulative addition of Ca2+ in depolarizing Kreb’s solution. However, in regular Kreb’s solution, ME-leaves pre-incubation did not affect PE-induced contraction but was able to significantly reduce the contraction induced by KCl. Calcium movement plays an important role in the NO synthesis and releases in endothelium cells, contributing to endothelium-dependent relaxation (Loeb et al. 1988; Fleming and Busse 1999). Otherwise, the contraction of smooth muscle is also regulated by cytosolic Ca2+ level and the sensitivity to Ca2+ of the contractile elements in response to changes in the environment surrounding the cell (Karaki et al. 1997). Studies show that lowering intracellular calcium ([Ca2+]o) reduced ACh-induced relaxation emphasizing the importance of extracellular Ca2+ in endothelium-dependent relaxation (Long and Stone 1985; Hayashi and Hester 2010), and it seems likely that [Ca2+]o plays an important role not only in contractile processes but also in relaxant processes as well (Godfraind et al. 1986). In the same way, contraction induced by KCl was reduced in the presence of ME-leaves. The cellular mechanism of contraction involved in response to KCl is that a high concentration of KCl causes a membrane depolarization, which leads to an increased Ca2+ influx through voltage-dependent calcium channels (VDCCs). Our data suggest that Ca2+ may be involved in the ME-leaves induced vasorelaxation.
We wondered if the aorta from hypertensive responds differently from the normotensive. Hypertension in SHR is associated with smooth muscle hypertrophy and changes in the structure and composition of the arterial wall (Levy et al. 1994), which leads to arterial rigidity and deficiency of the smooth muscle reduce the aortic compliance. Such changes when occurring in resistance vessels may lead to the change of peripheral resistance, and consequently hypertension (Wright and Angus 1999; Intengan and Schiffrin 2001). In our study, we found that aortas of hypertensive rats presented a reduction in acetylcholine-induced vasodilator response and a reduction of vasoconstrictor response to phenylephrine and KCl (data not shown). Differences in maximal responses between SHR and NTR tissues for contracting substances (PE and KCl) can be attributed to reduced smooth muscle contractility and/or changes in the mechanical properties of the isolated vessel.
Hypertension is a chronic and multifactorial disease and is a major risk factor for other severe cardiovascular conditions (Vanhoutte 1996). Hypertension has been associated with changes in vascular endothelium production and the release of vasoactive substances and is related to endothelial dysfunction and changes in vascular smooth muscle reactivity (Lunardi et al. 2009), both in animals and human models.
Spontaneously hypertensive rats (SHR) are useful for studying the mechanism involved in the endothelium dysfunction conditioned by hypertensive vasoconstriction (Pinto et al. 1998). Hypertension in SHR animals is like essential hypertension in humans (Gendron et al. 2004), in this way has become an experimental tool widely used for the study of hypertension in pre-clinical trials. Some studies demonstrated that aorta from SHR shown reduced ACh-induced vascular endothelium-dependent relaxation. This impairment is considered to be due to a decreased release of NO, a decreased release of EDHF, or an increased release of endothelium-dependent contraction factor (EDCF) (Diederich et al. 1990; Fujii et al. 1992; Hayakawa et al. 1993), and reduced contractility response to vasoconstrictor, in other words, vasoconstrictor are weaker in the aorta of SHR than in those from normotensive rats (Gendron et al. 2004). In SHR, in which hypertension develops gradually over weeks, alterations in aortic wall properties precede the development of hypertension, such vascular lesions should, therefore, be considered as a cause and not a consequence of high blood pressure (van Gorp et al. 2000; Gendron et al. 2004). Moreover, hypertension is associated with endothelium dysfunction, which is mainly defined by decreased vasodilator potential (Spijkers et al. 2010; Intengan and Schiffrin 2001). Thus, based on the participation of the NO/cGMP pathway in the vasorelaxation effect of ME-leaves, it was interesting to investigate if ME-leaves could induce vasorelaxation in aorta from SHR.
In our study, we found that the ME-leaves of E. mattosii was able to induce relaxation in aortic rings SHR pre-contracted with PE, in the same way as in normotensive rats, L-NAME, but not MB, completely inhibited ME-leaves-induced aorta relaxation. As already described above sGC present in vascular smooth muscle is the major target of NO to induce relaxation. The difference in relaxation inhibition is probably because L-NAME acts on NOS activity, inhibiting NO synthesis and production. Whereas, MB blocks the activation of guanylate cyclase by NO. In the presence of ME, NO is continuously being produced and diffuses into the vascular smooth muscle. NO is known to promote muscle relaxation at least in part through the activation of K+ channels (Faraci and Heistad 1998; Nelson and Quayle 1995). Opening a K+ channel present in the vascular smooth muscle membrane increases K+ efflux into the extracellular environment, leading to hyperpolarization (Sobey 2001). Hyperpolarization, in turn, leads to the closure of voltage-dependent calcium channels, reducing calcium intake and consequently inhibiting contraction (Nelson and Quayle 1995). Potassium channels also play an important role in vascular relaxation through vascular smooth muscle polarization. Probably it is through this mechanism that we still see some relaxation with methylene blue. Furthermore, they are direct or indirect targets of the actions of NO in the vasculature (Sobey 2001; Costa and Assreuy 2005). The production of NO by the ME-leaves may be modulating potassium channels and thus inducing vasodilation. As in normotensive, the presence of the extract reduced the contraction induced by the cumulative addition of CaCl2 in the aorta of SHR. Thus, it is reasonable to state that the relaxant effect of ME-leaves can be related to activation of NO/cGMP pathway and at least in part to some action on extracellular calcium uptake or changes in calcium mobilization from intracellular stores.
Taken together our study demonstrated that E. mattosii ME-leaves, fractions and isolated phenolic compounds were able to induce relaxation in aortic rings from normotensive and hypertensive rats. The major mechanism by which ME-leaves induce relaxation involves the NO/cGMP pathway, however, other studies are necessary to clarify the other possible mechanism of action, including those endothelium-independent. The pharmacological findings described in this study may be an important step for validation of the popular usage of E mattosii as a phytomedicine for the treatment of vascular diseases. Our findings may constitute the basis for further functional studies, which is going to reveal the mechanism by which cryptostrobin and catechin may induce vascular relaxation. Besides, both the efficacy and safety for acute and chronic usage of preparations obtained from E mattosii remains to be investigated. Indeed, the pharmacological treatment of cardiovascular diseases such as hypertension includes flavonoid-rich diets. Phenolic compounds are mostly ascribed to their antioxidant and vasodilator actions (Zenebe et al. 2001; Nijveldt et al. 2001; van den Elsen et al. 2014). Also, as already mentioned above, previous phytochemical studies have revealed the presence of steroids, terpenes and phenolic compounds as the main constituents from E. mattosii.
Conclusions
In conclusion, our study has demonstrated that ME-leaves from E. mattosii can induce a concentration-dependent relaxation on both endothelium-intact and denuded thoracic aortic rings pre-contracted by PE from normotensive and spontaneously hypertensive rats. The endothelium-dependent relaxation may be predominantly mediated by the NO/cGMP pathway. Furthermore, extracellular calcium uptake or changes in calcium mobilization from intracellular stores. The phenolic compounds cryptostrobin and catechin also induced endothelium-dependent relaxation an event that might also involve NO pathway. Moreover, cryptostrobin and catechin induced relaxation, which may contribute synergistically to the vasorelaxation effect of the ME-leaves. The finding we have presented suggest that E. mattosii has the potential for further studies in animal models of cardiovascular diseases.
References
Amat AG, Yajıá ME (1991) Medicinal plants and ethnopharmacology in the province of Misiones (Argentina). Acta Farm Bon 10:153–159
Bandoni AL, Mendiondo ME, Rondina RVD, Coussio JD (1972) Survey of Argentine medicinal plants. I. Folklore and phytochemical screening. Lloydia 35:69–80
Basu A, Rhone M, Lyons TJ (2010) Berries: emerging impact on cardiovascular health. Nutr Rev 68:168–177
Biavatti MW, Leite SN (2005) Práticas de Farmacognosia. Ed, 1 the edition. Universidade do Vale do Itajaí, Itajaí
Consolini EA, Sarubbio MG (2002) Pharmacological effects of Eugenia uniflora (Myrtaceae) aqueous crude extract on rat’s heart. J Ethnopharmacol 81:57–63
Consolini AE, Baldini OAN, Amat AG (1999) Pharmacological basis for the empirical use of Eugenia uniflora L. (Myrtaceae) as antihypertensive. J Ethnopharmacol 66:33–39
Costa RS, Assreuy J (2005) Multiple potassium channels mediate nitric oxide induced inhibition of rat vascular smooth muscle cell proliferation. Nitric Oxide 13:145–151
Diederich D, Yang ZH, Bühler FR, Lüscher TF (1990) Impaired endothelium dependent relaxations in hypertensive resistance arteries involve cyclooxygenase pathway. Am J Physiol 258:445–451
Faraci FM, Heistad DD (1998) Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev 78(1):53–97
Farrugia G, Szurszewski JH (2014) Carbon monoxide, hydrogen sulfide, and nitric oxide as signaling molecules in the gastrointestinal tract. Gastroenterology 147(2):303–313
Feletou M, Vanhoutte PM (1988) Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol 93:515–524
Fitzpatrick DF, Hirschfield SL, Coffey RG (1993) Endothelium-dependent vasorelaxing activity of wine and other grape products. Am J Physiol 265:774–778
Fiuza TS et al (2008) Pharmacognostic characterization of the leaves of Eugenia uniflora L. Rev Elet de farm 2:21–31
Fleming I, Busse R (1999) Signal transduction of eNOS activation. Cardiovasc Res 43:532–541
Fujii K et al (1992) Decreased endothelium-dependent hyperpolarization to acetylcholine in smooth muscle of the mesenteric artery of spontaneously hypertensive rats. Circ Res 70:660–669
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–376
Ganz P, Ganz E (2001) Coronary blood flow and myocardial ischemia. In: Braunwald E, Zipes E, Libby P (eds) Heart disease. A textbook of cardiovascular medicine, 6th edn. W.B. Saunders Company, London, pp 1091–1113
Gendron G et al (2004) Contractile responses of aortae from WKY and SHR to vasoconstrictors. Clin Exp Hypertens 26:511–523
Godfraind T, Miller R, Wibo M (1986) Calcium antagonism and calcium entry blockade. Pharmacol Rev 38:324–416
Hayakawa H et al (1993) Mechanisms of altered endothelium-dependent vasorelaxation in isolated kidneys from experimental hypertensive rats. Am J Physiol 264:1535–1541
Hayashi S, Hester RK (2010) Reduction in extracellular Ca2+ attenuates endothelium dependent relaxation more than nitroprusside-induced relaxation. Acta Pharmacol Sin 31(1):19–26
Intengan HD, Schiffrin EL (2001) Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 38(3 Pt. 2):581–587
Karaki H et al (1997) Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev 49(2):157–230
Landrum LR, Kawasaki ML (1997) The genera of Myrtaceaein Brazil: an illustrated synoptic treatment and identification keys. Brittonia 49:508–536
Levy BI et al (1994) Effect of chronic dihydropyridine (isradipine) on the large arterial walls of spontaneously hypertensive rats. Circulation 90(6):3024–3033
Limberger RP, Sobral M, Henriques AT (2004) Óleos voláteis de espécies de Myrcia nativas do Rio Grande do Sul. Quím Nova 27(6):916–919
Loeb AL et al (1988) Endothelium-derived relaxing factor release associated with increased endothelial cell inositoltrisphosphate and intracellular calcium. Am J Cardiol 62:36–40
Long CJ, Stone TW (1985) The release of endothelium-derived relaxant factor is calcium dependent. Blood Vessels 22(4):205–208
Lopes RM, Oliveira TT, Nagem TJ, Pinto AS (2000) Flavonóides: farmacologia de flavonóides no controle hiperlipidêmico em animais experimentais. Biotecnologia Cien Desenvolv 3(17):18–22
Lunardi CN, da Silva RS, Bendhack LM (2009) New nitric oxide donors based on ruthenium complex. Braz J Med Biol Res 42(1):87–93
Magierowski M et al (2017) Cross-talk between hydrogen sulfide and carbon monoxide in the mechanism of experimental gastric ulcers healing, regulation of gastric blood flow and accompanying inflammation. Biochem Pharmacol 149:131–142
Massaro CF et al (2014) Anti-staphylococcal activity of C-methyl flavanones from propolis of Australian stingless bees (Tetragonula carbonaria) and fruit resins of Corymbia torelliana (Myrtaceae). Fitoterapia 95:247–257
Moncada S, Higgs EA (2006) The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol 147:S193–S201
Moncada S, Vane JR (1978) Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev 30:293–331
Moresco HH et al (2016) Chemical constituents of Eugenia catharinae and their antioxidant activity. Nat Prod Res 30(22):2624–2628
Nelson MT, Quayle JM (1995) Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol 268(4):C799–C822
Nijveldt RJ et al (2001) Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 74:418–425
Oroian M, Escriche I (2015) Antioxidants: characterization, natural sources, extraction and analysis. Food Res Int 74:10–36
Pinto YM, Paul M, Ganten D (1998) Lessons from rat models of hypertension: from Goldblatt to genetic engineering. Cardiovasc Res 39(1):77–88
Ratera EE, Ratera MO (1980) Plantas de la Flora Argentina Empleadas en Medicina Popular. Editorial Hemisferio Sur, Buenos Aires, pp 128–129
Rosandy AR et al (2013) Isolation and characterization of compounds from the stem bark of Uvaria rufa (Annonaceae). MJAS 17(1):50–58
Santos L, Campos A, Cechinel Filho V, Nesello LAN (2018) Phytochemical profile and gastroprotective activity of Eugenia mattosii fruits. Arq Gastroenterol 55(2):138–141
Schini-Kerth VB, Auge RC, Kim JH, Etienne-Selloum N, Chataigneaun T (2010) Nutritional improvement of the endothelial control of vascular tone by polyphenols: role of NO and EDHF. Pflugers Arch 459:853–862
Shukla SK et al (2014) Eugenia jambolana pretreatment prevents isoproterenol-induced myocardial damage in rats: evidence from biochemical, molecular, and histopathological studies. J Med Food 17(2):244–253
Sobey CG (2001) Potassium channel function in vascular disease. Arterioscler Thromb Vasc Biol 21:28–38
Sofi F, Macchi C, Abbate R, Gensini GF, Casini A (2014) Mediterranean diet and health status: an updated metaánalysis and a proposal for a literature-based adherence score. Public Health Nutr 17(12):2769–2782
Solladié G, Gehrold N, Maignan J (1999) Biomimetic synthesis of the flavanone leridol, revision of the structure of the natural products. J Eur J Org Chem 9:2309–2314
Spijkers LJ, Alewijnse AE, Peters SL (2010) Sphingolipids and the orchestration of endothelium-derived vasoactive factors: when endothelial function demands greasing. Mol Cells 29:105–111
van den Elsen LW et al (2014) Dietary fish oil improves endothelial function and lowers blood pressure via suppression of sphingolipid-mediated contractions in spontaneously hypertensive rats. J Hypertens 32(5):1050–1058
van Gorp AW, Schenau DF, Hoeks A, Boudier H, de Mey JG, Reneman RS (2000) In spontaneously hypertensive rats alterations in aortic wall properties precede development of hypertension. Am J Physiol Heart Circ Physiol 278(4):1241–1247
Vanhoutte PM (1996) Endothelial dysfunction in hypertension. J Hypertens Suppl 14:S83–S93
Vechi G, Tenfen A, Boeder AM, Hernandez-Gómez L, de Córdova CMM, Delle Monache F, Cechinel Filho V (2019) Chemical composition and antimycoplasmic activity of Eugenia mattosii leaves, stems and isolated compounds. Nat Prod Commun 14:37–40
Wazlawik E et al (1997) Analysis of the role of nitric oxide in the relaxant effect of the crude extract and fractions from Eugenia uniflora in the rat thoracic aorta. J Pharm Pharmacol 49(4):433–437
Wishart DS et al (2009) HMDB a knowledgebase for the human metabolome. Nucleic Acids Res 37:D603–D610
Wright CE, Angus JA (1999) Enhanced total peripheral vascular responsiveness in hypertension accords with the amplifier hypothesis. J Hypertens 7(12 Pt. 1):1687–1696
Zenebe W, Pechanova O, Bernatova I (2001) Protective effects of red wine polyphenolic compounds on the cardiovascular system. Exp Clin Cardiol 6:153–158
Acknowledgements
The research work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Universidade do Vale do Itajaí (UNIVALI). Dr. Rita de Cássia Melo Vilhena de Andrade Fonseca da Silva is grateful for the Post doctoral scholar ship from PNPD/CAPES.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Vechi, G., de Souza, P., da Silva, L.M. et al. Mechanisms underlying Eugenia mattosii D. Legrand leaves extract, fractions and compounds induce relaxation of the aorta from normotensive and hypertensive rats. 3 Biotech 9, 445 (2019). https://doi.org/10.1007/s13205-019-1973-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-019-1973-4