Abstract
Waxy corn is popular beacuse of its high amylopectin due to mutation in granule-bound starch synthase-I or Waxy1 (Wx1) gene. Here, we characterized the wx1 allele among 24 diverse waxy inbreds using gene-based markers. A total of 29 alleles with average of 1.81 alleles/locus were observed. Major allele frequency varied from 0.42 to 1.00, with mean of 0.74. The polymorphism information content ranged from 0.00 to 0.56 (average 0.24). Three simple sequence repeat markers, viz., phi027, phi022 and phi061 were more polymorphic in the study. The mean heterozygosity was 0.04, which indicated attainment of higher levels of homozygosity. Dissimilarity coefficient varied from 0.00 to 0.90 with average of 0.51. Seventeen diverse haplotypes of wx1 allele were observed that was consistent with the pedigree. Cluster analyses grouped 24 genotypes into two main clusters each having sub-clusters. The information generated here possesses great potential for improvement of high amylopectin in maize through marker-assisted selection. This is the first report of molecular dissection of wx1 gene among the novel waxy inbreds developed in India.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Waxy maize possesses 95–100% of amylopectin compared to 70–75% in normal maize and has been abundantly used for cultivar development (Zhou et al. 2016). Waxy maize, also called as ‘sticky maize’ is a popular choice as food in China and other South Asian countries (Xiaoyang et al. 2017). It is also popular in North-Eastern parts of India as food prepared from waxy maize grains is widely preferred by the local people. Food prepared from waxy grains is easily digestible in human gut as compared to normal maize with higher amylose fractions (Fukunaga et al. 2002). Immature green ears of waxy maize are also gaining popularity as a breakfast item worldwide. Further, high viscosity of amylopectin makes the starch of waxy grains suitable for adhesive, paper and textile industries (Bao et al. 2012; Devi et al. 2017). Therefore, waxy maize possesses great potential as a high value crop (Tian et al. 2009).
Waxy maize was first discovered in 1909 at Yunnan–Guangxi region of China and subsequently disseminated to other Asian countries (Zheng et al. 2013). The coding region of Waxy1 (Wx1) gene (located on chromosome 9) consists of 14 exons spanning 3718 bp (Zheng et al. 2013). In the endosperm, Wx1 codes for the granule-bound starch synthase-I (GBSS-I), which catalyses amylose biosynthesis from ADP-glucose (Klosgen et al. 1986; Mason-Gamer et al. 1998). However, the mutant version which acts as a recessive allele (wx1) suppresses the action of GBSS-I that shifts ADP-glucose towards synthesis of amylopectin (Tsai 1974; Wessler et al. 1986; Bao et al. 2012; Zhang et al. 2013). The visual appearance of mutant waxy endosperm is distinct from normal maize endosperm, and can be easily identified through its cloudy appearance.
Waxy germplasm especially from China, Vietnam and Korea has been well characterized using simple sequence repeats (SSRs) distributed throughout the genome (Park et al. 2008; Hung et al. 2012; Zheng et al. 2013). However, molecular characterization of the waxy locus provides more useful information on variation of recessive wx1 alleles present in the diverse germplasm. Fan et al. (2008) studied nucleotide diversity at 9–14 exons of waxy locus, while Zheng et al. (2013) analysed sequence variation at 8–14 exons of the wx1 gene. So far, no study has been carried out to analyse the sequence variation of the entire wx1 gene, primarily due to involvement of considerable cost in sequencing. Shin et al. (2006) used only four single-nucleotide amplified polymorphism (SNAP) markers that led to the identification of four SNPs at waxy locus. In the present study, we used gene-based markers to assess the genetic variation in the entire length of wx1 locus in a panel of 24 diverse waxy inbreds developed at ICAR-Indian Agricultural Research Institute (IARI), New Delhi. The aims of present investigation were (1) to analyse the sequence variations in wx1 allele present in diverse waxy inbreds; (2) to identify haplotype patterns of wx1 allele, and (3) to study the genetic relationships among inbreds based on the polymorphism at wx1 locus.
Materials and methods
Panel of waxy maize inbreds
The pedigree details of waxy inbreds (MGUWX-101 to 124) developed at ICAR-IARI, New Delhi, are described in Table 1. Selection from the populations—and introgression—of wx1 strategy were employed for development of the waxy inbreds. Among the waxy inbreds, 15 were of white kernel colour, while nine inbreds of yellow kernel colour. The purity of inbreds was conserved through manual selfing.
Extraction of genomic DNA
Genomic DNA was isolated from the seeds using sodium dodecyl sulphate (SDS) extraction protocol (Dellaporta et al. 1983) and quality was checked on 0.8% agarose gel. The extracted DNA was quantified on UV-spectrophotometer (BT-UVS-SBA-E, G-Biosciences).
Primers used in the study
Markers designed by Shin et al. (2006), Liu et al. (2007) and Bao et al. (2012) were used to cover the major portion of Wx1 gene, (Table 2). Fourteen primers were selected from the studies of Shin et al. (2006), Liu et al. (2007) and Bao et al. (2012), and three Wx1-based SSRs retrieved from maize genome database (http://www.maizegdb.org) were also used in the present study. Primer binding sites of selected markers in the Wx1 gene are presented in Fig. 1. The purified and lyophilized form of oligonucleotide primers was synthesized from M/s. Macrogen. Final primer concentration of 10 µM in Milli-Q water was utilized.
Genotyping of waxy1 locus
The PCR reactions of final volume 20 µl consisting of 1X One PCR™ mix (Ready-to-use PCR mix, Gene Direx), 50 ng of template DNA, 0.5 µM of forward and reverse primers, were performed using Applied Biosystems’ Veriti96-thermal cycler. The amplification protocols were standardized for each of the primer-pairs as the size of amplicons varied from 70 to 1150 bp. Denaturation at 95 °C/5 min, 35 cycles consisting of denaturation at 95 °C/45 s, primer annealing ranged between 55 and 62 °C/45 s, primer extension at 72 °C/45–90 s and final extension at 72 °C/8 min was followed depending upon the amplicon size. The separation of PCR amplicons was performed using 4.0% agarose gel (Lonza, Rockland, ME USA).
Analysis of molecular data
The various genetic parameters (polymorphism information content, gene diversity, heterozygosity, total number of alleles, major allele frequency) were estimated by PowerMarker V3.0 (Liu and Muse 2005). DARwin-6.0 software (Perrier et al. 2003) was used for hierarchical clustering-based dendrogram construction. Jaccard’s coefficient was used to calculate dissimilarity matrix. The principal coordinate analysis (PCoA) was also performed to supplement the clustering pattern to further illustrate the diversity of the inbreds (Perrier et al. 2003).
Results and discussion
Genetic variation in wx1 locus
Of the 17 markers employed in the study, S2FR marker showed monomorphic pattern across the genotypes and therefore was not considered for data analysis. A total of 29 alleles within wx1 locus were detected among the 24 waxy maize inbreds, with mean of 1.81 alleles/locus (Table 3). Number of alleles/locus ranged from 1 to 3 among the inbreds. In contrast, higher numbers of alleles were identified in studies where waxy inbreds were characterized utilizing markers spread across the maize genome and not linked to wx1. For example, Yu et al. (2012) reported 60 alleles with a mean of 2.73 alleles/locus and a range of 2–4 alleles/locus. Similarly, Park et al. (2008), Hung et al. (2012) and Zheng et al. (2013) reported much higher values studying genetic characterization of waxy maize genotypes, viz., 127, 117 and 104 alleles; 4.20 alleles/locus, 3.26 alleles/locus and 5.20 alleles/locus; and range of 2–9 alleles/locus, 1–6 alleles/locus and 2–8 alleles/locus, respectively. Markers in non-coding region tend to show more polymorphism, as they are not likely to affect the fitness of the organism. The extent of polymorphism within any specific gene is low, as nucleotide variation affecting the fitness is generally lost and only beneficial or neutral mutations are retained in the populations. In the quality protein maize (QPM) inbreds, Babu et al. (2012a) also reported higher number of alleles/locus (3.35) using genome-based SSRs, while it was low (2.75 alleles/ locus) for lysine- and tryptophan-biosynthesis pathway-specific candidate gene-based SSR analyses (Babu et al. 2012b). Among the 16 polymorphic markers identified in the study, five markers [S1FR (1130 bp), P1/P2 (1070 bp), P5/P6 (1110 bp), S1/S2 (450 bp) and wx1-2507-F/RC (300 bp)] showed presence–absence polymorphism, eight markers showed co-dominant behaviour, while 3 markers exhibited both dominant and co-dominance pattern (Table 3). Two alleles were revealed by nine markers while three alleles were amplified with two markers (Fig. 2). Shin et al. (2006) analysed the diversity of wx1 gene using single nucleotide-amplified polymorphic (SNAP) markers, and showed allele-specific polymorphisms within waxy locus in maize. It is well established that molecular structure of waxy locus in rice and foxtail millet possesses co-linearity with that of maize (Fukunaga et al. 2002). Van et al. (2008) has reported 17 single nucleotide polymorphic (SNP) markers in non-coding and three SNPs in coding region of waxy locus of foxtail millet. Nucleotide variation in waxy locus of rice has also been reported by Chrungoo and Devi (2016). However, in our study, of the 16 polymorphic markers, three were SSRs which produced 2.67 alleles/locus compared to 1.61 alleles/locus for the rest 13 markers. The high level of allelic polymorphism detected by SSRs is possibly due to reasons like recombination errors, unequal crossing over and replication slippage at the SSR locus (Tautz and Schlotterer 1994).
The major allele frequency varied from 0.42 (phi027) to 1.00 (S1FR, P1/P2, P5/P6, S1/S2, wx1-2507-F/RC) with mean of 0.74 (Table 3). The range for gene diversity was from 0.00 (S1FR,P1/P2, P5/P6, S1/S2, wx1-2507-F/RC) to 0.64 (phi061), while the mean was 0.30. Polymorphism information content (PIC) among the markers varied from 0.00 (S1FR, P1/P2, P5/P6, S1/S2, wx1-2507-F/RC) to 0.56 (phi061) with an average of 0.24 (Table 3). Eight markers exhibited PIC > 0.30. The mean PIC of markers present within wx1 locus is lower than PIC of genome-based SSRs observed in waxy germplasm. For example, Hung et al. (2012) and Hao et al. (2015) reported PIC of 0.46 and 0.31 in waxy germplasm, respectively. The higher PIC values, 0.70 and 0.62 among waxy maize germplasm were observed by Zheng et al. (2013) and Sa et al. (2015), respectively. Low PIC is an indicator of lower diversity of the gene. This may be due to beneficial nucleotide variation fixed through strong positive selection for waxy locus. Whitt et al. (2002) also found low level of nucleotide diversity or ‘selective sweep’ at wx1 locus in maize. Similar observation in wx1 locus was also reported by Fan et al. (2008) where 25 Chinese glutinous maize accessions were characterized. Their results suggested that a genomic region of 53 kb or longer, was affected by selective sweep in the waxy maize germplasm. The phenomenon of selective sweep has also been described in teosinte branched1 (tb1) and yellow1 (y1) genes in maize (Wang et al. 1999; Palaisa et al. 2004). However, PIC among SSRs ranged from 0.37 to 0.56, compared to 0.00 to 0.38 in other markers, thereby suggesting the hyper-variability of SSR locus.
The lower heterozygosity (range 0.00–0.23; mean 0.04) among the set of inbreds indicates that the repeated selfing of inbreds has led to higher degree of homozygosity (Table 3). However, some primers, viz., R2 (0.23), D7 (0.23), D10 (0.13), phi022 (0.04) and wx-2507-F/RG (0.04) detected heterozygosity among the 24 waxy inbreds. Since maize is a highly cross-pollinated crop, residual heterozygosity at few loci remains and continues to perpetuate as breeders select lines based on morphological uniformity. This could be the possibility of heterozygosity at wx1 gene, although waxy phenotype in different lines may look similar. Several maize researchers have reported residual heterozygosity in maize inbreds despite of continuous inbreeding (Choudhary et al. 2015; Muthusamy et al. 2015; Zunjare et al. 2015; Mehta et al. 2017). Complete homozygosity across the genome is achieved through doubled haploid technique which requires 1–2 generations compared to 6–7 generations of selfing in conventional inbred development programme (Choudhary et al. 2015).
Haplotype variation in wx1 locus
A total of 17 haplotypes of recessive wx1 gene were detected among the 24 inbreds using 16 gene-based markers (Fig. 2). MGUWX-120, -121, -122 possessed same haplotype of waxy locus inherited from the parent germplasm, K027LOSBANOS (waxy). Similarly, (1) MGUWX-101 and MGUWX-102; (2) MGUWX-111 and MGUWX-112; (3) MGUWX-113 and MGUWX-114; (4) MGUWX-107 and MGUWX-108, and (5) MGUWX-103 and MGUWX-104, also had the same haplotype. Shin et al. (2006) working with 40 waxy accessions identified two haplotypes for wx1 allele using four SNAP markers. While three and four haplotypes in wild-type Wx1 gene were observed in 26 dent and 15 sweet corn accessions, respectively. Van et al. (2008) reported 23 SNP haplotypes in waxy locus in a set of 113 landraces of foxtail millet adapted in Korea. The haplotype patterns generated here, serve as the tool for the identification of specific wx1 alleles present in the waxy germplasm. The expression analyses of diverse wx1 would help in identifying the most desirable allele as it would possess the highest amylopectin. A combination of markers specific to a haplotype of the most desirable allele can be used in the marker-assisted breeding programme. Marker-based on single polymorphism is having less probability to be polymorphic between recipient and donor, than based on combination of markers. The haplotypes are the result of accumulation of different mutation types, viz., insertion of transposable elements, retroposons and fragments of few nucleotides and deletion of nucleotides in the wx1 allele (Bao et al. 2012; Devi et al. 2017). Reports suggest different types of spontaneous mutations including addition of transposable elements and few nucleotides into genic and intergenic regions of wx1 locus exist in maize germplasm. This may lead to (1) disruption of coding regions; (2) modified transcripts; (3) premature termination of polypeptide due to occurence of stop codon or alteration of amino acids in protein domain, and (4) alternative splicing and/or decreased expression of wx1 allele (Wessler and Varagona 1985; Okagaki et al. 1991; Marillonnet and Wessler 1997; Liu et al. 2007; Tian et al. 2008; Ding et al. 2009).
Relationships among waxy inbreds
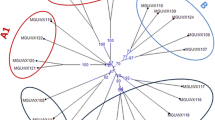
Genetic dissimilarity among the waxy inbreds ranged from 0.00 to 0.90 with an average of 0.51. Cluster diagram grouped 24 genotypes into two major clusters, viz., − A and − B (Fig. 3, Table S1). The genetic dissimilarity comparisons of the 276 pairs revealed presence of genetic variation in waxy locus. 23% of total pair of inbreds belonged to 0.70–0.79 dissimilarity thereby suggesting higher levels of genetic divergence among those inbreds (Fig. 4). Cluster-A had 12 genotypes with two sub-clusters (− A1 and − A2). Sub-cluster-A1 comprised 10 inbreds, viz., MGUWX-122, MGUWX-121, MGUWX-120, MGUWX-124, MGUWX-119, MGUWX-117, MGUWX-104, MGUWX-103, MGUWX-110, and MGUWX-109. Sub-cluster-A2 possessed only two inbreds, MGUWX-106 and MGUWX-107. Cluster-B had 12 genotypes with two sub-clusters, viz., -B1 and -B2 (Fig. 3). The B1 sub-cluster consisted of 10 waxy inbreds, viz., MGUWX-114, MGUWX-113, MGUWX-115, MGUWX-112, MGUWX-111, MGUWX-118, MGUWX-116, MGUWX-102, MGUWX-101, and MGUWX-105; while sub-cluster-B2 had only two inbreds (MGUWX-123 and MGUWX-106). Shin et al. (2006) analysed 81 maize accessions including 40 waxy types from South Korea using SNAP markers present in wx1 and other genes, viz., shrunken2 (sh2), brittle2 (bt2), sugary1 (su1) and amylose extender (ae1). Waxy germplasm existed in clusters I–I, I–II, II–I and II–II, and generally clustered away from the dent and sweet corn accessions. Fan et al. (2008) also analysed 25 Chinese waxy accessions along with normal maize and wild maize using wx1-based markers, and observed close relationship between waxy genotypes with cultivated maize but diverse from Coix lacryma-jobi. Their results suggested that domestication of cultivated maize from wild species occurred first followed by selection of Wx1 gene for glutinous characteristics. The clustering pattern observed in the study was also supported by PCoA (Fig. 5). The PCoA revealed genetic diversity for waxy locus as the inbreds were spread across the quadrangles.
Utilization of waxy inbreds in breeding programme
In a conventional waxy breeding programme, breeders rely upon presence of cloudy phenotypic expression in the endosperm for developing the waxy inbreds. Generally, breeders consider that waxy trait in the germplasm is due to one wx1 allele and various haplotypes of wx1 present in different germplasms. The study revealed that there are 17 haplotypes of wx1 allele which provides great advantage in the breeding programme. Among the 17 markers used, three markers, viz., R2, D7 and wx1-2507-F/RG showed both co-dominant as well as dominant nature, eight markers, viz., D10, phi022, phi027, phi061, S3FR, S4FR, S5FR and P3/P4 behaved as co-dominant marker, while, five markers, viz., S1FR, P1/P2, P5/P6, S1/S2 and wx1-2507-F/RC exhibited only dominant behaviour (Table 3). The co-dominance is due to insertion and deletion of nucleotide(s) within the amplicon generated by the marker, and is an ideal choice in marker-assisted selection (MAS), as it can differentiate the homozygotes from heterozygotes (Hossain et al. 2018). In a situation, where a normal maize hybrid is to be improved for amylopectin content, the parental lines can be introgressed with wx1 allele using marker-assisted backcross breeding (MABB) (Sarika et al. 2018). MABB is an accelerated method of breeding and requires only two generations of backcrossing compared to 6–7 generations in conventional methods (Muthusamy et al. 2014; Zunjare et al. 2018). As a first step, the recurrent parents and waxy donors should show polymorphism between Wx1 and wx1 alleles. Of the 16 gene-based markers, the ones that differentiate the Wx1 (from recurrent parent) and wx1 (from donor parent) can be used in the MAS programme. If the marker shows co-dominance, then heterozygotes (Wx1/wx1) can be identified from dominant homozygotes (Wx1/Wx1) in backcross generations (BC1F1 or BC2F1) under the MABB. In BC2F2, the recessive homozygotes (wx1/wx1) can also be easily identified and selected for further line derivation through repeated selfing. The dominant behaviour of the markers is primarily due to change in nucleotide sequence at the primer binding site, thereby showing presence and absence of polymorphism. The dominant markers can also be used in MABB programme, if the waxy donor produces a band, while recurrent parent shows absence of band. In BC1F1 or BC2F1, the heterozygotes can be selected by presence of allele. In BC2F2, the desirable homozygotes (wx1/wx1) can be selected by phenotype of the seeds having cloudy expression compared to glossy expression in wild type (Wx1/wx1 or Wx1/Wx1) seeds.
Conclusion
The present investigation is the first report on characterization of wx1 allele present in Indian waxy maize germplasm. It revealed the presence of 17 diverse haplotypes of wx1 which fall into two major clusters. Five of the markers showed dominant behaviour, eight markers showed co-dominant behaviour, while the rest three behaved in both co-dominant and dominant pattern in the present study. The results of our analysis can be effectively used in the breeding programme dealing with the improvement of amylopectin content in maize grains.
References
Babu BK, Pooja P, Bhatt JC, Agrawal PK (2012a) Characterization of Indian and exotic quality protein maize (QPM) and normal maize (Zea mays L.) inbreds using simple sequence repeat (SSR) markers. Afr J Biotechnol 11:9691–9700
Babu BK, Agrawal PK, Gupta HS, Kumar A, Bhatt JC (2012b) Identification of candidate gene-based markers for lysine and tryptophan metabolic pathways in maize (Zea mays). Plant Breed 131:20–27
Bao JD, Yao JQ, Zhu JQ (2012) Identification of glutinous maize landraces and inbred lines with altered transcription of waxy gene. Mol Breed 30:1707–1714
Choudhary M, Hossain F, Muthusamy V, Thirunavukkarasu N, Saha S, Pandey N, Jha SK, Gupta HS (2015) Microsatellite marker-based genetic diversity analyses of novel maize inbreds possessing rare allele of β-carotene hydroxylase (crtRB1) for their utilization in β-carotene enrichment. J Plant Biochem Biotechnol 25:12–20
Chrungoo NK, Devi AG (2016) Sequence polymorphism in the Waxy locus and its relationship with apparent amylose content of endosperm starch in cultivars of rice (Oryza sativa L.) from Northeast India. Indian J Plant Physiol 21:556–568
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol 1:19–21
Devi EL, Hossain F, Muthusamy V, Chhabra R, Zunjare RU et al (2017) Microsatellite marker-based characterization of waxy maize inbreds for their utilization in hybrid breeding. 3 Biotech 7:316. https://doi.org/10.1007/s13205-017-0946-8
Ding XZ, Wang BG, Gao QH, Zhang Q, Yan GQ, Duan K, Huan JH (2009) Molecular diversity and differential expression of starch synthesis genes in developing kernels of three maize hybrids. Plant Cell Rep 28:1485–1497
Fan LJ, Qan LY, Leng XD, Guo XY, Hu WM, Ruan S et al (2008) Molecular evidence for post-domestication selection in the Waxy gene of Chinese waxy maize. Mol Breed 22:329–338
Fukunaga K, Kawase M, Kato K (2002) Structural variation in the Waxy gene and differentiation in foxtail millet [Setaria italica (L.) P. Beauv.]: implications for multiple origins of the waxy phenotype. Mol Genet Genom 268:214–222
Hao D, Zhang Z, Cheng Y, Chen G, Lu H, Mao Y, Shi M, Huang X, Zhou G, Xue L (2015) Identification of genetic differentiation between waxy and common maize by SNP genotyping. PLoS One 10:e0142585. https://doi.org/10.1371/journal.pone.0142585
Hossain F, Muthusamy V, Pandey N, Vishwakarma AK, Baveja A, Zunjare RU, Thirunavukkarasu N, Saha S, Manjaiah KM, Prasanna BM, Gupta HS (2018) Marker-assisted introgression of opaque2 allele for rapid conversion of elite maize hybrids into quality protein maize. J Genet 97:287–298
Hung TN, Huyen TN, Loc NV, Cuong BM (2012) The application of SSR molecular indicator to assess the purity and genetic diversity of waxy corn inbred lines. J ISSASS 18:45–54
Klosgen RB, Gierl A, Schwarz-Sommer Z, Saedler H (1986) Molecular analysis of the waxy locus of Zea mays. Mol Genet Genom 203:237–244
Liu K, Muse SV (2005) PowerMarker: integrated analysis environment for genetic marker data. Bioinformatics 21:2128–2129
Liu J, Rong T, Li W (2007) Mutation loci and intragenic selection marker of the granule-bound starch synthase gene in waxy maize. Mol Breed 20:93–102
Marillonnet S, Wessler SR (1997) Retrotransposon insertion into the maize waxy gene results in tissue-specific RNA processing. Plant Cell 9:967–978
Mason-Gamer RJ, Well CF, Kellogg EA (1998) Granule-bound starch synthase: Structure, function, and phylogenetic utility. Mol Biol Evol 15:1658–1673
Mehta B, Hossain F, Muthusamy V, Baveja A, Zunjare RU, Jha SK, Gupta HS (2017) Microsatellite-based genetic diversity analyses of sugary1-, shrunken2- and double mutant- sweet corn inbreds for their utilization in breeding programme. Physiol Mol Biol Plants. https://doi.org/10.1007/s12298-017-0431-1
Muthusamy V, Hossain F, Thirunavukkarasu N, Choudhary M, Saha S, Bhat JS, Prasanna BM, Gupta HS (2014) Development of β-carotene rich maize hybrids through marker-assisted introgression of β-carotene hydroxylase allele. PLoS One 9:1–22. https://doi.org/10.1371/journal.pone.0113583
Muthusamy V, Hossain F, Thirunavukkarasu N, Pandey N, Vishwakarma AK, Saha S, Gupta HS (2015) Molecular characterization of exotic and indigenous maize inbreds for biofortification with kernel carotenoids. Food Biotechnol 29:276–295
Okagaki RJ, Neuffer MG, Wessler SR (1991) A deletion common to two independently derived common to two independently derived waxy mutations of maize. Genetics 128:425–431
Palaisa K, Morgante M, Tingey S, Rafalski A (2004) Long range patterns of diversity and linkage disequilibrium surrounding the maize Y1 gene are indicative of an asymmetric selective sweep. Proc Natl Acad Sci USA 101:9885–9890
Park JS, Park JY, Park KJ, Lee JK (2008) Genetic diversity among waxy corn accessions in Korea revealed by microsatellite markers. Korean J Breed Sci 40:250–257
Perrier X, Flori A, Bonnot F (2003) Data analysis methods. In: Hamon P, Seguin M, Perrier X, Glaszmann JC (eds) Genetic diversity of cultivated tropical plants. Science Publishers Montpellier, Enfield, pp 43–76
Sa KJ, Park JY, Choi SH, Kim BW, Park KJ, Lee JK (2015) Genetic diversity, population structure, and association mapping of agronomic traits in waxy and normal maize inbred lines. Genet Mol Res 14:7502–7518
Sarika K, Hossain F, Muthusamy V, Zunjare RU, Baveja A, Goswami R, Bhat JS, Saha S, Gupta HS (2018) Marker-assisted pyramiding of opaque2 and novel opaque16 genes for further enrichment of lysine and tryptophan in sub-tropical maize. Plant Sci 272:142–152
Shin JH, Kwon SJ, Lee JK, Min HK, Kim NS (2006) Genetic diversity of maize kernel starch synthesis genes with SNAPs. Genome 49:1287–1296
Tautz D, Schlotterer C (1994) Simple sequences. Curr Opin Genet Dev 4:832–837
Tian ML, Huang YB, Tan GX, Liu YJ, Rong TZ (2008) Sequence polymorphism of waxy genes in landraces of waxy maize from Southwest China. Acta Agron Sin 34:729–736 (in Chinese with an English abstract)
Tian ML, Tan GX, Liu YJ, Rong TZ, Huang YB (2009) Origin and evolution of Chinese waxy maize: evidence from the Globulin-1 gene. Genet Resour Crop Evol 56:247–255
Tsai PS (1974) The function of the waxy locus in starch synthesis in maize endosperms. Biochem Genet 11:83–96
Van K, Onoda S, Kim YM, Lee SH (2008) Allelic variation of the Waxy gene in foxtail millet [Setaria italica (L.) P. Beauv.] by single nucleotide polymorphisms. Mol Genet Genom 279:255–266
Wang RL, Stec A, Hey J, Lukens L, Doebley J (1999) The limits of selection during maize domestication. Nature 398:236–239
Wessler SR, Varagona MJ (1985) Molecular basis of mutations at the waxy locus of maize: correlation with the fine structure genetic map. Proc Natl Acad Sci USA 82:4177–4181
Wessler SR, Baran G, Varagona M, Dellaporta SL (1986) Excision of Ds produces waxy protein with a range of enzymatic activities. EMBO J 5:2427–2432
Whitt SR, Wilson LM, Tenaillon MI, Gaut BS, Buckler ES (2002) Genetic diversity and selection in the maize starch pathway. Proc Natl Acad Sci USA 99:12959–12962
Xiaoyang W, Dan C, Yuqing L, Weihua L, Xinming Y, Xiuquan L, Juan D, Lihui L (2017) Molecular characteristics of two new waxy mutations in China waxy maize. Mol Breed 37:27
Yu RH, Wang YL, Sun Y, Liu B (2012) Analysis of genetic distance by SSR in waxy maize. Genet Mol Res 11:254–260
Zhang W, Yang W, Wang M, Wang W, Zeng G, Chen Z et al (2013) Increasing lysine content of waxy maize through introgression of opaque-2 and opaque-16 genes using molecular assisted and biochemical development. PLoS One 8:e56227
Zheng H, Wang H, Yang H, Wu J, Shi B, Cai R, Xu Y, Wu A, Luo L (2013) Genetic diversity and molecular evolution of Chinese waxy maize germplasm. PLoS One 8:1–11
Zhou Z, Song L, Zhang X, Li X, Yan N, Xia R et al (2016) Introgression of opaque2 into waxy maize causes extensive biochemical and proteomic changes in endosperm. PLoS One. https://doi.org/10.1371/journal.pone.0158971
Zunjare R, Hossain F, Muthusamy V, Vishwakarma AK et al (2015) Analyses of genetic diversity among exotic- and indigenous- maize inbreds differing for responses to stored grain weevil (Sitophilus oryzae L.) infestation. Maydica 60:1–7
Zunjare RU, Hossain F, Muthusamy V, Baveja A, Chauhan HS, Bhat JS, Thirunavukkarasu N, Saha S, Gupta HS (2018) Development of biofortified maize hybrids through marker-assisted stacking of β-carotene hydroxylase lycopene-ε-cyclase and opaque2 genes. Front Plant Sci 9:178
Acknowledgements
We are grateful to ICAR-IARI, New Delhi, for funding the study. We also express thanks to Dr. B.M. Prasanna, CIMMYT for sharing the source waxy germplasm.
Author information
Authors and Affiliations
Contributions
Design of experiment: FH; development of waxy inbreds: FH; compilation of marker information from public domain: SKJ; genotyping: RC and ELD; data analysis: FH and RC; field evaluation and maintenance of lines: RUZ; drafting of manuscript: FH and VM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflict of interest exists.
Rights and permissions
About this article
Cite this article
Hossain, F., Chhabra, R., Devi, E.L. et al. Molecular analysis of mutant granule-bound starch synthase-I (waxy1) gene in diverse waxy maize inbreds. 3 Biotech 9, 3 (2019). https://doi.org/10.1007/s13205-018-1530-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1530-6