Abstract
The cyclodextrin glycosyltransferase (CGTase) was used to catalyze the conversion of starch into cyclodextrins (CD) in industry. Improving the activity of CGTase to produce more CD with relative low cost is intensely interesting and has drawn wide attention. Amino acid mutation of His167 into Cys significantly enhanced β-CGTase activity; however, optimization of culture conditions for β-CGTase-H167C remains unclear. To determine this, the medium and culture conditions for β-CGTase-H167C were optimized with response surface methodology. Maximum activity of β-CGTase-H167C was obtained with the medium containing 1.1% corn starch, 4.4% corn steep liquor, 1.1% peptone, 0.02% MgSO4·7H2O and 0.1% K2HPO4·3H2O that were cultured with the initial pH 8.4, incubation temperature at 37.4 °C, with 5% inoculation size and shaking speed at 202 r/min. Under the optimal conditions, the activity of β-CGTase-H167C was up to 4355 U/mL, which is 1.93-fold in comparison with the initial activity. Our results established the promising culture strategy for the production of cyclodextrins by β-CGTase-H167C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclodextrin glycosyltransferase (CGTase, 2.4.1.19) is a member of the α-amylase family of glycosyl hydrolases that catalyzes the formation of cyclic-oligosaccharides called cyclodextrins (CDs) from starch and related carbohydrates (Rendleman and Knutson 1998; Terada et al. 1997). The main natural CDs are classified into α, β and γ CD containing 6, 7, and 8 glucopyranose units, respectively (Alves-Prado et al. 2008). The structure of CD has a hydrophilic outer surface and a hydrophobic cavity (Lindner and Saenger 1980). Cyclodextrins are widely used in food, cosmetic, and pharmaceutical industries, because they have the ability to form water-soluble inclusion complex with a wild range of substances and reshape their physicochemical properties (Alves-Prado et al. 2008).
Developing approaches to improve the quality and production of CDs with low production cost has been a long-term target in the industries. The CGTase produced mainly by Bacillus is a key enzyme capable of converting starch and related substances to CDs (Rosso et al. 2002). This kind of microorganism can be isolated with the biotechnological potential for the production of CGTase from different environments. The protein of the CGTase enzyme has been purified and the properties including molecular weight, thermal stability and pI have been well characterized. Strategies that facilitate the growth of microbial and improve the output of the enzyme are quite necessary for the potential industrial roles of CGTase.
Conventionally, the production of CGTase was achieved by incubating the bacteria with the culture medium that contains essential components for the growth of the microbial. Among the elements in the medium, the carbon and nitrogen sources were obtained with high cost and complex technical issues (Coelho et al. 2016). To solve this problem, agro-industrial origins of carbon and nitrogen sources have drawn wide attention (Akindahunsi and Oboh 2003; Brauman et al. 1996; Lepiz-Aguilar et al. 2013). Cassava flour, an agro-industrial product rich in starch, can be simply obtained and has been an alternative candidate for the carbon source (Akindahunsi and Oboh 2003; Brauman et al. 1996; Lepiz-Aguilar et al. 2013). Additionally, it has been reported that the corn steep liquor originated from corn processing and is commonly used as the nitrogen source in fermentation (Coelho et al. 2016; Ebadipour et al. 2016; He et al. 2017; Wischral et al. 2016). To overall improve the growth of microbial and the synthesis of enzyme, other essential elements in the culture medium including metal ions and phosphate also need to the optimized. As the growth of the bacteria and the activity of the enzyme are affected by fermentation conditions, such as the pH value of the medium, the culture temperature, shaking speed as well as the inoculum concentration, these parameters also need to the considered in the fermentation process.
Our previous data demonstrated that the site-directed mutation at the amino acid residue of His167 into Cys significantly enhanced the enzymatic activity of β-CGTase. This study aimed to establish the optimal conditions for β-CGTase-H167C production. The effect of different medium composition and culture conditions on the enzymatic activity of β-CGTase-H167C was detected. The optimal parameters for the product of β-CGTase were determined by response surface methodology and central composition design.
Materials and methods
Reagents
Primer STAR® HS DNA polymerase, restriction enzymes, Molecular weight maker 250 were purchased form TaKaRa (Dalian, China). Agarose, peptone, yeast extraction and agar were obtained from Sigma-Aldrich. The plasmid min-pre kit and gel extraction kit were purchased from TianGen Biotech (Beijing) Co. Ltd. Ampicillin and isopropyl β-d-thiogalactopyranoside (IPTG) were provided by Beijing Dingguo Changsheng Biotechnology Co. Ltd.
Bacterial strain
The cDNA sequence of β-CGTase with site-directed mutation H167C was constructed into the expression plasmid vector pUC-18, respectively. The resulting expression vector was verified by restriction analysis and sequencing. The mutant enzymes were expressed by transforming the mutant plasmids to the host E. coli strain BL21 (DE3) and induced by lactose. The expression of β-CGTase was purified with affinity chromatography and detected by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with 12% separating gel. The band was visualized by Coomassie Brilliant Blue R-250 dye staining.
Fermentation assay
The glycerol stock of E. coli strain BL21 (DE3) harboring pUC-18/β-CGTase was incubated with 10 mL LB medium (yeast extract 5.0 g/L, tryptone 10.0 g/L and NaCl 10.0 g/L) supplemented with 100 μg/mL ampicillin. Cells were incubated at 37 °C for 8 h with shaking at 200 r/min to generate the seed culture. To express the protein, an aliquot of the seed culture (3%, v/v) was added to 100 mL LB medium and cultured at 37 °C until the optical density at 600 nm reaches 1.5–2.0. Lactose was added into the medium with the final concentration at 3% to induce protein expression for 12 h. Following the induction growth, 0.5% Triton X-100 and 1% glycine were added into the culture medium and cells were cultured for another 8 h with shaking at 200 r/min. The culture medium was centrifuged with 7000 r/min at 4 °C for 10 min to obtain the crude enzyme extraction of β-CGTase.
β-CGTase activity detection
Diluted crude enzyme solution (0.01 mL) was added into 0.2 mL Gly-NaOH buffer (pH 9.0) containing freshly prepared 0.2 mL of 0.2% potato starch to initiate the reaction. After incubation at 40 °C for 10 min, the reaction was stopped with 0.5 mL of 0.5 M glacial acetic acid. Afterwards, 3 mL of 0.005% iodine solution was added into the reaction buffer and the absorbance at 700 nm was measured using a spectrophotometer. Reaction buffer without crude enzyme solution was used as the zero control. One unit of enzyme activity was defined as the amount of enzyme required to decrease 10% of the absorbance value according to the equation:
(a the OD value of the control group; b the OD value of the experimental group).
Statistical analysis
The results were presented as mean ± S.D. and analyzed by one-way analysis of variance (ANOVA). P < 0.05 was considered as statistical significance.
Results and discussion
The protein expression of β-CGTase-H167C in E. coli strain BL21 (DE3)
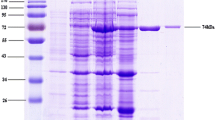
The full-length of cDNA sequence of β-CGTase with the indicated amino acid residue mutation H167C was inserted into the expression vector pUC-18-His. Afterwards transformed into the E. coli strain BL21 (DE3), the construct expressed β-CGTase. After 48 h of post-induction growth, the β-CGTase activity of the mutants H167C reached 2254 U/mL. The expressed protein product was purified by Ni2+ beads and further detected by western blot with anti-His antibody. As shown in Fig. 1a, a major band approximately 70 kDa was visualized with Coomassie blue staining, which is agreement with the molecular weight of β-CGTase-H167C previously described. The western blot showed that incubation with anti-His antibody generated a pure band of β-CGTase-H167C (Fig. 1b).
Expressing detection of β-CGTase-H167C. a The expression of β-CGTase was purified with His affinity chromatography and detected by SDS-PAGE with 12% separating gel. The band was visualized by Coomassie Brilliant Blue R-250 dye staining. b The production of β-CGTase-H167C was confirmed by western blot with anti-His antibody
Screen the alternative components of the culture medium
The achievement of high yield of β-CGTase-H167C requires efficient culture medium. Optimization of the medium components would benefit the outcome of β-CGTase-H167C and render the process more economical in the industry. To determine this, several different components of the medium including the carbon source, nitrogen source, metal ions as well as phosphorus were added into the culture medium and the enzymatic activity of β-CGTase-H167C was evaluated under each condition, respectively.
Carbon source
As shown in Fig. 2a, of all the different carbon sources tested, higher enzyme activity of the wild-type and mutant β-CGTase-H167C was obtained when corn starch was used as the carbon source. Considering the production cost, corn starch is an optimal candidate to provide carbon source in the production of β-CGTase-H167C. Consistently, optimization studies of the production media of β-CGTase by B. circulans ATCC and B. firmus also suggested that maximum production of β-CGTase was achieved with low substrate concentration of starch (Gawande et al. 1998; Pinto et al. 2007).
Screen the alternative components of the culture medium for the production of β-CGTase-H167C. a 1% glucose, 1% fructose, 1% soluble starch, 1% corn starch, 1% sucrose and 1% maltose were added to the basal culture medium, respectively. The enzymatic activity of β-CGTase-H167C was measured at each condition. b With 1% corn starch as the carbon source, 5% corn steep liquor plus 1% peptone, yeast extraction, 1% peptone, 1% urea, 1% (NH4)2SO4, and 1% NH4Cl were adapted as the nitrogen source and the enzymatic activity of β-CGTase-H167C was evaluated. c The effect of different metal ions including 0.02% K2SO4, 0.02% Na2SO4, 0.02% MgSO4, 0.02% MnSO4, 0.02% FeSO4, 0.02% ZnSO4 and 0.02% CuSO4 on the activity of β-CGTase-H167C was determined with 1% corn starch and 5% corn steep liquor plus 1% peptone. The data were presented from three independent experiments. d 0.1% K2HPO4, 0.1% Na2HPO4 and 0.1% (NH4)2PO4 were added into the culture medium and the activity of β-CGTase-H167C was detected, respectively
Nitrogen source
Organic nitrogen is another essential element in the enzyme expression. Previous studies have demonstrated that among the complex organic nitrogen sources tested, yeast extract was found to be the most significant component in the synthesis of β-CGTase. To search for the alternative nitrogen source in the β-CGTase-H167C production, 5% corn steep liquor plus 1% peptone, yeast extraction, 1% peptone, 1% urea, (NH4)2SO4, NH4Cl was used in the medium as the nitrogen source. The experimental results indicated that the highest enzyme activity of β-CGTase-H167C was observed when compound 5% corn steep liquor and 1% peptone were added into the culture medium (Fig. 2b). This result differs from that of the other group that the increased enzymatic activity of β-CGTase in the presence of ammonium salt was repressed by additional supplementation with organic nitrogen such as tyrptone or corn-steep liquor (Rosso et al. 2002). However, consistent with our data, previous study also suggested that organic nitrogen is required for the growth and enzyme production.
Metal ions
In addition to the carbon and nitrogen sources, metal ions are also key elements for the growth of organisms. To determine the effect of different metal ions on the production of β-CGTase-H167C, several mineral salts prepared in deionizer water were added into the basal medium with the indicated concentration. As shown in Fig. 2c, 0.02% MgSO4·7H2O presented significant promotion effect on the enzymatic activity of β-CGTase-H167C with the maximum activity up to approximately 3050 U/mL. This result is supported by previous data that found magnesium was essential for enzyme production (Gawande et al. 1998).
Phosphate
Beyond the above components of the medium, phosphate was also found to be essential for the microbial growth and metabolite biosynthesis. The influence of different phosphate including 0.1% K2HPO4·3H2O, 0.1% Na2HPO4, and 0.1% (NH4)2PO4 on the activity of β-CGTase-H167C was assessed, respectively. As shown in Fig. 2d, for the wild-type and mutant β-CGTase-H167C increased activity was produced when K2HPO4·3H2O was used as the phosphate source. Maximum enzymatic activity reached up to 3230 U/mL by adding 0.1% K2HPO4·3H2O into the culture medium. Consistently, Ibrahim et al. have reported the importance of K2HPO4 in the production of CGTase by Bacillus sp. G1 (Ibrahim et al. 2005).
Screen the culture conditions to improve the activity of β-CGTase-H167C
Conventionally, the highly efficient production of β-CGTase is tightly associated with the medium composition, as well as the culture conditions. It has been documented that initial pH value, incubation temperature, inoculum concentration, and shaking speed regulate the growth of microbial and the biosynthesis of β-CGTase (Kimura et al. 1990; Lee et al. 2007; Ong et al. 2008; Rosso et al. 2002).
Initial pH
To determine the optimum fermentation pH value for the wild-type and mutant strain, microbial were cultured in the screened optimum medium with the initial pH of 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, respectively. Crude enzyme solution was obtained and the activity of β-CGTase-H167C was measured. The β-CGTase activity of both wild-type and mutant β-CGTase produced in cultures was maximal with an initial pH of 8.0 (Fig. 3a), which indicated that pH 8.0 is more appropriate for the growth of microbial and the production of β-CGTase in comparison with that of other initial pH values. Approximately, Rosso et al. demonstrate the CGTase activity produced by B. circulans DF 9R was maximal with an initial pH of 8.3 (Rosso et al. 2002). It is also worth mentioning that another group reported that the enzyme produced by a new alkalophilic isolate of Bacillus represented optimum activity at pH 5.0 (Coelho et al. 2016). These results indicated that the optimal pH values of CGTase produced by different microbial strains are variable.
Screen the culture conditions for β-CGTase-H167C. a Bacterial strain harboring β-CGTase-H167C was cultured with the medium containing the selected components as described in Fig. 2 with the indicated pH value. The enzymatic activity of β-CGTase-H167C was examined. Results were obtained from three independent experiments. b β-CGTase-H167C was produced with different fermentation temperature with initial pH value at 6.0. c Bacterial strain expressing β-CGTase-H167C was seeded with the indicated inoculum size and the activity of β-CGTase-H167C was detected at each condition. d Different shaking speed was adapted and the production of β-CGTase-H167C was examined
Incubation temperature
To elucidate the influence of culture temperature on the enzymatic activity of β-CGTase, both wild-type and mutant strain-H167C were cultured at various incubation temperature from 28 to 38 °C. As presented in Fig. 3b, increasing enzymatic activity of β-CGTase-H167C was observed at 30 °C, and the maximal activity up to 3335 U/mL was achieved when microbial were cultured at 38 °C. However, the activity of both wild-type and β-CGTase-H167C decreased significantly with further increased incubation temperature, which suggested that CGTase may be rapidly get inactivated when the temperature was higher than 38 °C. Interestingly, optimal reaction temperature of CGTase from alkalophilic microorganisms has been reported among 45–60 °C. Additionally, some rare strains such as Thermoanaerobacter kivui have considerable activity under extreme temperature (Avci and Donmez 2009). There data indicated that the enzyme produced by different microbial strains have different thermal stability.
Inoculum concentration
To evaluate the response obtained through various inoculum concentrations, the inoculation size that ranged from 1 to 8% was added in the shaking bottle. Enhanced enzymatic activity of wild-type and mutant β-CGTase was observed within the range of 1–5%. And the highest enzymatic activity was obtained when the inoculum size was 5% (Fig. 3c). Further increased dose of inoculum led to the decrease of β-CGTase-H167C. This result suggested that inoculum concentration at 5% is optimal for the growth of microbial and the production of β-CGTase-H167C.
Shaking speed
As the wild-type and mutant strains expressing β-CGTase used in this study are aerobic bacteria, the dissolved oxygen content takes great effect on the microbial growth and the enzyme biosynthesis. The concentration of dissolved oxygen in the lipid culture medium is associated with the shaking speed of the incubator. To assess the regulation of agitation speed of rotary shaker on the enzymatic activity of β-CGTase-H167C, speed ranging from 140 to 220 rpm/min was adapted. A shown in Fig. 3d, the activity of β-CGTase-H167C was enhanced with the speed between 140 and 200 rpm/min, and the maximal activity was produced at the speed of 200 rpm/min. It is mentioned that when the speed is above 200 rpm/min, the decrease in the activity of β-CGTase-H167C was observed. This phenomenon may be due to great shearing force that inhibited the growth of the microbial.
Optimization of the medium components by response surface methodology and Box–Benhnken central composite design
Based on the single-factor experiment, the carbon and nitrogen sources, the metal ions and the phosphate were identified as important factors in regulating the activity of β-CGTase-H167C. To further optimize the combined effects of these factors on this enzyme, the experiments were designed with Box–Behnken central composite, and the four factors and three levels of response surface analysis were employed to establish the multinomial regression model. The regression model obtained after analysis with the SAS8.0 software gives the production of β-CGTase-H167C. Four important parameters namely different carbon (X1) and nitrogen sources (X2), the metal ions (X3) and the phosphate (X4) were selected as the independent variables and the β-CGTase activity (U/mL) was the dependent response variable as shown in Table 1. Each of these independent variables was studied at five different levels as per CCD in four variables with a total of 27 experimental runs as shown in Table 2. These factors were represented in the following second-order polynomial equation where the activity of β-CGTase-H167C was expressed as:
The variance analysis of the regression model was shown as Table 3. Statistical significance of the model was evaluated by F test on the analysis of variance estimates of mean squares, which demonstrated that the regression is statistically significant (P < 0.01). At the 5% probability level, the liner and quadratic coefficient of carbon (X1) and nitrogen sources (X2), the quadratic coefficient of the metal ions (X3) and phosphate (X4) were significant (P < 0.01) for the enzymatic activity of β-CGTase-H167C (Table 3). However, the linear coefficient of metal ions (X3) and phosphate (X4) and the interactions of any two of these four factors were not significant in the regulation of the β-CGTase-H167C activity (Table 3). These results suggested that the correlation between the tested factors and response values can be described as quadratic paraboloid regression equation. The extent of effect levels of these elements on the production of β-CGTase was corn starch (X1) > corn steep liquor + peptone (X2) > K2HPO4·3H2O > MgSO4·7H2O.
Credibility of the regression model indicated in Table 4 revealed that the model fitted the data well with a high coefficient of determination (R2 = 0.9457), which explains the 88.24% of the variability in the production of β-CGTase-H167C. Therefore, this model can be used to analyze and predict the optimal medium components of β-CGTase-H167C. To obtain the coded values for each optimal point, the first partial derivatives of the regression model was set to 0, which was showed as below:
From this model, the values for the X1, X2, X3 and X4 are 0.4559, 0.4503, 0.0112 and − 0.0277, respectively. Correspondingly, the values for carbon and nitrogen sources, metal ions and phosphate were 1.1, 5.5, 0.02, and 0.1%, respectively. Under this circumstance, the predicted optimal enzymatic activity of β-CGTase-H167C reached 3913.33 U/mL. To validate the precision of this model, the bacteria strain expressing β-CGTase-H167C was cultured with the above-predicted parameters and the enzyme activity of β-CGTase-H167C was obtained as 3910.24 U/mL. This result suggested that the regression equation is a good model for the production of β-CGTase-H167C.
With the regression model, the response surface methodology and the contour plot analysis by SAS software were shown as Fig. 4. Combined this analysis with Box–Benhnken central composite design, the optimal medium components for producing β-CGTase-H167C consisted of 1.1% corn starch, 4.4% corn steep liquor, 1.1% peptone, 0.02% MgSO4·7H2O and 0.1% K2HPO4·3H2O. Previous studies by other research groups also improved enzyme production with the response surface methodology. Gawande et al. showed that the optimum composition of the culture medium consisted of corn starch 21.0 g/L, yeast extract 23.0 g/L, and pharmamedia 22.0 g/L (Gawande et al. 1998). Another example is that the optimized culture medium for β-CGTase produced by B. stearothermophilus HR1 was made up of sago starch 16.02 g/L, peptone from casein 20.0 g/L, K2HPO4 1.4 g/L, CaCl2 0.2 g/L and initial pH 7.54 (Abd Rahman et al. 2004). These findings illustrated the wild application of response surface methodology in searching for the optimal culture conditions that improved the activity of β-CGTase produced by different microbial strains.
Optimization of the culture conditions by statistical analysis
Similar to the culture components, to determine the optimal culture conditions for β-CGTase-H167C, the effect of the initial pH (X1), incubation temperature (X2), inoculation concentration (X3) and shaking speed (X4), were evaluated by the central composite design and RSM analysis as shown in Table 5. Each of these independent variables was studied at five different levels as per CCD in four variables with a total of 27 experimental runs as shown in Table 2. The second-order polynomial equation where the activity of β-CGTase-H167C was expressed as:
Variance analysis of regression model for the fermentation conditions of β-CGTase-H167C was presented as Table 6, which indicated that the regression model is statistically significant (P < 0.01). Among those fermentation conditions, the liner and quadratic coefficient of initial pH (X1) and incubation temperature (X2), the quadratic coefficient of seeding concentration (X3) and shaking speed (X4) significantly influenced the enzymatic activity of β-CGTase-H167C (P < 0.01). However, the liner coefficient of inoculation concentration (X3) and shaking speed (X4) as well as the interaction between any of these two elements has no significant effect on the activity of β-CGTase-H167C. These results suggested the correlation between these four factors and enzymatic activity was presented as quadratic paraboloid regression equation. As shown in Table 6, the influence of these factors on β-CGTase-H167C activity was, in order, initial pH (X1), incubation temperature (X2), shaking speed (X4) and inoculation concentration (X3).
As shown in Table 7, the high coefficient of determination (R2 = 0.9457) of this regression model indicated that it was able to explain the 85.80% of the variability in the production of β-CGTase-H167C. Therefore, we used this model to predict the optimal culture conditions of β-CGTase-H167C with the following equation:
The optimum coded values of the variables X1, X2, X3 and X4 was determined as 0.4193, 0.4348, 0.0185 and 0.0951, which corresponded to initial pH 8.4, incubation temperature 37.4 °C, inoculation concentration 5.0% and shaking speed 202 r/min. Under these conditions, the predicted activity of β-CGTase-H167C achieved the value of 3895.33 U/mL. To validate the accuracy of this model, we measured the activity of β-CGTase-H167C under these culture conditions. The activity of β-CGTase-H167C was detected as 3892.45 U/mL by three independent experiments, which fitted well to the predicted value 3895.33 U/mL. Finally, with the analysis of response surface methodology and the central composite design shown in Fig. 5, optimal conditions for β-CGTase-H167C included an initial pH of 8.4, incubation temperature at 37.4 °C, 5% of inoculation concentration and shaking speed of 202 r/min. Notably, under the optimal medium components and the culture condition, the activity of β-CGTase-H167C was up to 4355 U/mL, which is 1.93-fold in comparison with the initial enzymatic activity.
Determination of CDs by HPLC–MS
For the HPLC analysis, 500 μL of crude liquid of β-CGTase-H167C was mixed with 2.5 mL 6.0% corn starch solution at 60 °C for 24 h. Afterwards, the mixture was centrifuged at 4500 rpm for 15 min at 4 °C. The supernatant was incubated with 0.1 mL α-glucamylase for 1 h and then filtered with 0.45 μm membrane for HPLC–MS analysis. The HPLC map showed that the time for the peak of β-CD was similar the standard control of β-CD as shown Fig. 6a. The peak was collected and confirmed by MS. The data showed that the molecular weight of the sample was 1134.4, which was similar to that of the β-CD (Fig. 6b). Collectively, these results suggested the product of β-CGTase was β-CD.
Determination of β-CD by the HPLC–MS analysis. a The HPLC map showed that the time for the peak of β-CD was similar to the standard control of β-CD. b The peak was collected and confirmed by MS. The data showed that the molecular weight of the sample was 1134.4, which was similar to that of the β-CD
Conclusion
In this study, we screened the medium components and the culture conditions for β-CGTase-H167C. With the response surface methodology and central composite design analysis, optimum conditions selected in this study were: 1.1% corn starch, 4.4% corn steep liquor, 1.1% peptone, 0.02% MgSO4·7H2O and 0.1% K2HPO4·3H2O with the initial pH 8.4, incubation temperature 37.4 °C, inoculation size 5% and shaking speed of 202 r/min. The production of β-CD was confirmed by HPLC–MS analysis. Our results provide efficient strategy for the production of CGTase by the mutant strain β-CGTase -H167C with relative low cost.
References
Abd Rahman R, Illias RM, Nawawi MGM, Ismail AF, Hassan O, Kamaruddin K (2004) Optimisation of growth medium for the production of cyclodextrin glucanotransferase from Bacillus stearothermophilus HR1 using response surface methodology. Process Biochem 39:2053–2060
Akindahunsi AA, Oboh G (2003) Effect of fungi fermentation on organoleptic properties, energy content and in vitro multienzyme digestibility of cassava products (flour and gari). Nutr Health 17:131–138. https://doi.org/10.1177/026010600301700204
Alves-Prado HF, Carneiro AA, Pavezzi FC, Gomes E, Boscolo M, Franco CM, da Silva R (2008) Production of cyclodextrins by CGTase from Bacillus clausii using different starches as substrates. Appl Biochem Biotechnol 146:3–13. https://doi.org/10.1007/s12010-007-8093-z
Avci A, Donmez S (2009) A novel thermophilic anaerobic bacteria producing cyclodextrin glycosyltransferase. Process Biochem 44:36–42
Brauman A, Keleke S, Malonga M, Miambi E, Ampe F (1996) Microbiological and biochemical characterization of cassava retting, a traditional lactic Acid fermentation for foo-foo (cassava flour) production. Appl Environ Microbiol 62:2854–2858
Coelho SL, Magalhaes VC, Marbach PA, Cazetta ML (2016) A new alkalophilic isolate of Bacillus as a producer of cyclodextrin glycosyltransferase using cassava flour. Braz J Microbiol 47:120–128. https://doi.org/10.1016/j.bjm.2015.11.018
Ebadipour N, Lotfabad TB, Yaghmaei S, RoostaAzad R (2016) Optimization of low-cost biosurfactant production from agricultural residues through response surface methodology. Prep Biochem Biotechnol 46:30–38. https://doi.org/10.1080/10826068.2014.979204
Gawande BN, Singh RK, Chauhan AK, Goel A, Patkar AY (1998) Optimization of cyclomaltodextrin glucanotransferase production from Bacillus firmus. Enzyme Microb Tech 22:288–291
He Q et al (2017) Production of chlorothalonil hydrolytic dehalogenase from agro-industrial wastewater and its application in raw food cleaning. J Sci Food Agric 97:2582–2587. https://doi.org/10.1002/jsfa.8079
Ibrahim HM, Yusoff WMW, Hamid AA, Illias RM, Hassan O, Omar O (2005) Optimization of medium for the production of beta-cyclodextrin glucanotransferase using central composite design (CCD). Process Biochem 40:753–758
Kimura K, Ishii Y, Kataoka S, Takano T, Yamane K (1990) Expression of the beta-cyclodextrin glucanotransferase gene of an alkalophilic Bacillus sp. #1011 in Escherichia coli cells and characterization of the synthesized enzyme. Agric Biol Chem 54:641–648
Lee MH, Yang SJ, Kim JW, Lee HS, Kim JW, Park KH (2007) Characterization of a thermostable cyclodextrin glucanotransferase from Pyrococcus furiosus DSM3638. Extremophiles 11:537–541. https://doi.org/10.1007/s00792-007-0061-6
Lepiz-Aguilar L, Rodriguez-Rodriguez CE, Arias ML, Lutz G (2013) Acetone–Butanol–Ethanol (ABE) production in fermentation of enzymatically hydrolyzed cassava flour by Clostridium beijerinckii BA101 and solvent separation. J Microbiol Biotechnol 23:1092–1098
Lindner K, Saenger W (1980) Crystal structure of the gamma-cyclodextrin n-propanol inclusion complex; correlation of α-, β-, γ- cyclodextrin geometries. Biochem Biophys Res Commun 92:933–938
Ong RM, Goh KM, Mahadi NM, Hassan O, Rahman RN, Illias RM (2008) Cloning, extracellular expression and characterization of a predominant β-CGTase from Bacillus sp. G1 in E. coli. J Ind Microbiol Biotechnol 35:1705–1714. https://doi.org/10.1007/s10295-008-0462-2
Pinto FS, Flores SH, Ayub MA, Hertz PF (2007) Production of cyclodextrin glycosyltransferase by alkaliphilic Bacillus circulans in submerged and solid-state cultivation. Bioprocess Biosyst Eng 30:377–382. https://doi.org/10.1007/s00449-007-0134-z
Rendleman JA Jr, Knutson CA Jr (1998) Conversion of cyclodextrin into high-amylose starch of low molecular mass by means of cyclodextrin glucanotransferase. Biotechnol Appl Biochem 28(Pt 3):219–228
Rosso AM, Ferrarotti SA, Krymkiewicz N, Nudel BC (2002) Optimisation of batch culture conditions for cyclodextrin glucanotransferase production from Bacillus circulans DF 9R. Microb Cell Fact 1:3
Terada Y, Yanase M, Takata H, Takaha T, Okada S (1997) Cyclodextrins are not the major cyclic alpha-1,4-glucans produced by the initial action of cyclodextrin glucanotransferase on amylose. J Biol Chem 272:15729–15733
Wischral D et al (2016) Production of 1,3-propanediol by Clostridium beijerinckii DSM 791 from crude glycerol and corn steep liquor: process optimization and metabolic engineering. Bioresour Technol 212:100–110. https://doi.org/10.1016/j.biortech.2016.04.020
Acknowledgements
The work was supported by Natural Science Foundation of Inner Mongolia (2015BS0312). The sponsors of financial support have no involvement in the study design, data collection and analysis, writing of the report and decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of the paper.
Rights and permissions
About this article
Cite this article
Wang, H., Zhou, W., Li, H. et al. Optimization of the fermentation conditions for the mutant strain of β-cyclodextrin glycosyltransferase H167C to produce cyclodextrins. 3 Biotech 8, 165 (2018). https://doi.org/10.1007/s13205-018-1182-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1182-6