Abstract
The lipase from Aspergillus oryzae was modified with a surfactant and then observed to exhibit high catalytic efficiency and enantioselectivity for the kinetic resolution of (RS)-1-phenylethanol. The influential factors of the modified-lipase preparation were investigated, including the surfactant source, the organic cosolvent, and the buffer pH. The optimum modification conditions were found with a surfactant of polyoxyethylene sorbitan monopalmitate, an organic cosolvent of tetrahydrofuran and a phosphate buffer of pH 7.0. In the transesterification of (RS)-1-phenylethanol with vinyl acetate, the surfactant-modified lipase showed excellent enantioselectivity for the R-isomer (E > 200), giving an enantiomeric excess of higher than 99% for (R)-1-phenylethyl acetate at 46.8% conversion with the reaction time of 2 h at 30 °C. The enzymatic activity had barely altered after 30 days even at 50 °C when it was saved in a powdered state. The results indicated that the modification strategy was useful and highly efficient, and that modified A. oryzae lipase was a promising biocatalyst in the kinetic resolution of (RS)-1-phenylethanol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The lipase from Aspergillus oryzae (AOL) has been proven as being a versatile biocatalyst. It can be used in the kinetic resolution of a biotin intermediate lactone (Zheng et al. 2009), α-lipoic acid (Yan et al. 2009) and 1-phenylethanol (Yan et al. 2017). It can also stereoselectively catalyze the hydrolysis of ethyl 2-(4-hydroxyphenoxy) propanoate and its analogues, which are key intermediates in the synthesis of aryloxyphenoxy propionate herbicides (Zheng et al. 2013). In addition, it is a promising biocatalyst in the synthesis of flavour esters by the esterification of a series of short-chain acids and alcohols (Yan et al. 2014). All of the above used enzymes are involved in the mycelium-bound lipase, but A. oryzae is able to produce lipases simultaneously in mycelium and fermentation broth (Yan et al. 2015). An extracellular enzyme is the one employed in the fermentation broth. Due to the complexity and liquid form of the fermentation broth, the extracellular enzyme solution cannot be employed directly in organic solvent for the esterification or transesterification reactions. Generally, an enzyme solution can be immobilized on a solid carrier or precipitated with salts, organic solvents and hydrophobic support materials, and then dried to undergo reaction in an organic solvent. The hydrophobic support materials, such as lipids or surfactants, are mixed with a lipase solution to form a lipase-lipid complex (LLC) or lipase-surfactant complex (LSC) precipitate. In this so-formed precipitate, the hydrophilic head groups of lipid or surfactant interact with the hydrophilic surface of the enzyme, while the lipophilic alkyl chains extend away from its surface and solubilize the enzyme in hydrophobic organic solvents (Okahata et al. 1995). Consequently, the LLC or LSC exhibit good solubility and catalytic activity in organic media (Isono et al. 1995a). The LLC and LSC were applied to the catalysis of esterification (Okahata et al. 1995; Isono et al. 1995a; Goto et al. 1996; Kamiya et al. 1996; Basheer et al. 1996; Okazaki et al. 1997; Huang et al. 1998; Wu et al. 2002; Hsieh et al. 2006), transesterification (Wu et al. 2004; Hama et al. 2010; Zhong et al. 2014) and hydrolysis reactions (Mogi et al. 1999; Isono et al. 1996) for the production of structured lipids (Isono et al. 1995a; Hama et al. 2010; Mogi and Nakajima 1996), sugar ester (Zhong et al. 2014) and kinetic resolution of chiral compounds (Okahata et al. 1995; Goto et al. 1996; Okazaki et al. 1997; Wu et al. 2004). Their activity or enantioselectivity was found to be higher than that of the native powdered or other forms of enzymes. The lipids or surfactants modified lipases originate from Pseudomonas sp. (Okahata et al. 1995; Isono et al. 1995a, 1996; Wu et al. 2004), Candida rugosa (formerly C. cylindracea) (Goto et al. 1996; Kamiya et al. 1996; Huang et al. 1998; Wu et al. 2002; Zhong et al. 2014), Rhizopus sp. (Basheer et al. 1996; Okazaki et al. 1997; Hama et al. 2010; Mogi and Nakajima 1996; Mogi et al. 1999), Burkholderia cepacia (formly Pseudomonas cepacia) (Hsieh et al. 2006) and others. Nonetheless, to date, no studies have been conducted concerning the surfactant modified lipase derived from A. oryzae. To exploit the extracellular lipase sourced from A. oryzae, the enzyme was modified by various surfactants and used in the kinetic resolution of (RS)-1-phenylethanol ((RS)-1-PE). Enantiomerically pure 1-PE is a useful building block for the preparation of the chiral compounds such as numerous pharmaceuticals. In addition, (R)-1-PE is widely used as fragrance in cosmetic industry owing to its mild floral odor. Other applications include solvatochromic dye, ophthalmic preservative, and inhibitor of cholesterol intestinal adsorption (Suan and Sarmidi 2004). Performance of this surfactant-modified AOL was investigated for enantioselective transesterification of (R)-1-PE, leaving the (S)-1-PE in unreacted form (Scheme 1). Investigation has been performed into the effects of various modification conditions and reaction parameters on the enzyme’s activity and enantioselectivity were investigated.
Materials and methods
Chemicals
(RS)-1-PE, vinyl acetate, methyl tert-butyl ether (MTBE), molecular sieve (4 Å), polyoxyethylene sorbitan monolaurate (Tween 20), polyoxyethylene sorbitan monopalmitate (Tween 40), polyoxyethylene sorbitan monooleate (Tween 80), sorbitan monolaurate (Span 20), sorbitan monooleate (Span 80) and sorbitan trioleate (Span 85) were purchased from Aladdin Chemistry Co. Ltd (Shanghai, China). Poly(vinyl alcohol) (Mw = 1750 ± 50) was purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). All other chemicals used were of analytical grade. (RS)-1-PE, vinyl acetate and the reaction solvent were dried over a molecular sieve (4 Å) prior to use.
Production of AOL
The strain of A. oryzae WZ007 was isolated from soil and stored in the China Centre for Type Culture Collection with the accession number of CCTCC no. M206105 (Zheng et al. 2009). The strain was then transferred into the cultivation media. These cultivations were performed in cotton-stopped shake flasks at 30 °C, 200 rpm for 48 h in an orbital shaker. The cultivation medium was composed of 20 g/L peptone, 1 g/L KH2PO4, 0.5 g/L MgSO4, 0.5 g/L NaCl and 10 mL/L olive oil at an initial pH of 5.0. After moving the mycelium by filtration from the fermentation cultures, the resultant fermentation broth was used as the extracellular lipase for modification by the surfactant.
Preparation of LSC
LSC was prepared according to the method employed by Kamiya et al. (1996), with some modifications. A solution containing 0.5 g of surfactant in 5 mL of water or organic solvent was mixed with 50 mL of 0.1 M phosphate buffer (pH 7.0). Then 50 mL of enzyme solution (460 U) was added to the above mixed solution and sonicated in an ultrasonic bath for 20 min. After incubating it for 24 h at 4 °C, the precipitates were collected by centrifugation at 4 °C (20,000×g for 10 min) and lyophilized. A solution subject to the same treatment but without the surfactant served as the control.
LSC-catalyzed kinetic resolution of (RS)-1-PE
In a typical experiment, the reactions were performed in a 50 mL conical flask with a stopper. The reaction mixtures were composed of (RS)-1-PE (1 mmol), vinyl acetate (1 mmol), organic solvent (10 mL) and lyophilized LSC (100 mg, 420 U) and were incubated at 200 rpm and 30 °C on a shaking water bath. Reaction mixtures without LSC were also run to exclude for any possible spontaneous non-enzymatic reactions. At appropriate intervals, the samples were withdrawn and diluted with ethyl acetate, and then analyzed by gas chromatography.
Analysis
The lipase activity was assayed by titration method using olive oil as substrate (Yan et al. 2015). An olive oil emulsion was prepared by mixing 50 mL of olive oil and 150 mL of 4% poly(vinyl alcohol) solution. The poly(vinyl alcohol) solution was prepared by dissolving poly(vinyl alcohol) in 50 mM phosphate buffer (pH 7.5). Prior to the assay, the olive oil emulsion was homogenized. The reaction mixture containing 5 mL of olive oil emulsion, 4 mL of 50 mM phosphate buffer (pH 7.5) and 1 mL of enzyme solution (or 0.01 g LSC) was incubated at 40 °C and 150 rpm for 10 min in a shaker. The enzyme reaction was terminated by adding 15 mL of ethanol to the reaction mixture. Then the liberated free fatty acids were titrated with 0.05 M NaOH. One unit of enzyme activity was defined as the amount of enzyme required to liberate 1 μmol of fatty acid per minute under the assay conditions. The surfactants were assayed by HPLC 1100 (Agilent, Wilmington, DE) with a Zorbax SB- C18 column (250 mm × 4.6 mm, 5 μm, Agilent, Wilmington, DE). The mobile phase was composed of acetonitrile/water at a ratio of 5/95. The flow rate was 1 mL/min. Absorbance of column effluents was monitored at 210 nm. The concentrations of (RS)-1-PE and (RS)-1-phenylethyl acetate ((RS)-1-PEA) were determined in a gas chromatograph (GC-2010 plus, SHIMADZU, Japan) equipped with a flame ionization detector (FID) and a CP-Chirasil-Dex CB capillary column (0.25 μm film thickness, 25 m length, 0.25 mm I.D., Varian, Madrid, Spain). The temperatures of the injector and detector were both set to 250 °C. Nitrogen was used as the carrier gas, and the split ratio was 100. The oven temperature program was kept at 120 °C for 11 min. The retention time were found to be 5.551 and 6.269 min for (S)- and (R)-1-PEA, and 8.579 and 9.254 min for (R)- and (S)-1-PE, respectively. The enantiomeric excess of the substrate (ees) and the product (eep) were individually defined as ees (%) = (SS − SR)/(SS + SR) × 100 and eep (%) = (PR − PS)/(PR + PS) × 100, where SS and SR represented the peak areas of (S)- and (R)-PE, and PR and PS the peak areas of (R)- and (S)-1-PEA, respectively. The conversion (c) and enantioselectivity (E) were calculated using c (%) = ees/(ees + eep) × 100 and E = ln[1 − c(1 + eep)]/ln[1 − c(1 − eep)], respectively (Chen et al. 1982). The protein content of the original enzyme solution was determined according to Bradford’s method (Bradford 1976). The protein content of LSC was determined using an elementary analyzer (vario EL cube, elementar, Germany) (Huang et al. 1998). The protein recovery is defined as the protein amount of LSC divided by the original protein amount.
Characterization
Fourier transform infrared (FTIR) spectra of free AOL and LSC were obtained using a TENSOR27 infrared spectrometer (Bruker, Germany) equipped with a nitrogen-cooled, mercury-cadmium-tellurium (MCT) detector. The spectral resolution was 4 cm−1 between 4000 and 400 cm−1. The standard KBr pellet technique was applied for sample preparation (Zhang et al. 2015).
Results and discussion
Characterization of LSC
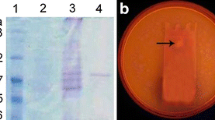
The FTIR spectra of the free AOL, LSC and Tween 40 were presented in Fig. 1. The amide I region (C=O stretching, 1690–1600 cm−1) and amide II region (C–N stretching and N–H bending, 1575–1480 cm−1) were found in the A and B spectra, which had primarily been used to assign secondary structures to proteins (Carbonaro and Nucara 2010). In Fig. 1A, the typical absorption peaks of the free AOL occurred at 1641 and 1539 cm−1 for CONH and 2800–3000 cm−1 for CH2 and CH3, respectively (Yu et al. 2015). The same absorption peaks were also maintained within the LSC spectra (Fig. 1B). However, the absorption peaks of 1641 and 1539 cm−1 were weakened, and the absorption bands in the regions of 2800 and 3000 cm−1 were enhanced. The results indicated that AOL had been coated with the surfactant.
Effect of different types of surfactants on the preparation of LSC
The activity and stability displayed by the LSC is intrinsically connected to the type and structure of the surfactant (Hsieh et al. 2006). The effect of the surfactants on the preparation of LSC and the activity of LSC in the resolution of (RS)-1-PE are outlined in Table 1. When no surfactant was added, no LSC precipitate was formed. Tween 40 gave a higher LSC yield and protein recovery, and the highest catalytic activity of the six tested surfactants. Span 20 gave the highest LSC yield and protein recovery, but exhibited a low activity. Tween 20 gave a low LSC yield and protein recovery as well as low activity. Tween 80, Span 80 and Span 85 were barely able to form any LSC precipitate, and cloudy solutions were observed following centrifugation. The results indicated that the LSC yield, protein recovery and LSC activity were influenced by the kinds and hydrophobic groups of the surfactants used (Hsieh et al. 2006; Mogi and Nakajima 1996), and not only by the hydrophile-lipophile balance (HLB) of the surfactants (Basheer et al. 1996). And a surfactant with the proper number of hydrophobic groups is beneficial towards AOL modification. For example, Tween 40 gave a high yield of LSC with a high activity. The HLB (15.6) and the number of hydrophobic groups (C16) of Tween 40 fell between those of Tween 20 (HLB 16.7, C12) and those of Tween 80 (HLB 15, C18). Compared to Tween, Span were unsuitable for the modification of AOL, although Span 20 gave a high LSC yield but a low activity. As a result, Tween 40 was selected as the most suitable surfactant for the preparation of the LSC.
It is generally known that polymeric surfactants and low molecular amphiphiles are known to interact strongly unless they carry the same charge. Therefore, it is not surprising that lipase, a typical amphiphilic protein, can interact strongly with many surfactants (Holmberg 2018). In addition, surfactants at low concentration levels can also activate lipases, via binding to a site in the lid (Mogensen et al. 2005). Alam et al. (2015) have investigated the activity and structural stability of Rhizopus niveus lipase in the presence of different types of surfactants. It was noted that both anionic surfactant SDS and the cationic surfactant cetyltrimethylammonium bromide (CTAB) deactivated the lipase while a nonionic surfactant improved its activity despite the partial unfolding of the protein. Our group previously reported that nonionic surfactants provided the LSC with a higher catalytic activity level than did the ionic surfactants (Zhong et al. 2014). Similar results were recorded in Huang’s and Okahata’s reports (Huang et al. 1998; Okahata and Ijiro 1988). Thus, in this study, we selected the most common surfactants, Tween and Span, which were all nonionic. Nonionic surfactants only bind to the lipase through hydrophobic, whereas an ionic surfactant can bind by a combination of electrostatic attraction and hydrophobic interactions. This different in binding mode contributes to the positive effect of the nonionic surfactants (Holmberg 2018). Although nonionic surfactants can be regarded as more benign towards an enzyme than anionic and cationic ones (Holmberg 2018), not all nonionic surfactants are suitable for all lipases. In this study, Tween 40 was a good choice for the AOL, but the Span was unsuitable. The reduced enzyme activity with Span may not be a result of conformational changes in the protein, but since the surfactant bound with one or more lipase-binding sites close to the active site (Gabriele et al. 2018).
Effect of organic cosolvent on the preparation of LSC
The variety of cosolvent employed in the preparation of LSC was found to have a decisive effect in obtaining an active LSC complex (Kamiya et al. 1996). The aim of using an organic solvent is to increase the dispersibility of a surfactant in an aqueous medium containing lipase and also to change the solubility of the enzyme, which allows both the solvent and enzyme to associate more easily (Kamiya et al. 1996). The logarithm of the partition coefficient, log P, was taken as an indicator of solvent polarity (Laane et al. 1987). Eight types of water-miscible solvents having different log P values were employed to investigate the effects of a cosolvent on the LSC yield, protein recovery and catalytic activity. Table 2 demonstrates that all of the tested solvents including water gave close LSC yields and protein recovery values. These results were inconsistent with Kamiya’s report, where the LSC complexes were obtained at high yields with hydrophilic solvents as cosolvents, but low yield was obtained when water was utilized as a cosolvent (Kamiya et al. 1996). These differences might result from the nature of the enzyme and surfactant used. In this study, Tween 40 as a water-miscible surfactant has a good solubility in water. However, the activity of the LSC complex was still affected by the cosolvent employed. The LSC prepared with tetrahydrofuran (THF) as a cosolvent exhibited the highest activity in resolution of (RS)-1-PE, giving a conversion of 46.8% with a reaction time of 2 h. The activity of the LSC was significantly higher than that of natural lipase (mycelium-bound lipase). The natural lipase required about 24 h to achieve the same conversion rate (Yan et al. 2017). The LSC prepared without an organic cosolvent likewise displayed a higher conversion of 31.6% with the same reaction time (at 2 h of reaction). The other solvents gave lower conversions than THF and water. Dimethyl sulfoxide (DMSO), a typical protein-dissolving nonaqueous solvent, exhibited the lowest activity. This indicated that this solvent might have a higher affinity than water, making it possible for it to interact strongly with lipase and disrupt the three-dimensional structure of the LSC during the preparation process (Kamiya et al. 1996). The ee value of the formed product (eep > 99%) and the enantioselectivity (E > 200) did not affect from the cosolvents used. Finally, THF was selected as being the most suitable cosolvent in the preparation of the LSC.
Effect of buffer on the preparation of LSC
The buffer solution plays an important role as the charge density of the enzyme surface changes with the variation of pH (Wu et al. 2002). Table 3 relates the dependence of the LSC yield, the protein recovery and the enzymatic activity on the buffer used to prepare the LSC. The protein recovery was affected by the pH value and the kind of the buffer chosen. The Tris–HCl buffers at the pH of 7.0–8.0 gave the higher LSC yields and protein recoveries, while the phosphate buffers at the pH of 6.0–8.0 and acetate buffers at the pH of 4.0–5.0 gave the relatively low LSC yields and protein recoveries. The lowest LSC yield and protein recovery were obtained at the high pH value of 9.0. The same behaviour was observed when the lipase from Rhizomucor miehei was modified with Span 60. It was inferred that a spontaneous hydrolysis of the surfactant might occur at a high pH value, which would decrease the amount of surfactant in the solution (Persson et al. 2002). The pH value and buffer type also strongly affected the enzyme’s activity. No enzymatic activity was observed when the LSC was prepared in Tris–HCl buffers. The phosphate buffers at the pH of 6.0–7.0 and acetate buffers at the pH of 4.0–5.0 gave the high enzymatic activities, but at pH values of 8.0, the enzymatic activity decreased drastically. The highest enzymatic activity of the LSC was obtained in phosphate buffer at a pH of 7.0. This pH-dependence of the LSC activity was similarly reported by the other studies (Huang et al. 1998; Wu et al. 2002; Mogi and Nakajima 1996; Sugimura et al. 1998). This phenomenon is analogous to that referred to by Klibanov as the “pH-memory of enzymes” (Sugimura et al. 1998). The activity of the LSC was likewise influenced by the pH environment to which the enzyme had last been exposed (Wu et al. 2002). Therefore, the LSC was prepared for the following experiments in a phosphate buffer at a pH of 7.0.
Effect of reaction solvent on LSC-catalyzed resolution of (RS)-1-PE
To assess the effects of the diverse reaction solvents on the activity and enantioselectivity of the LSC, eight organic solvents with log P values of from − 0.33 to 3.2 were employed. Table 4 displayed that MTBE was overwhelmingly superior to the other solvents, affording the highest LSC activity. The activity of the LSC did not appear in some hydrophilic organic solvents such as acetonitrile, acetone or pyridine. An aromatic solvent such as toluene and aliphatic solvents such as hexane and cyclohexane revealed decreased activities. This demonstrates a lack of correlation between the enzymatic activity and the log P value of the solvent. Indeed, some studies have reported that solvents’ the molecular structure played an essential role in affecting enzyme activity (Wu et al. 2002; Secundo et al. 1992; Chua and Sarmidi 2006). According to the hypothesis of Secundo et al. (1992), some solvent molecules may interact with the binding site of enzyme to form a solvent-enzyme complex. This resulting complex would behave differently depending on the nature of solvent. Although the solvent’s nature is shown to affect the catalytic activity of the LSC, the enantioselectivity (E > 200) appears independent of the solvent chosen (except for pyridine). Based on our results, MTBE was selected as the optimum reaction solvent for the following tests.
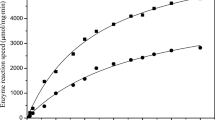
Effect of reaction temperature on LSC-catalyzed resolution of (RS)-1-PE
To investigate the influence of temperature on the resolution of (RS)-1-PE by the LSC, the reaction temperature was varied within the range of 20–50 °C. The results in Fig. 2 indicated a marked tendency for the catalytic activity to rise as the temperature increased up to 45 °C. Then, the catalytic activity decreased with subsequent increases in temperature. The optimal temperature was lower than that of the native lipase (Yan et al. 2017). Wu et al. also discovered that the LSC displayed a lower optimal temperature than the native one (Wu et al. 2004). This may be due to the exposure of the active site of lipase following modification by the surfactant (Wu et al. 2004). However, Okahata et al. found that the LLC exhibited high activity for broader temperature profiles (40–60 °C) than did the native one (40 °C) (Okahata and Ijiro 1992). This marked high activity at a broader temperature range was maintained from restricting the protein configuration and preventing the autolysis of lipase following its coating with surfactant (Li et al. 2019). Some other reports observed that LSC’s optimum reaction temperature equaled that of the native lipase (Huang et al. 1998; Wu et al. 2002; Isono et al. 1995b). It was proposed that the modification of enzymes with surfactants is through hydrogen bond linkages between the enzyme and surfactant molecules and does not affect the enzyme’s active site (Wu et al. 2002). Thus, the intrinsic properties of lipase such as the optimum reaction temperature were less affected by the modification (Wu et al. 2002).
LSC stability
To test the stability of the LSC, the LSC was stored in a powdered state at different temperatures and maintained in MTBE at 30 °C. Then, the enzymatic activity for the resolution of the (RS)-1-PE was determined and the results were presented in Fig. 3. When the LSC was stored in a powdered state at temperatures ranging from 4 to 50 °C, the enzymatic activity was found to barely alter after a period of 30 days. Alternately, when the LSC was kept in MTBE at 30 °C, the enzymatic activity diminished gradually with incubation time. After incubation of the LSC in MTBE for 2 weeks, approximately 30% of the original enzymatic activity was lost. The results are in agreement with those of Okahata et al. (1995), where by the lipid-coated lipase similarly displayed good stability in its powdered state, but lower stability in isooctane. This might be since the organic solvent dissolved the surfactant and led to the denaturation of the LSC (Okahata et al. 1995).
Conclusion
The present work showed that the AOL was modified with the surfactant and used as a catalyst for the kinetic resolution of (RS)-1-PE in an organic solvent. It was found the AOL interacted with the nonionic surfactant, Tween 40, with THF as an organic cosolvent in a phosphate buffer of pH 7.0 to form a LSC which possessed high activity and high enantioselectivity for the transesterification resolution of (RS)-1-PE. The LSC displayed the highest activity in MTBE at 45 °C. This activity was significantly higher than that of the native lipase, but the optimal temperature was lower than that of the native one. The LSC exhibited good stability in a powdered state even at 50 °C. These results implied that the surfactant-modified AOL was a good biocatalyst in the kinetic resolution of (RS)-1-PE.
References
Alam P, Rabbani G, Badr G, Badr BM, Khan RH (2015) The surfactant-induced conformational and activity alterations in Rhizopus niveus lipase. Cell Biochem Biophys 71:1199–1206
Basheer S, Nakajima M, Cogan U (1996) Sugar ester-modified lipase for the esterification of fatty acids and long-chain alcohols. J Am Oil Chem Soc 73(11):1475–1479
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carbonaro M, Nucara A (2010) Secondary structure of food proteins by Fourier transform spectroscopy in the mid-infrared region. Amino Acids 38:679–690
Chen C-S, Fujimoto Y, Girdaukas G, Sih CJ (1982) Quantitative analyses of biochemical kinetic resolutions of enantiomers. J Am Chem Soc 104:7294–7299
Chua LS, Sarmidi MR (2006) Effect of solvent and initial water content on (R,S)-1-phenylethanol resolution. Enzym Microb Tech 38:551–556
Gabriele F, Spreti N, Giacco TD, Germani R, Tiecco M (2018) Effect of surfactant structure on the superactivity of Candida rugosa lipase. Langmuir 34:11510–11517
Goto M, Noda S, Kamiya N, Nakashio F (1996) Enzymatic resolution of racemic ibuprofen by surfactant-coated lipases in organic media. Biotechnol Lett 18(7):839–844
Hama S, Yoshida A, Nakashima K, Noda H, Fukuda H, Kondo A (2010) Surfactant-modified yeast whole-cell biocatalyst displaying lipase on cell surface for enzymatic production of structured lipids in organic media. Appl Microbiol Biotechnol 87:537–543
Holmberg K (2018) Interactions between surfactants and hydrolytic enzymes. Colloids Surf B 168:169–177
Hsieh H-J, Nair GR, Wu W-T (2006) Production of ascorbyl palmitate by surfactant-coated lipase in organic media. J Agric Food Chem 54:5777–5781
Huang SY, Chang HL, Goto M (1998) Preparation of surfactant-coated lipase for the esterification of geraniol and acetic acid in organic solvents. Enzym Microb Tech 22:552–557
Isono Y, Nabetani H, Nakajima M (1995a) Lipase-surfactant complex as catalyst of interesterification and esterification in organic media. J Ferment Bioeng 80(2):170–175
Isono Y, Nabetani H, Nakajima M (1995b) Interesterification of triglyceride and fatty acid in a microaqueous reaction system using lipase-surfactant complex. Biosci Biotechnol Biochem 59:1632–1635
Isono Y, Nabetani H, Nakajima M (1996) Preparation of lipase-surfactant complex for the catalysis of triglyceride hydrolysis in heterogeneous reaction systems. Bioprocess Eng 15:133–137
Kamiya N, Murakami E, Goto M, Nakashio F (1996) Effect of using a co-solvent in the preparation of surfactant-coated lipases on catalytic activity in organic media. J Ferment Bioeng 82(1):37–41
Laane C, Boeren S, Vos K, Veeger C (1987) Rules for optimization of biocatalysis in organic solvents. Biotechnol Bioeng 30:81–87
Li H, Ni Y, Cao X, He X, Li G, Chen K, Ouyang P, Yang J, Tan W (2019) Highly active nanobiocatalysis in deep eutectic solvents via metal-driven enzyme-surfactant nanocomposite. J Biotechnol 292:39–49
Mogensen JE, Sehgal P, Otzen DE (2005) Activation, inhibition, and destabilization of Thermomyces lanuginosus lipase by detergents. Biochemistry 44:1719–1730
Mogi K, Nakajima M (1996) Selection of surfactant-modified lipases for interesterification of triglyceride and fatty acid. J Am Oil Chem Soc 73:1505–1512
Mogi K, Nakajima M, Mukataka S (1999) Surfactant modification of lipases for lipid interesterification and hydrolysis reactions. J Am Oil Chem Soc 76:1259–1264
Okahata Y, Ijiro K (1988) A lipid-coated lipase as a new catalyst for triglyceride synthesis in organic solvents. J Chem Soc Chem Commun 20:1392–1394
Okahata Y, Ijiro K (1992) Preparation of a lipid-coated lipase and catalysis of glyceride ester syntheses in homogeneous organic solvent. Bull Chem Soc Jpn 65:2411–2420
Okahata Y, Fujimoto Y, Ijiro K (1995) A lipid-coated lipase as an enantioselective ester synthesis catalyst in homogeneous organic solvents. J Org Chem 60:2244–2250
Okazaki S, Kamiya N, Goto M, Nakashio F (1997) Enantioselective esterification of glycidol by surfactant-lipase complexes in organic media. Biotechnol Lett 19(6):541–543
Persson M, Mladenoska I, Wehtje E, Adlercreutz P (2002) Preparation of lipases for use in organic solvents. Enzym Microb Tech 31:833–841
Secundo F, Riva S, Carrea G (1992) Effects of medium and of reaction conditions on the enantioselectivity of lipases in organic solvents and possible rationales. Tetrahedron Asymmetry 3:267–280
Suan C, Sarmidi MR (2004) Immobilised lipase-catalysed resolution of (R,S)-1-phenylethanol in recirculated packed bed reactor. J Mol Catal B Enzym 28:111–119
Sugimura Y, Fukunaga K, Matsuno T, Nakao K, Goto M, Nakashio F (1998) A study on the lipid-coating of lipases. Biochem Eng J 2:137–143
Wu JC, Song BD, Xing AH, Hayashi Y, Talukder MMR, Wang SC (2002) Esterification reactions catalyzed by surfactant-coated Candida rugosa lipase in organic solvents. Process Biochem 37:1229–1233
Wu H-Y, Xu J-H, Tsang S-F (2004) Efficient resolution of a chiral alcohol (RS)-HMPC by enzymatic transesterification with vinyl acetate using surfactant-modified lipase. Enzym Microb Technol 34:523–528
Yan HD, Wang Z, Chen LJ (2009) Kinetic resolution of α-lipoic acid via enzymatic differentiation of a remote stereocenter. J Ind Microbiol Biotechnol 36:643–648
Yan HD, Zhang Q, Wang Z (2014) Biocatalytic synthesis of short-chain flavor esters with high substrate loading by a whole-cell lipase from Aspergillus oryzae. Catal Commun 45:59–62
Yan HD, Liu HC, Wang Z (2015) Optimization of the fermentation conditions and substrate specificity of mycelium-bound ester hydrolases of Aspergillus oryzae Cs007. J Serb Chem Soc 80(1):1–8
Yan HD, Wang Z, Qian JQ (2017) Efficient kinetic resolution of (RS)-1-phenylethanol by a mycelium-bound lipase from a wild-type Aspergillus oryzae strain. Biotechnol Appl Biochem 64(2):251–259
Yu Y, Fei X, Tian J, Xu LQ, Wang XY, Wang Y (2015) Self-assembled enzyme–inorganic hybrid nanoflowers and their application to enzyme purification. Colloids Surf B 130:299–304
Zhang WW, Yang XL, Jia JQ, Wang N, Hu CL, Yu XQ (2015) Surfactant-activated magnetic cross-linked enzyme aggregates (magnetic CLEAs) of Thermomyces lanuginosus lipase for biodiesel production. J Mol Catal B Enzym 115:83–89
Zheng JY, Wang Z, Zhu Q, Zhang YJ, Yan HD (2009) Resolution of biotin intermediate lactone by enzyme-catalyzed stereoselective lactonization in organic solvent. J Mol Catal B Enzym 56:20–23
Zheng JY, Wu JY, Zhang YJ, Wang Z (2013) Resolution of (R,S)-ethyl-2-(4-hydroxyphenoxy) propanoate using lyophilized mycelium of Aspergillus oryzae WZ007. J Mol Catal B Enzym 97:62–66
Zhong X, Qian JQ, Guo H, Hu YY, Liu M (2014) Biosynthesis of sucrose-6-acetate catalyzed by surfactant-coated Candida rugosa lipase immobilized on sol–gel supports. Bioprocess Biosyst Eng 37:813–818
Acknowledgements
This research was supported by the National Key Research and Development Program (no. 2016YFD0400803).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yan, H.D., Guo, B.H., Wang, Z. et al. Surfactant-modified Aspergillus oryzae lipase as a highly active and enantioselective catalyst for the kinetic resolution of (RS)-1-phenylethanol. 3 Biotech 9, 265 (2019). https://doi.org/10.1007/s13205-019-1796-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-019-1796-3