Abstract
Anthracnose, caused by Colletotrichum spp. is the most devastating disease of chili (Capsicum annuum) in the tropical and subtropical regions of the world. The present study aimed at molecular mapping and development of markers linked to a new gene for anthracnose resistance in the chili cultivar ‘Punjab Lal’. Phenotypic evaluation of F1, F2, and BC1F1 populations derived from a cross between ‘Punjab Lal’ and susceptible cultivar ‘Arka Lohit’ against a virulent isolate of C. truncatum revealed that anthracnose resistance in Punjab Lal is governed by a monogenic-dominant gene designated as RCt1. Forty-four (28 ISSRs and 16 AFLPs) out of 201 markers exhibited parental polymorphism and were used in bulk segregant analysis. Three ISSRs (ISSR411493, ISSR581485, and ISSR1121857) and one AFLP marker (E-ACA/M-CTG516) showed precise polymorphism between resistant and susceptible bulks, and were used for genotyping F2 and BC1 populations. The four putative fragments were converted into sequence-tagged site (STS) markers and southern blotting confirmed their association with the resistance locus. Molecular mapping revealed that the STS markers CtR-431 and CtR-594 were closely linked to the RCt1 locus in coupling at distances of 1.8 and 2.3 cM, respectively. Furthermore, both of these markers showed the presence of resistance-linked allele in seven genotypes including the highly resistant C. chinnese ‘PBC932’ and C. baccatum ‘PBC80’ while negatively validated in 32 susceptible genotypes. Therefore, CtR431 and CtR-594 could be recommended as efficient diagnostic markers to facilitate the introgression of RCt1 locus into susceptible chili variants towards the development of high-yielding anthracnose resistance genotypes in C. annuum background.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Capsium annuum (family Solanaceae) is the most commonly cultivated chili species and an indispensable source of vegetable and spice across the tropical and subtropical regions of the world. India is the second largest producer, consumer, and exporter of chili, accounting for 1.39 million tons and contributing 21.5% of the world’s total chili production (FAOSTAT 2016). However, a significant decline in chili productivity has been realized in the recent times due to the anthracnose disease caused by the Colletotrichum species complex (Kim et al. 2008a; Than et al. 2008a). The pathogen causes both pre and post-harvest fruit rot and spots and blights of aerial plant parts resulting in a significant crop loss across the world including 10% in Korea (Byung 2007), 10 to 54% in India (Ramachandran and Rathnamma 2006), and 20 to 80% in Vietnam (Don et al. 2007). Although a number of Colletotrichum species are linked to chili anthracnose, C. truncatum (Schwein) (Andrus and Moore 1935) (formerly referred as C. capsici) is most predominant and belligerent in India (Ranathunge et al. 2012; Saxena et al. 2014). An estimated yield loss of 29.5%, amounting to US$ 491.67 million, has been reported due to chili anthracnose in India (Garg et al. 2014). Although the management of chili anthracnose has been a burning issue for more than a decade, no effective control measures have, so far, been proposed. Currently, practiced application measures for controlling chili anthracnose with fungicides such as Azoxyxtrobin and Mancozeb is costly and only partially effective under environmental conditions that are favorable for pathogen infection (Goswami et al. 2013). Besides, the biological control of anthracnose by inoculation of antagonistic fungi and bacteria is not 100% effective due to variability in the antagonistic effect against different Colletotrichum species (Vasanthakumari and Shivana 2013). Thus, molecular breeding through the deployment of resistant cultivars could be the most efficacious and safer management strategy for the economically important C. truncatum infection problem.
The inheritance to anthracnose resistance is highly variable based on resistance source and Colletotrichum species involved. For instance, resistance of C. chinense ‘PBC932’ and the derived C. annuum progressive line ‘Daepong-cho’ to C. truncatum is controlled by a single recessive gene, while that in C. annuum’83-168′ is governed by monogenic-dominant gene (Lin et al. 2002; Pakdeevaraporn et al. 2005; Kim et al. 2008a, b). Similarly, C. baccatum ‘PBC80’ exhibit resistance to C. acutatum via a dominant gene, while the same in C. chinense ‘PBC932’ and the C. annuum ‘AR’ is controlled by a single recessive factor (Yoon and Park 2005; Kim et al. 2007). In addition, the recent evidences indicated a polygenic inheritance of anthracnose resistance. Voorrips et al. (2004) reported one major and one minor resistant QTL for C. truncatum and one major and three minor resistant QTLs for C. gloeosporoides in C. chinense ‘PRI95030’ lines. Recently, Chunying et al. (2015) reported one major QTL in chromosome 5 of C. chinense ‘PBC932’ exhibiting broad spectrum resistance against C. acutatum. However, none of the above-mentioned genes or QTLs have so far been linked with the C. annuum cultivars of Indian subcontinent and their inheritance pattern of anthracnose resistance is still unknown. The recent identification of new natural sources of resistance in the C. annuum background (Garg et al. 2013; Mishra 2017, 2018) makes it essential to understand the resistance mechanism in these genotypes for their suitability in marker-assisted breeding programs.
A combination of PCR-based molecular markers and high-throughput phenotyping offer the possibility of identifying disease resistance loci through molecular breeding (Ashkani et al. 2015). The molecular systems such as the amplified fragment length polymorphism (AFLP), inter-simple sequence repeats (ISSRs), short-sequence repeats (SSRs), and single-nucleotide polymorphism (SNP) have been widely used to develop markers linked to disease-resistant traits in many plant species due to their high accuracy and reproducibility (Agarwal et al. 2008). Furthermore, the subsequent cloning and conversion of the ISSRs and AFLPs into sequence-tagged sites (STS) or sequenced characterized amplified regions (SCARs) makes them more reliable, less cumbersome, and breeder friendly markers that increases their specificity in the identification of genetic loci linked to disease resistance (Kar et al. 2014). Therefore, a comprehensive characterization of C. annuum genotypes with suitable molecular markers will play an important role in the early identification of C. truncatum-resistant chili genotypes in the Indian subcontinent for their successful utilization in the marker-assisted selection program. In the present study, we have ascertained the genetics of anthracnose resistance in the C. annuum cultivar ‘Punjab Lal’ which is designated as resistant to C. truncatum in India (Garg et al. 2013; Mishra et al. 2018). We also employed a wide set of ISSR and AFLP markers in a bulk segregant analysis to identify putative resistant fragments linked to anthracnose resistance and their subsequent validation in chili genotypes from different genetic backgrounds.
Materials and methods
Plant material and disease evaluation
The plant materials used in this study included chili cultivars—Punjab Lal and Arka Lohit. Punjab Lal is a natural landrace developed at the Punjab Agricultural University, Ludhiana, India. It is a cayenne-type cultivar with medium-sized plants which produces two inch erect fruits that turn green to purple to red. Arka Lohit is a pure line selection variety developed at the Indian Institute of Horticulture Research (IIHR), Bangalore, India, through a mass selection from the local collection line IHR-324. It has straight dark green fruits that turn deep red on maturity which high pungency. Disease reaction from field evaluation and glass house screening has revealed that Punjab Lal is resistant, while Arka Lohit is highly susceptible to anthracnose caused by C. truncatum (Garg et al. 2013; Mishra 2017; Mishra et al. 2018). To analyze the inheritance of anthracnose resistance, Punjab Lal was crossed with Arka Lohit and the plants from F1 (54 plants), F2 (255 plants), and BC1F1 (99 plants) segregating populations were labeled for phenotypic identification during disease reactions. The F2 generation chili population was also used for molecular mapping of the anthracnose resistance gene.
Capsicum truncatum virulent isolate MTCC-3414 (obtained from Microbial type Culture Collection, Institute of Microbial Technology, Chandigarh, India) was used for resistance evaluation of the parental lines along with the F1, F2, and BC1 materials. Prior to inoculation, the identity of the pathogen isolate was confirmed through PCR amplification using species-specific primers as described previously (Thind and Jhooty 1990). The disease inoculation experiments were conducted in a temperature-controlled greenhouse at the Centre for Biotechnology, Siksha O Anusandhan University, Bhubaneswar, India. The microbial isolate was cultured on potato dextrose agar (PDA) medium at 25 °C under 12 h fluorescent light/12 h dark in an incubation chamber for 7 days. A conidial suspension was made by flooding the plates with distilled water and scraping the surface to collect the conidia, and the final concentration was adjusted to 106 conidia/ml. Three fruits of mature red ripened stage from each plant were harvested, surface sterilized with 0.1% mercuric chloride (HgCl2), and rinsed twice with distilled water. The fruit pericarp was injected with 1000 spores in 1 µl at both the proximal and distal end using a micro-injector consisting of a micro-syringe model 1705 TLL fitted with a 1 mm micro needle (1 mm length) and a PB600-1 dispenser (Hamilton company, Switzerland). The inoculated fruits were placed in acrylic boxes that were tightly sealed with plastic bags to maintain more than 90% humidity and kept in dark for 48 h. The boxes were then removed from plastic bags and incubated at 25 ± 1 °C with a 12 h dark/ light cycle in a small moist chamber with relative humidity (RH) of 95%. The disease severity was recorded 9 days after inoculation (DAI) and the disease reactions of the plants were determined according to Montri et al. (2009). The frequency of each phenotype was assessed, and a Chi-square test was used to establish the goodness of fit for a Mendelian segregation ratio in the F2 and BC1F1 generations.
DNA isolation and marker analysis
Genomic DNA was extracted from the fresh leaf tissues of young seedlings following the Cetyltrimethyl ammonium bromide (CTAB) method with the desired modifications (Doyle and Doyle 1990). The DNA quality was determined on a 0.8% ethidium bromide stained agarose gel using uncut lambda DNA (Fermentas, USA) as standard. In addition, the DNA concentration was assessed on a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, USA), and the DNA samples were diluted to 25 ng/µl in TE buffer for PCR amplification of molecular markers.
A total of 201 molecular markers including 154 ISSRs and 47 AFLPs were used for screening of polymorphism between Punjab Lal and Arka Lohit. Based on stable amplification in C. annuum genotypes, additional 86 SSR markers including 49 genomic SSRs and 37 EST-SSRs were also included in the parental polymorphic survey. These markers were retrieved from previously published data based on their stable amplification in C. annuum genotypes (Kumar et al. 2001; Rai et al. 2013; Krishnamurthy et al. 2015). However, none of the SSRs could result in any polymorphism between the parents and, therefore, were exempted from further analysis.
ISSR and AFLP analyses
Equal amount of DNA from ten resistant and ten susceptible F2 plants were bulked to produce resistant and susceptible pools for bulk segregation analysis (BSA) (Michelmore et al. 1991). Based on parental screening, 28 ISSRs and 16 AFLP primer combinations were used to amplify DNA from the two parents and resistant and susceptible bulks for a co-segregating polymorphic marker study. ISSR amplification was carried out in a 25 µL reaction system consisting of 2 µL of 10× reaction buffer (16 mM (NH)2SO4, 67 mM Tris–HCl with 25 mM MgCl2), 20 pM of ISSR primer, 200 µM dNTP mix, 25 ng of template DNA, and 1 unit of Taq DNA polymerase. The PCR reaction was executed in a Veriti thermal cycler (Applied Biosystems, Foster City, CA, USA) with the following conditions: 94 °C for 5 min tagged on by 40 cycles at 94 °C for 1 min, 45–58 °C (for different primers) for 1 min, 72 °C for 2 min followed by a final extension at 72 °C for 10 min. The amplified products were resolved on 1.5% agarose gel in 1× TBE buffer at 60 volts for 3 h.
The AFLP analysis was performed using the standard protocol (Vos et al. 1995) with desired modifications. 100 ng of genomic DNA of each sample was digested with EcoRI/MseI restriction endonuclease solution and linked with the corresponding block adapters. Pre-amplification reaction was performed using EcoRI primer (5′-GACTGCGTACCAATTCA-3′) and MseI primer (5′-GATGAGTCCTGAGTAAC-3′) for 30 cycles at a melting temperature of 94 °C for 30 s, an annealing temperature of 56 °C for 30 s, and elongation temperature of 72 °C for 1 min. A 20-fold dilution of the pre-amplification product was used as template for selective PCR amplification involving 35 cycles including 10 touchdown cycles with annealing temperature reducing from 65 °C to 56 °C (0.7 °C per cycle) followed by 20 cycles with annealing temperature at 56 °C for 30 s. PCR products were separated on a 5% polyacylamide gel with a 50 bp DNA ladder (Fermentas, USA). The co-segregating ISSR and AFLP primers were analyzed at least twice in the entire F2 population to confirm the putatively linked marker for anthracnose resistance.
Development and analysis of STS marker
The polymorphic-resistant specific ISSR and AFLP fragments were eluted from the gel and purified using the Wizard SV gel and PCR cleanup system (Promega, USA). The purified product was cloned into the pTZ57R/T vector (Insta clone TA cloning kit, Thermo-Fischer Scientific, Waltham, USA) and transformed into competent Escherichia coli strain JM109 cells. The positively cloned DNA samples were sequenced using the BigDye Terminator v 3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA). The sequenced fragments were subjected to similarity check in the National Centre for Biotechnology Information (NCBI) database using the BLASTp tool. Sequence-tagged site markers were designed using the Primer3 software (Untergrasser et al. 2012) based on the sequence alignment information.
A set of 20 resistant and susceptible individuals randomly chosen from the F2 population was used to validate the resistance specificity of the STS markers. PCR was performed on 25 µL reaction volume containing 25 ng of template DNA, 200 µM dNTP mix, 10 µM forward and reverse primers, and 0.5 units of Taq Polymerase in 10× PCR buffer. The PCR temperature conditions were: 94 °C for 5 min followed by 30 cycles of 94 °C for 1 min, 55 °C for 45 s, 72 °C for 1 min, and final extension at 72 °C for 7 min. The amplified product was resolved through agarose gel electrophoresis as described earlier. Subsequently, the putatively linked STS markers were also used to analyze the entire F2 population to confirm their linkage with anthracnose resistance locus.
Southern blot analysis
Southern blot analysis was carried out according to the protocol described by Kar et al. (2014). Three different restriction endonucleases- EcoRI, HinDIII and XbaI (Thermo Fischer Scientific, USA) were used to digest the genomic DNA from the resistant line ‘Punjab Lal’ and the susceptible line ‘Arka Lohit’. The electrophoretically separated DNA digest was subsequently blotted onto a nylon membrane filter (Hybond-N+, Amersham Pharmacia Biotech) and baked at 80 °C for 2 h. Digoxigenin labeled probes were designed from STS amplified DNA sequence using a digoxigenin DNA labeling and detection kit (Roche Diagnostics, Basel, Switzerland). The nylon membrane blocked with DIG Easy Hyb was subsequently hybridized with DIG labeled STS probe in a hybridization chamber and incubated at 65 °C for 15 h. This was followed by repeated washing of the hybridized nylon membrane with 0.1× SSC buffer (15 mM sodium chloride, 1.5 mM sodium citrate, pH 7.0) containing 0.1% sodium dodecyl sulfate. The possible hybridization between the genomic DNA and STS probe was detected as per the manufacturer’s instructions.
Data analysis and genetic mapping
Chi-square test was performed with a probability of 0.05 to determine the goodness of fit for the observed and expected segregation of anthracnose resistance gene using the anthracnose disease phenotype of each F2 and BC1F1 plant derived from Punjab Lal/Arka Lohit cross. Linkage analysis was performed by importing a matrix carrying the F2 disease phenotype and marker genotype data into the MAPMAKER software ver 3.0 (Lander et al. 1987). A logarithm of odds (LOD) score of > 3.0 was used as threshold to order the segregating markers within the linkage group. The Kosambi mapping function was applied to convert the recombination frequency into map distances in centi Morgans (cM).
Results
Genetics of anthracnose resistance in Punjab Lal
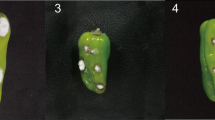
All plants in F1, F2, and BC1 generations were phenotyped as resistant and susceptible based on anthracnose disease score. When inoculated with C. truncatum isolate MTCC-3414, Punjab Lal showed a hypersensitive reaction, whereas Arka Lohit was highly susceptible (Fig. S1A and B). The inoculated fruits in the susceptible genotypes started developing sunken necrotic tissues within 3 days after inoculation followed by concentric rings of acervuli in the following days. The disease scoring was recorded at 9 days after infection, and the plants were grouped as resistant and susceptible based on the percentage disease index (PDI) and disease score of ‘1’ and ‘9’, respectively. The susceptible genotypes, including ‘Arka Lohit’, were heavily infected with PDI ranging between 26.4–73.2%, while the resistant parent ‘Punjab Lal’ remained largely uninfected with a minimum PDI value of 1.1%. All F1 genotypes, including the reciprocal F1, resembled the resistant parent ‘Punjab Lal’, and the 255 plants from the F2 population segregated as 190 resistant to 65 susceptible (χ23:1 = 0.033, df = 1, P = 0.857) fitting well with the single dominant gene segregation ratio (Table 1). When F2 plants were transplanted to the field, 197 plants survived to produce F3 seeds. The evaluation of anthracnose response in the F2:3 plants revealed that the ratio of homozygous resistant (RR):segregating resistant (Rr):homozygous susceptible from Punjab Lal/Arka Lohit was consistent with the expected ratio of 1:2:1 (Table 1) (Fig. S1C to S1F). Furthermore, the BC1F1 population consisting of 99 plants segregated as 49 resistant and 50 susceptible, thereby satisfying the expected ratio of 1:1 (χ2 = 0.011, df = 1 P = 0.921). Therefore, we concluded that anthracnose resistance in C. annuum cv. Punjab Lal is conferred by a single dominant gene, which was named as Resistance to Colletotrichum truncatum 1 (RCt1).
Identification of molecular markers linked to RCt1 locus
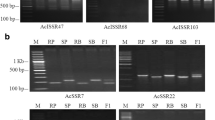
Out of 201 (154 ISSRs and 47 AFLPs) molecular markers used in parental polymorphic analysis, 44 (including 28 ISSRs and 16 AFLPs) differentiated the parental lines and were identified as polymorphic markers (Table S1 and S2). 154 ISSR markers resulted in 703 amplified bands in a size range of 200–3500 bp. The ISSR bands varied between 2 and 14 with an average of 6 bands per primer. 28 ISSR bands were found to be polymorphic resulting in 3.98% polymorphism between the parents. Likewise, 1038 fragments were amplified from 47 AFLP primer combinations with an average of 22 bands per marker. Although the majority of AFLP bands were similar in both the parents, 16 were polymorphic with respect to resistant and susceptible parents. Evaluation of 44 polymorphic markers with contrasting resistant (RB) and susceptible bulks (SB) revealed only four of them (three ISSRs and one AFLP) that were specifically distinguished in the resistant and susceptible bulks (Figs. 1, S2). The three ISSR markers, viz., ISSR411493, ISSR581485, and ISSR1121857, were amplified specific to resistant parent ‘Punjab Lal’, resistant bulk and 10 resistant F2 individuals while absent in the susceptible genotypes as well as the bulk (Fig. S2A–C). All the same, the AFLP primer E-ACA/M-CTG generated a specific amplicon of 516 bp approximately only in the resistant individual and resistant bulk (Fig. S2D). Thus, we reasoned that these four markers were co-segregating with the resistant gene.
Bulk segregant analysis towards identification of ISSR (a) and AFLP (b) markers linked to anthracnose resistance in Capsicum annuum. Amplification was performed with a total of 44 polymorphic markers (28 ISSR and 16 AFLP) with individual and bulked DNA from chili genotypes either susceptible or resistant to anthracnose. M, molecular weight marker; R, Resistant parent ‘Punjab Lal’; S, Susceptible parent ‘Arka Lohit’; RB, bulk DNA from ten resistant F2 individuals; SB, bulk DNA from ten susceptible F2 individuals. The arrow represents the polymorphic band in the resistant individual. The number represents the size of the polymorphic band
The resistance-specific fragments corresponding to the three ISSR and one AFLP markers were cloned into pTZ57R/T vector and sequenced (Figs. S3–S6). Homology assessment of the four sequenced fragment ISSR411493 (Accn no. MF581010), ISSR581485 (MF581014), ISSR1121857 (MF581012), and E-ACA/M-CTG516 (MF581016) using BLASTn against the nucleotide and EST databases in the NCBI homepage revealed no significant similarity between the cloned fragments and coding sequences of any functional genes. In addition, the four sequences did not possess any distinctive open reading frames and have multiple stop codons all along the reading structures. Based on the complete sequence information of the cloned fragments, four pairs of STS markers (CtR-431, CtR-594, CtR-496, and AFLP-376) were developed (Fig. S3–S6). PCR amplification with the four STS markers resulted in distinct unambiguous bands of 431 bp, 594 bp, 496 bp, and 376 bp, respectively, in the resistant parent ‘Punjab Lal’ and ten resistant F2 individuals with no bands in the susceptible parent DNA or susceptible F2 individuals (Fig. 2). To confirm the genomic position of the cloned fragments, the genomic DNA of resistant and susceptible cultivar was allowed to hybridize with digoxigenin labeled STS probes. DNA digested with three different restriction endonucleases (EcoRI, XbaI and HinDIII) revealed single-resistant specific bands for all the four STS probes in the southern blots (Fig. 3). This implies that the four STS markers are characterized by single copy sequence within the genome of the resistant cultivar and could be recognized as exclusive markers for the RCt1 dominant allele conferring resistance to chili anthracnose.
Validation of STS markers CtR-431 (a), CtR-594 (b), CtR-496, and AFLP-376 in F2 individuals either resistant or susceptible to chili anthracnose. M, molecular weight marker; R resistant genotype- Punjab Lal; S susceptible genotype- Arka Lohit, F1, F1 individual; 1–10, F2 individuals resistant to anthracnose; 11–20, F2 individuals susceptible to anthracnose
Southern blot analysis of the four STS markers- CtR-431, CtR-594., CtR-496, and AFLP-376. Restriction enzymes used are EcoRI, HinDIII and XbaI. M, molecular weight marker; R resistant genotype Punjab Lal; S susceptible genotype Arka Lohit. Hybridization patterns of the genomic DNA digested with all the three enzymes revealed single band only in the resistant genotype
Furthermore, the entire 255 plants from the F2 population were screened with the four STS markers and the segregation of the marker locus was assessed for goodness of fit to the expected Mendelian ratio of 3:1 to confirm their linkage with anthracnose resistance locus RCt1. Individual F2 plants were scored for the presence or absence of four amplicons, i.e., CtR-431, CtR-594, CtR-496, and AFLP-376. The genotyping pattern of F2 individuals with the molecular markers CtR-431 and CtR-594 is represented in Fig. 4. The observed phenotypes (anthracnose disease reaction) and the segregation ratio of four molecular markers fitted well with the expected ratio of 3:1 (Tables 2, S3). The information on the STS markers linked to RCt1 locus conferring anthracnose resistance in C. annuum cv. Punjab Lal in provided in Table S4.
Genotyping of F2 mapping population derived from the cross Punjab Lal × Arka Lohit using closely linked markers CtR-431 (a) and CtR-594 (b). M, molecular weight marker; R, resistant genotype- Punjab Lal; S, susceptible genotype- Arka Lohit, F1, F1 individual; F2 lines, individuals from the F2 populations. The details of the phenotypic expression of the F2 genotypes are represented in supplementary table S3
Molecular mapping and linkage assignment to RCt1 locus
The MAPMAKER compare and map commands were used to correlate the genotypic and phenotypic data of the F2 population to determine the most probable arrangement within the linkage group for the resistance gene RCt1. The analysis resulted in a linkage map of four linked markers with the resistance RCt1 locus spanning a distance of 16.9 cM (Fig. 5). Two flanking STS markers, CtR-431 and CtR594, were estimated to be located at a genetic distance of 1.8 cM and 2.3 cM from the resistant gene, respectively. Similarly, CtR496 was situated at 7.2 cM and AFLP-376 lay at a distance of 7.9 cM from the RCt1 locus. This is the first report of the development of molecular marker linked to anthracnose resistance locus RCt1 in C. annuum.
Validation of RCt1-linked markers
The two closely linked markers CtR-431 and CtR-594 were further genotyped on a set of 41 chili pepper lines to verify the presence or absence of a resistant RCt1 allele (Table 3). The marker CtR-431 and CtR-594 amplified a fragment of 431 and 594 bp, respectively, in the resistant parent ‘Punjab Lal’ as well as in another five chili genotypes, including BS35, Pant-C1, Achar Lanka, CA-4, and Bhut Jolokia, that has been previously identified as resistant to anthracnose (Garg et al. 2013; Mishra 2017). Interestingly, both the alleles were also amplified in the C. chinense var. PBC932 and C. baccatum var. PBC80, which have been formerly identified as natural sources of resistance to chili anthracnose (AVRDC 2003). However, the remaining 32 susceptible genotypes, including the susceptible parent ‘Arka Lohit’ could not amply either of the RCt1-linked markers. Therefore, the dominant markers CtR-431 and CtR-594 could be highly useful for marker-assisted selection of RCt1 gene in the background of these chili genotypes.
Discussion
Although a combination of different strategies, including chemical, physical, and biological approaches, has been recommended for managing chili anthracnose, the complexity of the causal pathogen and emergence of insensitive races makes these techniques only partially effective. Hence, the most practical, cost-effective, and safer disease management strategy involves the development of high-yielding anthracnose-resistant cultivars. Comprehensive understanding about the genetic basis of resistance in the parental genotype is a prerequisite for effective molecular tagging (Dhole and Reddy 2013). C. annuum cv. ‘Punjab Lal’ that was previously reported as a resistant genotype to C. truncatum infection (Garg et al. 2013; Mishra 2017; Mishra et al. 2018) was equally vindicated by disease reaction in the present study. Therefore, ‘Punjab Lal’ could be used for transferring resistance against C. truncatum into commercial high-yielding susceptible chili varieties. Phenotypic reaction of F1, F2, and BC1F1 population for C. truncatum resistance and susceptibility revealed that the resistance in ‘Punjab Lal’ is governed by a single dominant gene named as RCt1. C. chinense as well as the progressive lines of C. annuum developed from it exhibited resistance against C. truncatum by effect of a single recessive gene in green and red fruits (Pakdeevaraporn et al. 2005; Kim et al. 2008a, b; Mahasuk et al. 2009). In contrast, the C. annuum breeding lines’83-168′ and ‘Chungryong’ reported monogenic-dominant resistance (Lin e al. 2002). In addition, progressive lines of chili developed from a cross between C. annuum and C. chinense reported polygenic inheritance for C. truncatum resistance with at least one major QTL linked to infection frequency and lesion diameter (Voorrips et al 2004). These reports together with our findings from the present study suggest that inheritance of resistance to anthracnose caused by C. truncatum is highly variable with respect to resistance sources, pathotypes, and fruit maturity stages (Chunying et al. 2015).
Two SSR markers, an SCAR-insertion/deletion (Indel) marker and an STS marker, have been previously developed for the selection of C. chinense and C. baccatum specific fragment in progressive lines of C. annuum (Lee et al. 2010, 2011; Wang 2011). However, the SCAR indel and the particular STS marker are no longer reliable, since the positive PCR product tagging the resistance gene has become obvious in susceptible chili accessions (Suwor et al. 2015). Besides, the two SSR markers also failed to amplify the expected fragments in the C. annuum accessions used in the present study, even though the PCR reaction was successful. Therefore, a new set of reliable PCR-based markers for the identification of C. annuum fragments containing the gene conferring resistance to anthracnose in chili were developed in this study. Forty-four polymorphic primers including 28 ISSRs and 16 AFLPs were used to identify molecular markers associated with anthracnose resistance in C. annuum. Usage of ISSR in gene tagging experiments have several advantages such as high reproducibility, no requirement for prior sequence information, and multilocus amplification from both coding and non-coding regions (Agarwal et al. 2008). In addition, the amplification of fragments delimited by simple repeat sequences using ISSR primers is quick and easy given that there is a large abundance of SSRs in the eukaryotic genomes (Kar et al. 2014). Likewise, AFLPs are also useful in the analysis of genetic relationship between individuals in a population owing to high stability, rapid assay for detection of a wide range of polymorphism and efficient genome coverage for the creation of quick genetic maps (Chial 2008). ISSR- and AFLP-based molecular tagging of resistance genes has been carried out in multiple plant species including rice (Gowda et al. 2006), wheat (Li et al. 2007; Balta et al. 2014), tobacco (Zhang et al. 2012), turmeric (Kar et al. 2014), and more recently in black gram (Gupta et al. 2015) and cauliflower (Singh et al. 2015).
Broad spectrum information about the genetics of pathogen resistance and location of resistant gene is crucial for marker-assisted selection towards the development of durable resistance in susceptible genotypes (Sharma et al. 2016). The rapid detection of highly enriched DNA polymorphism linked to anthracnose resistance RCt1 locus was made possible using a BSA approach as have been reported earlier by several researchers (Kar et al. 2014; Sharma et al. 2016; Zou et al. 2016). In the recent times, the congregation of BSA with high-throughput genotyping approaches like microarrays and next-generation sequencing has resulted in rapid identification of genomic regions linked to desired phenotypes in numerous species (Becker et al. 2011; Kim et al. 2015; Win et al. 2016). The four putatively linked markers (ISSR411493, ISSR581485, ISSR1121857 and E-ACA/M-CTG516) as identified through BSA were co-segregated with the disease reactions in the F2 individuals and showed a good fit of 3:1 ratio. A further segregation of these markers in 1:1 ratio in the BC1F1 population confirmed their linkage with anthracnose resistance locus. As the ISSR and AFLP marker assays are complex, expensive, and generate multiple genetic loci, it is essential that they are converted into easy and reliable STSs to increase the efficiency and effectiveness for selecting resistant genotypes. Therefore, the four putative ISSR and AFLP markers identified in the present study were subsequently converted into PCR-based STS markers, viz., CtR431, CtR-594, CtR-496, and AFLP-376. Several studies have reported the development and utilization of STS markers in molecular mapping and marker-assisted selection of disease-resistant traits in multiple species including chili (Lee et al. 2011a, b; Chunying et al. 2015), wheat (Goutam et al. 2015) and rice (Luo et al. 2016). The four STSs demonstrated specific amplification only in the resistant genotypes which was further confirmed by the development of single hybridization fragment through gel blot assay. In addition, several primer sets designed for amplifying the internal and flanking regions of STSs produced the expected PCR bands in only the resistant parent and not in the susceptible parent (data not shown). This implies that the sequences of the four STSs were only present in the resistant parent and could be localized to a chromosomal region that is tightly linked to anthracnose resistance locus RCt1. In addition, the STSs were arbitrary fragments and revealed no sequence similarity with any known genes, suggesting that they are probably located in the intronic or non-coding regions as has been previously reported in the other plant species (Nanda et al. 2013; Kar et al. 2014). This is interesting, because multiple introns have been identified as regulators in the eukaryotic gene expression as well as the facilitators of foreign gene expression in transgenic tissues (Jeong et al. 2006; Lu et al. 2008). A similar role for the STSs identified in the present study cannot be ruled out given their unique linkage with anthracnose resistance response. However, additional experimental validation through knockout analysis of these fragments is needed to confirm the role of such intronic sequences in the regulation of resistance gene expression.
Molecular markers identified within 5 cM from the linked locus have a greater accuracy for indirect selection through MAS as they exhibit minimum recombination frequency (Randhawa et al. 2014; Ma et al. 2015). Besides, the accuracy for selection of resistance trait could be considerably increased using a pair of markers flanking the gene of interest. Therefore, the two linked markers—CtR-431 and CtR-594—closely flanking the RCt1 locus within a span of 4.1 cM could be recommended for resistance breeding in chili as they are expected to increase the accuracy for selecting anthracnose-resistant lines in segregating population. Successful application of MAS in plant breeding programs demands for precise identification and validation of linked markers in genotypes with different genetic background (Randhawa et al. 2014). The validation of the STS markers CtR-431 and CtR-594 linked to anthracnose resistance in different genotypes with the known anthracnose reaction revealed steady association of the markers in seven anthracnose-resistant genotypes and absent in all the 32 anthracnose susceptible genotypes. This confirms the presence of these markers with anthracnose resistance gene in different genetic backgrounds and, therefore, could be used to speed up the introgression of RCt1 locus in the susceptible chili variants for the development of high-yielding anthracnose resistance genotypes through MAS.
Conclusion
In conclusion, the molecular and phenotypic segregation of the F2 and BC1F1 population suggested that resistance to C. truncatum infection in C. annuum cultivar’Punjab Lal’ is governed by a monogenic-dominant gene RCt1. Although several anthracnose resistance genes and QTLs have been previously identified from allied species and progressive lines of cultivated chili, this is the first report of molecular tagging of an anthracnose resistance locus in the C. annuum background. RCt1 gene is flanked by four putatively linked STS markers, two of which (CtR-431 and CtR-594) could be significantly applied in MAS with high accuracy for selection of resistant trait. As we could not amplify or link any SSR markers to the resistance locus, the chromosomal location of the RCt1 in the C. annuum genome is yet to be ascertained. However, it is necessary to employ more robust and advanced DNA marker such as single-nucleotide polymorphism (SNPs) to fine map the resistance locus with more closely linked markers. Hence, the identification of new anthracnose resistance gene(s) in the C. annuum background together with development of tightly linked markers would be of huge significance towards development of pre-breeding anthracnose-resistant genetic lines in cultivated chili through marker-assisted breeding.
References
Agarwal M, Shrivastava N, Padh H (2008) Advances in molecular marker techniques and their application in plant sciences. Plant Cell Rep 27:617–631
Andrus CF, Moore WD (1935) Colletotrichum truncatum (Schw.). n. comb., on garden and lima beans. Phytopathology 25:121–125
Ashkani S, Rafii MY, Shabanimofrad M, Miah G, Sahebi M, Azizi P, Tanweer FA, Akhtar MS, Nasehi A (2015) Molecular breeding strategy and challenges towards improvement of blast disease resistance in rice crop. Front Plant Sci 6:886
AVRDC (2003) AVRDC report 2002, AVRDC publication number 03-563, Shanhua. AVRDC-The World Vegetable Center, Taiwan, pp 29–30
Balta H, Metin OK, Akfirat FS, Ertugrul F, Hasancebi S, Aydin Y, Akan K, Mert Z, Turet M, Uncuoglu AA (2014) Identification of an AFLP marker linked with yello rust resistance in wheat (Triticum aesivum L.). Turk J Biol 38:371–379
Becker A, Chao DY, Zhang X, Salt DE, Baxter I (2011) Bulk segregant analysis using single nucleotide polymorphism microarrays. PLoS ONE 6:15993
Byung SK (2007) Country report of Anthracnose research in Korea. In: First international symposium on chili anthracnose, Hoam Faculty House, Seoul National University, Seoul, 24
Chial H (2008) DNA fingerprinting using amplified fragment length polymorphisms (AFLP): no genome sequence required. Nat Educ 1:176
Chunying S, Li MS, Hai ZZ, Alain P, Hao WL, Xi ZB (2015) Resistances to anthracnose (Colletotrichum acutatum) of Capsicum mature green and ripe fruit are controlled by a major dominant cluster of QTLs on chromosome P5. Sci Hort 181:81–88
Dhole VJ, Reddy KS (2013) Development of a SCAR marker linked with a MYMV resistance gene in mungbean (Vigna radiate L. Wilczek). Plant Breed 132:127–132
Don LD, Van TT, Phuong VTT, Kieu PTM (2007) Colletotrichum spp. attacking on chilli pepper growing in Vietnam, country report. In: Oh DG, Kim KT (eds) Abstracts of the first international symposium on chilli anthracnose, Seoul National University, Seoul, 24
Doyle JJ, Doyle JL (1990) Isolation of plant genomic DNA from fresh tissue. Focus 12:13–15
Food and agriculture organization of the United Nations statistics (FAOSTAT) (2016) Available at http://faostat.fao.org/faostat/collections/subset/agriculture. Accessed 26 Sept 2017
Garg R, Kumar S, Kumar R, Loganathan M, Saha S, Kumar S, Awadhesh RB, Roy BK (2013) Novel source of resistance and differential reactions on chili fruit infected by Colletotrichum capsici. Aus Plant Pathol 42:227–233
Garg R, Loganathan M, Saha S, Roy BK (2014) Chili anthracnose: a review of causal organism, resistance source and mapping of gene. In: Kharwar RN, Upadhyay R, Dubey N, Raguwanshi R (eds) Microbial diversity and biotechnology in food security. Springer, Heidelberg, pp 589–610
Goswami S, Thind TS, Nagrale DT (2013) Efficacy of new fungicides against anthracnose of chili (Capsicum annuum) caused by Colletotrichum capsici. Indian J Phytopath 66:207–208
Goutam U, Kukreja S, Yadav R, Salaria N, Thakur K, Goyal AK (2015) Recent trends and perspectives of molecular markers against fungal diseases in wheat. Front Microbiol 6:861
Gowda M, Roy-Barman S, Chattoo BB (2006) Molecular mapping of a novel blast resistance gene Pi38 in rice using SSLP and AFLP markers. Plant Breed 125:596–599
Gupta SK, Souframanien K, Reddy KS (2015) Validation of molecular markers linked with yellow mosaic virus disease resistance in diverse genetic background of black gram (Vigna mungo). Electron J Plant Breed 6:755–763
Jeong YM, Mun JH, Lee I, Woo JC, Hong CB, Kim SG (2006) Distinct roles of the first introns on the expression of Arabidopsis profilin gene Family members. Plant Physiol 140:196–209
Kar B, Nayak S, Joshi RK (2014) Development and evaluation of STS diagnostic marker to track turmeric (Curcuma longa L.) resistance against rhizome rot caused by Pythium aphanidermatum. Aust Plant Pathol 43:167–175
Kim SH, Yoon JB, Do JW, Park HG (2007) Resistance to anthracnose caused by Colletotrichum acutatum in chili pepper (Capsicum annuum L.). J Crop Sci Biotech 10:277–280
Kim JT, Park SY, Choi W, Lee YH, Kim HT (2008a) Characterization of Colletotrichum isolates causing anthracnose of pepper in Korea. Plant Pathol 24:17–23
Kim SH, Yoon JB, Do JW, Park HG (2008b) A major recessive gene associated with anthracnose resistance to Colletotrichum capsici in chili pepper (Capsicum annuum L.). Breeding Sci 58:131–141
Kim S, Kim CW, Park M, Choi D (2015) Identification of candidate genes associated with fertility restoration of cytoplasmic male-sterility in onion (Allium cepa L.) using a combination of bulked segregant analysis and RNA-sEq. Theor Appl Genet 128:2289–2299
Krishnamurthy SL, Prasanth Y, Rao AM, Reddy KM, Ramachandra R (2015) Assesment f AFLP marker based genetic diversity in chilli (Capsicum annuum L. & C. baccatum L.). Indian J Biotechnol 14:49–54
Kumar LD, Kathirvel M, Rao GV, Nagaraju J (2001) DNA profiling of disputed chilli samples (Capsicum annuum) using ISSR-PCR and FISSR-PCR marker assays. Forensic Sci Int 116:63–68
Lee J, Hong JH, Do JW, Yoon JB (2010) Identification of QTLs for resistance to anthracnose to two Colletotrichum species in pepper. J Crop Sci Biotech 13:227–233
Lee J, Do JW, Yoon JB (2011a) Development of STS markers linked to the major QTLs for resistance to the pepper anthracnose caused by Colletotrichum acutatum and C. capsici. Hort Environ Biotechnol 52:596–601
Lee J, Jee HH, Jae WD, Jae BY (2011b) Development of STS markers linked to the major QTLs for resistance to the pepper anthracnose caused by Colletotrichum acutatum and C. capsici. Hort Environ Biotech 52:596–601
Li LI, Ynag WX, Li YN, Liu DQ, Yan HF, Meng QF, Zhang T (2007) Identification of AFLP markers linked to Lr19 resistance to wheat leaf rust. Agric Sci China 6:311–315
Lin Q, Kanchana UC, Jaunet T, Mongkolporn O (2002) Genetic analysis of resistance to pepper anthracnose caused by Colletotrichum capsici. Thai J Agric Sci 35:259–264
Lu J, Sivamani E, Azhakanandam K, Samadder P, Li X, Qu R (2008) Gene expression enhancement mediated by the 5′ UTR intron of the rice rubi3 gene varied remarkably among tissues in transgenic rice plants. Mol Genet Genom 279:563–572
Luo Y, Ma T, Zhang A, Ong KH, Li Z, Yang J, Yin Z (2016) Marker-assisted breeding of the rice restorer line Wanhui 6725 for disease resistance, submergence tolerance and aromatic fragrance. Rice 9:66
Ma P, Xu H, Xu Y, Li L, Qie Y, Luo Q, Zhang X, Li X, Zhou Y, An D (2015) Molecular mapping of a new powdery mildew resistance gene Pm2b in Chinese breeding line KM2939. Theor Appl Genet 128:613–622
Mahasuk P, Khumpeng N, Wasee S, Taylor PWJ, Mongkolporn O (2009) Inheritance of resistance to anthracnose (Colletotrichum capsici) at seedling andfruiting stages in chili pepper (Capsicum spp.). Plant Breed 128:701–706
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88(21):9828–9632
Mishra R (2017) Development of molecular markers and marker assisted selection towards anthracnose resistance in chili pepper (Capsicum annuum L.). Project completion report, SERB project no. SB/YS/LS-171, Science and Engineering Research Board. Ministry of Science and Technology, Govt. of India, New Delhi
Mishra R, Rout E, Joshi RK (2018) Identification of resistance sources against anthracnose disease caused by Colletotrichum truncatum and Colletotrichum gloeosporioides in Capsicum annuum L. Proc Natl Acad Sci India. https://doi.org/10.1007/s40011-018-0965-1
Montri P, Taylor PWJ, Mongkolporn O (2009) Pathotypes of Colletotrichum capsici, the causal agent of chili anthracnose, in Thailand. Plant Dis 93:17–20
Nanda S, Kar B, Nayak S, Jha S, Joshi RK (2013) Development of an ISSR based STS marker for sex identification in pointed gourd (Trichosanthes dioica Roxb.). Sci Hort 150:11–15
Pakdeevaraporn P, Wasee S, Taylor PWJ, Mongkolporn O (2005) Inheritance ofresistance to anthracnose caused by Colletotrichum capsici in Capsicum. Plant Breed 124:206–208
Rai VP, Kumar R, Kumar S, Rai A, Kumar S, Singh M, Singh SP, Rai AB, Paliwal R (2013) Genetic diversity in Capsicum germplasm based on microsatellite and random amplified microsatellite polymorphism markers. Physiol Mol Biol Plants 19:575–586
Ramachandran N, Rathnamma K (2006) Colletotrichum acutatum—a new addition to the species of chilli anthracnose pathogen in India. In: Paper presented at the annual meeting and symposium of Indian Phytopathological Society, Central Plantation Crops Research Institute, Kasarago
Ranathunge NP, Mongkolporn O, Ford R, Taylor PWJ (2012) Colletotrichum truncatum pathosystem on Capsicum spp.: infection, colonization and defence mechanisms. Austral Plant Path 41:463–473
Randhawa M, Bansal U, Valarik M, Klocova B, Dolezel J, Bariana H (2014) Molecular mapping of stripe rust resistance gene Yr51 in chromosome 4AL of wheat. Theor Appl Genet 127:317–324
Saxena A, Raghuvanshi R, Singh HB (2014) Molecular, phenotypic and pathogenic variability in Colletotrichum isolates of subtropical region in North Eastern India, causing fruit rot of chilies. J Appl Microbiol 117:1422–1434
Sharma BB, Kalia P, Yadava DK, Singh D, Sharma TR (2016) Genetics and molecular mapping of black rot resistance locus Xca1bc on chromosome B-7 in ethiopian mustard (Brassica carinata A. Braun). PLoS ONE 11:e0152290
Singh S, Sharma SR, Kalia P, Deshmukh P, Katara J, Sharma P, Sharma TR (2015) Identification of putative DNA markers for disease resistance breeding in Indian cauliflower (Brassica oleracea var. Botrytis L.). Indian J Biotech 14:455–460
Suwor P, Thummabenjapone P, Sanitchon J, Kumar S, Techawongstien S (2015) Phenotypic and genotypic responses of chili (Capsicum annuum L.) progressive lines with different resistant genes against anthracnose pathogen (Colletotrichum spp.). Eur J Plant Pathol 143:725–736
Than PP, Prihastuti H, Phoulivong S, Taylor PWJ, Hyde KD (2008) Chili anthracnose disease caused by Colletotrichum species. J Zhejiang Univ Sci Biomed Biotechnol 9:764–778
Thind TS, Jhooty JS (1990) Studies on variability in two Colletotrichum spp. causing anthracnose and fruit rot of chili in Punjab. Indian Phytopathol 43:53–58
Untergrasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3-new capabilities and interfaces. Nucleic Acids Res 40(15):e115
Vasanthakumari MM, Shivana MN (2013) Biological control of anthracnose of chili with rhizosphere and rhizoplane fungal isolates from grasses. Arch Phytopath Plant Protect 46:1641–1666
Voorrips RE, Finkers R, Sanjaya L, Groenwold R (2004) QTL mapping of anthracnose (Colletotrichum spp.) resistance in a cross between Capsicum annuum and C. chinense. Theor Appl Genet 109:1275–1282
Vos P, Hogers R, Bleeker M, Reijans M, Van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23(21):4407–4414
Wang YW (2011) Development of sequence characterized amplified region (SCAR) markers associated with pepper anthracnose (Colletotrichum acutatum) resistance. Master Thesis, Department of Agronomy, National Chiayi University, Taiwan
Win KT, Vegas J, Zhang C, Song K, Lee S (2016) QTL mapping for downy mildew resistance in cucumber via bulked segregant analysis using next-generation sequencing and conventional methods. Theor Appl Genet 130:199–211
Yoon JB, Park HG (2005) Trispecies bridge crosses, (Capsicum annuum × C. chinense) × C. baccatum, as an alternative for introgression of anthracnose resistance from C. baccatum into C. annuum. Hort Environ Biotechnol 46:5–9
Zhang S, Gao M, Zaitlin D (2012) Molecular linkage mapping and marker-trait associations with NiRPT, a downy mildew resistance gene in Nicotiana langsdorffi. Front Plant Sci 3:185
Zou C, Wang P, Xu Y (2016) Bulked sample analysis in genetics, genomics and crop improvement. Plant Biotechnol J 14:1941–1955
Acknowledgements
The study is funded by a research grant (FT/YS/LS-171/2013) from the Science and Engineering Research Board (SERB), Dept. Of Science and Technology (DST), Government of India. RM is grateful to SERB, DST, India for the financial support in the form of Young Scientist Fellowship. We also thank DST-FIST, Govt. of India, for the research infrastructure facilities provided to Centre of Biotechnology, Siksha O Anusandhan University.
Author information
Authors and Affiliations
Contributions
RM and RKJ conceived and supervised the project. RM collected samples, isolated DNA, and performed molecular marker analysis and detection of linked markers. ER and JNM cloned the STS fragments and performed marker validation. RM and RKJ interpreted the data and prepared the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13205_2018_1552_MOESM1_ESM.tif
Figure S1: Representative images depicting the screening of resistant and susceptible parents and F2 genotypes (Punjab Lal × Arka Lohit) of Capsicum annuum against anthracnose pathogen Colletotrichum truncatum. Inoculated matured red fruits of (A) Resistant parent ‘Punjab Lal’; (B) Susceptible parent ‘Arka Lohit’; (C–D) Resistance reaction in segregating F2 plants; (E–F) Susceptible reaction in the segregating F2 plants (TIF 1594 KB)
13205_2018_1552_MOESM2_ESM.tif
Figure S2: Bulk segregant analysis and amplification pattern of polymorphic ISSR and AFLP markers in F2 individuals either resistant or susceptible to chili anthracnose. (A) Amplification with Ca-ISSR41 [(GACA)4]; (B) Amplification with Ca-ISSR112 [(GT)8C]; (C) Amplification with Ca-ISSR58 [(CA)8T]; (D) Amplification with AFLP marker E-ACA/M-CTG. M, molecular weight marker; R, Resistant genotype—Punjab Lal; S, susceptible genotype—Arka Lohit, RB, bulk DNA from ten resistant F2 individuals; SB, bulk DNA from ten susceptible F2 individuals. 1–10, F2 individuals resistant to anthracnose; 11–20, F2 individuals susceptible to anthracnose. Please note that 18 F2 individuals were amplified using Ca-ISSR112 and E-ACA/M-CTG markers (TIF 528 KB)
13205_2018_1552_MOESM3_ESM.tif
Figure S3: DNA sequence of the Capsicum annuum resistance-specific markers ISSR41 [(GACA)4]-1493. The underlined region represents the position of the specific ISSR primers. The arrow with the shaded region indicates the position of specific primers for amplifying the sequenced tagged sites marker CtR-431 (TIF 102 KB)
13205_2018_1552_MOESM4_ESM.tif
Figure S4: DNA sequence of the Capsicum annuum resistance-specific markers ISSR112 [(GT)8C]-1857. The underlined region represents the position of the specific ISSR primers. The arrow with the shaded region indicates the position of specific primers for amplifying the sequenced tagged sites marker CtR-594 (TIF 117 KB)
13205_2018_1552_MOESM5_ESM.tif
Figure S5: DNA sequence of the Capsicum annuum resistance-specific markers ISSR58 [(CA)8T]-1485. The underlined region represents the position of the specific ISSR primers. The arrow with the shaded region indicates the position of specific primers for amplifying the sequenced tagged sites marker CtR-496 (TIF 100 KB)
13205_2018_1552_MOESM6_ESM.tif
Figure S6: DNA sequence of the Capsicum annuum resistance-specific AFLP marker E-ACA/M-CTG-516. The underlined region represents the position of the specific AFLP primers. The arrow with the shaded region indicates the position of specific primers for amplifying the sequenced tagged sites marker AFLP-376 (TIF 55 KB)
Rights and permissions
About this article
Cite this article
Mishra, R., Rout, E., Mohanty, J.N. et al. Sequence-tagged site-based diagnostic markers linked to a novel anthracnose resistance gene RCt1 in chili pepper (Capsicum annuum L.). 3 Biotech 9, 9 (2019). https://doi.org/10.1007/s13205-018-1552-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1552-0