Abstract
Experimental investigations were carried out for Cupriavidus necator (MTCC 1472)-based improved production of poly-3 hydroxy butyrate (PHB) through induced nitrogen limiting fed-batch cultivation strategies. Initially Plackett–Burman design and response surface methodology were implemented to optimize most influencing process parameters. With optimized process parameter values, continuous feeding strategies ware applied in a 5-l fermenter with table sugar concentration of 100 g/l, nitrogen concentration of 0.12 g/l for fed-batch fermentation with varying dilution rates of 0.02 and 0.046 1/h. To get enriched production of PHB, concentration of the sugar was further increased to 150 and 200 g/l in feeding. Maximum concentrations of PHB achieved were 22.35 and 23.07 g/l at those dilution rates when sugar concentration maintains at 200 g/l in feeding. At maximum concentration of PHB (23.07 g/l), productivity of 0.58 g/l h was achieved with maximum PHB accumulation efficiency up to 64% of the dry weight of biomass. High purity of PHB, close to medical grade was achieved after surfactant hypochlorite extraction method, and it was further confirmed by SEM, EDX, and XRD studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyhydroxyalkanoates (PHAs) have been long regarded as the best substitute for traditional petroleum-based plastics. Poly-3-hydroxy butyrate (PHB), which is a completely biodegradable thermoplastic material and considered as an important family member of PHAs, can be synthesized and accumulated by numerous microorganisms as their own energy reserve material under nutritional stress conditions. Limiting conditions of essential nutrients like nitrogen, phosphorous, and magnesium in the presence of excess carbon source in the growth environment triggers the formation of PHB as intracellular granules inside the cell (Khanna and Srivastava 2005a; Koller et al. 2005). Such specific nutritional condition can be effectively maintained during feeding process by fed-batch cultivation mode. Possessing some qualities like biodegradability, biocompatibility, and thermoplasticity, PHB-based polymer composites can participate in broad range of applications in packaging industry, agriculture, paint industry, pharmacy, and food industry (Valappil et al. 2007). In spite of these advantages of PHB compared with petroleum-derived plastics and its wide range of applications, its market commercialization is still limited due to its relatively high cost with respect to petroleum-derived plastics (Khanna and Srivastava 2005b). Hence, several attempts have been made in this direction to develop processes for cost-effective production of PHB. Major advanced attempts include finding low-cost feed stocks, selection of robust microorganisms capable of synthesizing large amounts of pure PHB, and suitable fermentation strategy with efficient recovery process of PHB (Choi and Lee 1999).
As almost half of the production cost can be attributed to raw materials, in order to reduce the production cost of PHB, plenty of carbon sources like glucose, lactose, and fructose already have been investigated as feed stocks. Many waste materials and byproducts of industries such as molasses, cheese whey, plant oils, and soya milk wastes have also been tried (Bengtsson et al. 2008, 2010; Kulpreecha et al. 2009). However, cost factor persists to be high as pretreatment requirements are often expensive. Table sugar despite being one of the cheapest carbon source has been rarely investigated (Rohini et al. 2006).The price of table sugar is significantly lower than those of pure sucrose and other pure sugars. As a producer, the microorganism, Alcaligenes eutrophus, commonly known as Ralstonia eutropha, is considered as the most efficient model organism for PHB synthesis (Khanna and Srivastava 2005a; Pohlmann et al. 2006). This microorganism has been utilized for the first time in the present study for conversion of sucrose-based low-cost sugar substrate to PHB although the ability of this microorganism has been largely investigated using other carbon sources like glucose, fructose, and acetic acid. In a complete nitrogen- or phosphorous-depleted condition, A. eutrophus can accumulate PHB up to 80% of the total dry weight of the cell. In producing high-density PHB under nutritional stressed condition, fed-batch cultivation strategies are considered to be the most ideal methods. Fed-batch fermentations with specific feeding strategies have been attempted in achieving high cell densities with high intracellular PHB productivity (Lee et al. 1999, 2000; Ahn et al. 2001; Kulpreecha et al. 2009; Arifin et al. 2010; Cui and Muralidhara 2010; Hafuka et al. 2011; García et al. 2014; Mozumder et al. 2014; Schmidt et al. 2016; Ienczak et al. 2016). The main challenge associated with fed-batch fermentation is controlling the feeding substrate concentration within an optimal range in terms of limiting and inhibiting levels. Therefore, the substrate feeding strategy plays a crucial role in achieving high cell density cultures with PHB successfully. Several feeding strategies including continuous feeding (Hafuka et al. 2011), pH stat (Kulpreecha et al. 2009; Arifin et al. 2010), and dissolved oxygen (DO) stat (Lee et al. 2000) have been investigated to improve PHB productivity and yield. Among all the methods, continuous feeding is considered to be the simplest and the most ideal method in comparison with others. In pH or DO stat mechanism, substrate-feeding strategies with indirect feedback control are based on the levels of DO or pH which are finally dependent on the depletion of a carbon source. In such processes, nutrient is added at a predetermined rate into the fermenter (Lee et al. 1999) when pH or DO becomes higher than its set point. Due to such nature of this feeding mechanism, the substrate concentration cannot be maintained at the desired level, and hence the biomass growth rate and final PHB productivity can be adversely affected (Mozumder et al. 2014). Similarly, membrane cell recycle fed-batch culture system to achieve high-density PHB has been also implemented by some researchers (Ahn et al. 2001; Ienczak et al. 2016; Schmidt et al. 2016). Problems regarding oxygen limitation and sterilization in the external loop are the major drawbacks in the cell recycle fed-batch culture system (Cui and Muralidhara 2010). Continuous fermentations facilitate high productivity of PHB, particularly for the cultures of high specific growth rates, but the execution of such cultivation strategy in industry for PHB production has been limited as the continuous process is highly prone to microbial contamination which may lead to huge financial losses (Koller and Muhr 2014).
This study attempts to develop a novel, robust, and much improved continuous feeding strategy for high cell density fed-batch fermentation process for the production of highly concentrated PHB with high purity and productivity, where initial optimization of most significant process parameters ware achieved using response surface methodology (RSM) and Plackett–Burman methodology. An optimized medium always helps in enhancing PHA content inside the cell biomass, and in order to achieve this, RSM plays a key role to study the interactive effects of parameters involved in fermentation process while seeking optimized conditions for improved product formation (Narayanan and Ramana 2012). Adoption of surfactant—sodium hypochlorite-based extraction strategy leads to the separation and purification of high-quality PHB from cell biomass, thereby making the overall process economically viable.
Materials and methods
Microorganism
Cupriavidus necator (MTCC 1472), an intracellular PHB producing microorganism, was used throughout this work. It was purchased from Microbial Type Culture Collection (MTCC), Chandigarh in lyophilized condition. It was earlier known as Ralstonia eutropha or Wautersia eutropha or A. eutrophus. It is equivalent to ATCC 17699. It was maintained on nutrient agar slants at 4 °C and sub-cultured monthly.
Media for fermentation and inoculum development
The medium which was used during fermentation, was consisted of the following components: sucrose (table sugar) 20 g/l, (NH4)2SO4 1.4 g/l, KH2PO4 1.5 g/l, Na2HPO4 1.8 g/l, MgSO4 0.2 g/l, trace metal solution 1 ml/l, yeast extract 0.1 g/l. Trace metal solution consists of ammonium Fe(III) citrate 6 g/l, CaCl2·2H2O 10 g/l, H3BO3 0.3 g/l, CoCl2·6H2O 0.2 g/l, ZnSO4·7H2O 0.1 g/l, MnCl2·4H2O 0.03 g/l, Na2MoO4·2H2O 0.03 g/l, NiSO4·7H2O 0.02 g/l, and CuSO4·5H2O 0.01 g/l. After the addition of supplements, solutions were sterilized for 15 min prior to every fermentation. The phosphate components of the medium were autoclaved individually to avoid precipitation. Trace metal solution was sterilized by filter sterilization method. After that, all the components of the medium were mixed under aseptic conditions, i.e., in the Laminar flow hood. pH of the fermentation broth was adjusted to 7.0 with 2 N NaOH/2N HCl solution. For inoculum development, same media composition with 10 g/l sucrose (table sugar) was used. A loopful of culture was inoculated into the 100 ml of this medium, taken in 250-ml conical flask and kept in incubator shaker for 24 h at 30 °C, 150 rpm till log phase was obtained. For fermentation, 5% (v/v) inoculum volume was always maintained. For bioreactor operation, similar inoculum volume was prepared at similar operating conditions in 1-l fermenter.
Experimental conditions maintained during fermentations
Medium optimization studies were initially done at shake flask level. To determine substrate inhibition effects, sucrose concentration was varied from 10 to 30 g/l while the concentrations of the other components were kept constant. Second, to know the influence of nitrogen concentration, concentration of ammonium sulphate was varied from 0.2 to 6 g/l, and the concentrations of the other components including sucrose were kept constant. Initial batch experiments for optimization were conducted in 250-ml flasks containing 100-ml media, and then productions were scaled up in 5-l sartorius fermenter with such optimized media.
Screening of most significant parameters by Plackett–Burman method
Plackett–Burman design under Design Expert software (version 8.0.4) was used for the determination of most influencing parameters in present study. Eleven factors including nutrients and physical parameters were examined to know the most influencing parameters for production of PHB by C. Necator from table sugar-based media. Among all the nutrients, sucrose, MgSO4.7H2O, Na2HPO4, KH2PO4, trace element solution, (NH4)2SO4, yeast extract, peptone, CaCl2, and the physical parameters like temperature and rpm were considered in this method. Amount of PHB formation was considered as the response in designing the experiments. Each of the factor was tested at two levels, high (+1) and low (−1) (Table 1) by means of twelve experiments, which were designed by Plackett–Burman design. The experiments were performed in 250-ml flasks containing 100-ml media. Samples were withdrawn at the end of 48 h fermentation and analyzed for the responses in terms of software-based t coefficient values. High positive t values of some factors with respect to the formation of PHB demonstrate that they are statistically important and thus they were selected for further analysis.
Optimization of the major influencing parameters by RSM
Response surface methodology (RSM) under same Design Expert software (version 8.0.4) was used to optimize the concentrations of the four influencing factors namely, peptone, trace metal solution, yeast extract, (NH4)2SO4 which were initially screened by the Plackett–Burman design. PHB production was taken as the response in designing the experiments. The upper (+1) and the lower (−1) limits of the factors are shown in Table 2. Using Design Expert software (version 8.0.4) a 2n-factorial central composite design (CCD) was developed which lead to total number of 30 experiments with different combinations of concentrations of the selected variables. The initial fermentation experiments were carried out in 250-ml flasks with working volume of 100-ml using shaker incubator at 150 rpm at 30 °C. Samples were withdrawn at the end of 48 h and analyzed for the response. The optimal concentrations of the factors were obtained by the regression and graphical analysis.
Experimental conditions maintained during fed-batch fermentations
For fed-batch fermentation studies, same 5-l fermenter (Sartorious, Germany) was used. For such fermentation studies, experiments were started under batch cultivation mode with 3 l of media optimized by previous method. Samples were collected after every 3 h of interval for analyses. After 20 h of fermentation, 1.5-l culture was removed from the fermenter and stored in aseptic condition for another set of fed-batch operation with different dilution rate. The nutrient feeding was initiated at 20 h as it was seen from the batch cultivation studies that the culture was in actively growing state at 20 h. For fed-batch fermentation studies, feed solutions ware provided with intermediate flow rates of 70 and 30 ml/h with the help of a peristaltic pump. The corresponding dilution rates were 0.046 and 0.02 1/h. Initially sucrose and nitrogen concentrations maintained in the feed solution were 100 and 0.32 g/l at C:N ratio of 131:1. Similar experiments were conducted with same nitrogen concentration and higher sucrose concentrations of 150 and 200 g/l. Corresponding C:N ratios in those feed compositions were 197:1 and 263:1. Normally feeding was continued for 20 h continuously (from 20 to 40 h). After 40 h, both the fermenters were once more operated in batch mode up to 44 h for the consumption of remaining substrates. After such operations, samples were collected, fermenters were cleaned, and prepared for next operation.
Experimental equipment
Fermentations ware carried out in a 5-l fermenter (Sartorius, Germany) equipped with pH, DO and temperature probe. The fermenter was fully automated, and temperature was maintained by circulating water from a thermostatically controlled water bath. For aeration, compressed air was initially passed to controller and then to fermenter at one VVM during fermentation. For the calibration of DO probe, separate provision of N2 sparging was present in the controller. Temperature and agitation speed were maintained at 303 K and 150 rpm, respectively, during fermentation. To maintain DO during fermentation, cascade control system was applied with agitation speed and aeration speed with DO level in fermenter. Inoculum was separately prepared in 1-l fermenter and aseptically transferred to 5-l fermenter. For fed-batch fermentation, fresh feeds with specific flow rates were supplied to fermenter by means of peristaltic pump attached to controller. Schematic representation of the overall experimental system is presented in Fig. 1. Mixing time and k L a of the fermenter were determined according to the standard procedure (Doran 1995).
Analytic methods
The samples from fermentation broth were taken out at predetermined time intervals, and the cell growth was monitored by measuring the absorbance of the culture broth at 600 nm. Absorbencies of those samples were measured by UV spectrophotometer (CECIL, 7000 Series, India). To separate cell biomass from fermentation broth, samples of fermentation broth were centrifuged. The cell pellet obtained after centrifugation (10,000 rpm for 10 min at 4 °C) was dried at 90 °C until constant weight was obtained. Supernatant after centrifugation was analyzed for residual sucrose estimation by dinitrosalicylic acid (DNS) method (Miller 1959). PHB was separated from the cell pellet by most efficient SDS—sodium hypochlorite digestion method (Ramsay et al. 1990). Total nitrogen analysis in sample was done by measuring the residual ammonia by Kjeldahl method (Horwitz 1980). To estimate PHB concentration in the dried pellet, 1 ml of 96% H2SO4 was first added to cell pellet for digestion, and the solution was kept at 100 °C for 1 h to form crotonic acid. The samples were then cooled down to room temperature, and 1 ml 0.014 M H2SO4 was added. The solutions were then filtered through PVDF membrane, and dilution was maintained at 1:10 with 0.014 M H2SO4 and crotonic acid. It was finally determined by Agilent HPLC (Series 1200) with C18 column. The solvent used for elution was 0.014 N H2SO4 and flow rate was maintained at 0.7 ml/min. Monitoring of the elution peaks was done at 210 nm with a photodiode array detector. To calculate the PHB content, standard curve of commercial PHB (Sigma Aldrich,) was prepared by treating samples in the same way as the experimental samples. Sucrose concentrations were analyzed by Agilent HPLC (Series 1200) with Zorbax, carbohydrate column, and RID. The column temperature was maintained to 30 °C. The mobile phase was 75% acetonitrile (sigma Aldrich) and 25% ultrapure water (milli Q) at a flow rate of 1.4 ml/min. An injection volume of the sample was always maintained to 10 μl. Morphological characteristics of PHB nanoparticle surfaces were analyzed via scanning electron microscopy (SEM) (Shimadzu model Vega 3LM) at an accelerating voltage of 10 kV. The samples were coated with gold by a Bio-Rad coating system before viewing. The scanning electron micrographs were recorded at the magnification of 25,000×. Energy-dispersive X-ray spectroscopy (EDS), generated from HITACHI SU5000, field emission scanning electron microscope, was used to identify the elemental composition of PHB molecules. Crystallographic structure of PHB nanomolecules was verified by using an X-ray Diffractometer (Shimadzu, model XRD-6000) equipped with a Cu tube operating at 45 kV and 40 mA. Data ware recorded in 2θ range of 2°–30° using the scan rate of 2°/min. Molecular weights of the PHB samples were determined by Gel Permeation Chromatography (GPC) technique while using a Varian GPC50 with an RI detector. Samples were passed through 30 cm PLGel Mixed C columns (Agilent) at 30 °C with distilled chloroform as the mobile phase. Molecular weights were determined based on the retention times (30 min) and after comparing with the standard curve. Pure PHB from Sigma-Aldrich of known molecular weight was used for standard preparations.
Results and discussion
Assessment of substrate inhibition effect
Batch cultivation studies were performed for initial assessment of substrate inhibition effects. To detect inhibitory effect of sucrose in the form of table sugar on microbial growth, sucrose concentration was varied from 10 to 30 g/l. It was observed that the maximum specific growth rate of C. necator increased as the sucrose concentration was increased from 10 to 20 g/l but as the sucrose concentration was increased further, the value of specific growth was found to decrease gradually due to substrate inhibition effect. The maximum value of specific growth rate achieved was 0.096 1/h when table sugar concentration of 20 g/l. The effects of varying concentrations of table sugar in the form of sucrose on microbial specific growth rate are presented in Fig. 2a. Similarly, like sucrose concentration, nitrogen concentration was increased from 0.2 to 6 g/l by varying (NH4)2SO4 concentrations. Maximum specific growth rate of microorganism gradually increases, but when nitrogen concentration was increased above 2 g/l, negative effect was observed on maximum specific growth rate due to substrate inhibition effect of nitrogen (Fig. 2b). The highest value of maximum specific growth rate was achieved 0.11 1/h at nitrogen concentration of 2 g/l. How variation of nitrogen concentration affects in microbial growth rate and conjugated PHB production has been similarly demonstrated by Mozumder et al. (2014).
Optimization of process parameters
Identification of influencing media components by Plackett–Burman method
According to the experimental design by central composite design (CCD), 12 experiments were conducted with 11 variables with different levels. High positive t values of the variables with respect to the biomass and PHB responses were the judgemental parameters to determine their overall influences in PHB production process. All the coded factors (A–L) are mentioned in Table 1. Corresponding experimental response values according to the design are represented in Table 3. The best response with respect to PHB production was achieved in the tenth row of Table 3. For overall analysis, order—linear, model—polynomial, transformation—none were selected. From the fit summary section in design menu, Model F value 853.80 and P (probability) value less than 0.05 clearly indicate that polynomial model terms are significant in terms of analysis. The high significance of the model is also established in the plot of predicted against actual response curves of PHB production (Fig. 3). Clustering of points around the diagonal line indicated capability of the model to predict the experiments. In Plackett–Burman method, Pareto chart helps to determine t value effects with respect to two limit lines, namely, the Bonferroni limit line (t value of effect = 14.71) and t limit line (t value of effect = 4.33) (Fahmy et al. 2012). Factors which have t value of effect above the Bonferroni limit line are termed as certainly significant effective factors, whereas the ones with t value of effect between Bonferroni limit line and t limit line are designated as likely to be significant factors. As the variables like J, H, F have t values far above the Bonferroni limit line, they are considered as certainly significant parameters (Fig. 4). The t value of G is equivalent to Bonferroni limit line, and thus, it is also considered as a significant parameter. Therefore, among all 11 parameters, most significant parameters are J (peptone), F (trace metal solution), H (yeast extract), and G ((NH4)2SO4). Usually yeast extracts and peptones are rich in peptides, amino acids, nucleotides, vitamins, and trace elements. Hence, utilization of peptone and yeast extract largely affects in microbial growth rate, rate of product formation, and product yield. As a result, influences of yeast extract and peptone in Plackett–Burman experiments are comparatively larger.
Optimization of the significant media components by RSM
Response surface methodology (RSM), most efficient and commonly used statistical tool (Osman et al. 2016) was used to optimize the effects of four most influencing parameters namely, peptone, trace metal solution, yeast extract, (NH4)2SO4 on PHB production. PHB production was taken as the response in designing the experiments. 2n-factorial central composite design (CCD) was developed which lead to a total number of 30 experiments with different combinations of concentrations of the selected variables. The list of experiments according to the Central Composite design and the corresponding values of responses are represented in Table 4. The best response with respect to PHB production was obtained in serial no 21 in Table 4. To determine the interrelationship between these variables, transformation: none and quadratic model was selected. In the fit summary section, model F value 32.22 and P value 0.001 being less than 0.050 indicate that the model terms are significant. The empirical relationship between PHB production and the four variables is represented by the final regression equation as shown below:
The adjusted determination coefficient value (adj. R 2 = 0.9378) was within reasonable agreement with the predicted R 2 of 0.8154. In this case, A, B, C, D, AC, A 2, B 2, C 2, D 2 are significant model terms. Analysis of variance (ANOVA) helps to determine the combined effect of varying peptone concentrations with trace metal solution concentration, yeast extract concentration with trace metal solution concentration, peptone concentration with yeast extract concentration, and (NH4)2SO4 concentration with peptone concentration on PHB production. At higher concentrations of trace metal solution, PHB concentration increased slightly with small increase of peptone concentration and then decreased rapidly with further increase of peptone concentration (Fig. 5a). At trace metal solution around 10 ml/l and even with initial peptone concentration, comparatively high concentration of PHB was synthesized. As the peptone concentration increased slightly and reached to around 2.5 g/l, synthesis of PHB was enhanced. Similar trend of effects were observed for the combined effects of trace metal solution concentration and yeast extract concentration (Fig. 5b), peptone concentration with yeast extract concentration (Fig. 5c), and (NH4)2SO4 concentration with peptone concentration on PHB production (Fig. 5d). To optimize the result, criteria selected in the design for expensive yeast extract and peptone concentration were “minimized” and for (NH)4SO4, the trace metal solution was “within range.” To obtain maximum production of PHB, criteria selected for PHB were “maximized.” Among the suggested optimized results, one solution was selected, and experiments were performed in triplicate with same values of experimental variables according to the selected optimized solution. After 48 h of experiment, maximum PHB concentration achieved was 1.86 ± 0.05 g/l, whereas the predicted response was 1.93 g/l. Such maximum PHB concentration was achieved from the optimized peptone concentration of 2 g/l (A), Trace metal solution of 9.15 ml/l (B), yeast extract concentration of 3.10 g/l (C) and (NH4)2SO4 concentration of 2.18 g/l. Similar type of optimization studies for PHB production were conducted by Vijayendra et al. (2007) where sucrose as a substrate was considered separately as a parameter in RSM whereas in our present study, inhibitory effect of substrate was separately investigated. As sucrose in the form of table sugar is the major carbon source in our study, separate determination of substrate inhibitory effect on microbial growth of C. necator (MTCC 1472) is more valuable rather than the determination of combine effect of sucrose.
Enhanced batch production of PHB through fermenter
In PHB production, dissolved oxygen plays a critical effective role as low dissolved oxygen concentration promotes the PHB synthesis; whereas at high values of it mainly promotes cell growth (Sabra et al. 2000). It was clearly observed from the experiment that mixing time (T m) is inversely proportional to the rotation speed of the impeller and at 150 rpm, mixing was achieved with proper distribution and dispersion of bubbles. The mixing time was calculated as 4.04 s at that specified rpm. Just like total mixing time, k L a was determined 0.037 1/s at 1 VVM. Similar type of study related to how better oxygen mass transfer and mixing efficiency influences in the accumulation of PHB in bacteria was performed by a few researchers (van Wegen et al. 2001; Gahlawat et al. 2012). During PHB production, individual growth of C. necator was monitored. Figure 6a represents the initial 23 h of growth curve of C. necator in the fermenter during batch cultivation. Cell growth curve clearly indicate that the lag phase was considerably minimized in the bioreactor due to inoculation of the microorganism which was grown in experimentally similar environment and maintained at exponential growth phase. (Gahlawat et al. 2012) showed that using similar type of media in inoculum development was quite effective to reduce the lag phase in microbial production of industrially important product. Maximum specific growth rate was calculated as 0.32 h−1 at 12 h. It was observed that the concentration of dissolved oxygen falls suddenly after 6 h of batch cultivation, while the culture enters to the log phase, and as the culture is in actively growing stage, oxygen consumption increases with time. After 40 h of operation, in spite of continuous aeration, DO levels in the fermenter almost drop down to zero. After 40 h of batch cultivation, sucrose (table sugar) concentration remained in the fermenter was almost 5 g/l. Initially during lag phase of batch cultivation in fermenter, the production of PHB along with biomass was started very slowly but just after 10 h of batch cultivation, concentration of biomass has been increased very rapidly. Even after achieving maximum specific growth rate of biomass at 12 h, biomass production continues. After 40 h of cultivation, the maximum value of biomass achieved was 4.81 g/l. Conjugate PHB accumulation was also induced with increased growth rate of biomass. After 40 h of batch cultivation total amount of PHB accumulated in the biomass was 2.85 g/l. Total 14.75 g/l sucrose was consumed during this batch cultivation of PHB from initial value of 20 g/l. The biomass yield (Y X/S) was observed to be 0.33 g/g while the product yield (Y P/S) was found to be 0.20 g/g. Figure 6b clearly elucidates the variation in concentration of sucrose (table sugar), biomass, PHB with time during batch cultivation of C. necator for PHB production. In few cases (Grothe et al. 1999; van Wegen et al. 2001) where pure sucrose was used as substrate, slightly higher concentrations of PHB were achieved in batch cultivation although comparatively better yield (Y PS) of PHB was achieved in our research. The common reasons which were analyzed behind such observations were the uses of pure and expensive sucrose substrate, and utilization of comparatively higher amount of batch production time.
Improved fed-batch fermentation to enhance PHB production
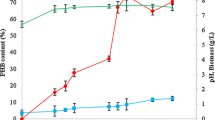
Significantly higher concentration of PHB was generated during induced nutritional limiting condition through fed-batch fermentation strategies. According to the main aim of our research to improve PHB production, two different continuous feeding strategies were implemented through fed-batch fermentation process. In the first case, concentration of sucrose and nitrogen maintained in the fresh feed solution ware 100 and 0.12 g/l, respectively. Nitrogen concentration of 0.12 g/l translates to 0.56 g/l of ammonium sulfate which was used as the sole source of nitrogen. At different set of experiments, feeding rates were varied from 30 up to 80 ml/h. The corresponding dilution rates maintained from 0.02 to 0.05 1/h, respectively. It was observed that at low dilution rate of 0.02 1/h, maximum biomass concentration achieved was 13.20 g/l, and the corresponding PHB concentration was 8.17 g/l. As the dilution rate increased beyond 0.046 1/h, a negative impact was observed on biomass production and PHB synthesis. At the dilution rate of 0.046 1/h (feed flow rate of 70 ml/h), maximum concentration of biomass achieved was 14.18 g/l, and corresponding PHB concentration was 9.15 g/l after 44 h of operation. During such fed-batch operation, sugar consumption, PHB and Biomass production with respect to time for both two dilution rates are clearly shown in Fig. 7. It is observed from the Fig. 7 that overall sucrose (table sugar) concentration in fermenter was increased after 20 h of time interval with the addition of fresh feed solution containing higher sucrose concentration. After 28 h of time interval, concentration of sucrose in the fermenter started to decrease as sucrose consumption by highly danced microorganism was effectively dominant over the lower dilution rate. Even after 40 h of time interval, similar decrease in sucrose concentration level was achieved as last 4 h were specifically conducted in batch mode for the consumption of residual sugar. Overall increases in the PHB production level and PHB containing biomass were obtained throughout the fermentation period. Similar effect of dilution rates on maximum PHB production was observed by Du et al. (2001). In this study, comparatively higher dilution rate of 0.14 1/h was maintained in a continuous production of PHB with expensive glucose as a substrate. Using relatively similar dilution rate of 0.1 1/h and almost similar concentration of expensive pure fructose of 90 g/l, comparatively lower concentration of PHB production (5.5 g/l) was achieved by Khanna and Srivastava (2008a).
In fed-batch fermentation process, for farther improvement in production of PHB, feeding strategies with higher sucrose (table sugar) concentration were carried out with 150 and 200 g/l at the same time interval between 20 and 40 h. Just like the previous feeding strategy, those feeding strategies also ensured the maintenance of limiting nutrient concentration at higher range and nitrogen concentration at the same stressed level of 0.32 g/l. For both the cases, continues feeding strategies at 20 h interval of time was quite beneficial as at that time interval sucrose concentration almost drops down to 12 g/l, and biomass was in the exponentially growth phase. For both concentrations of sucrose, same dilution rates of 0.02 and 0.046 1/h were maintained. At sucrose concentration of 150 g/l in feed solution and dilution rate of 0.2 1/h, PHB concentration achieved was 15.20 g/l, and the corresponding biomass concentration was 24.02 g/l. PHB concentration was comparatively increased at 40 h of fermentation period when dilution rate was separately maintained to 0.046 1/h instead of 0.02 1/h. At the dilution rate of 0.046 1/h, the concentration of the produced PHB reached to 19.08 g/l with dry biomass concentration of 28.82 g/l. To ensure high biomass production and enhancement in PHB accumulation, sucrose concentration in the feed was further increased to 200 g/l, and same two dilution rates were maintained. At the dilution rate of 0.02 1/h, biomass concentration in the fermenter had reached to 34.74 g/l. and the accumulated PHB concentration was 22.35 g/l at the end of fermentation. Maximum cell density of 37.56 g/l with maximum PHB concentration of 23.07 g/l was achieved when fresh feed concentration was maintained at 200 g/l with dilution rate of 0.046 1/h. For the sucrose concentration of 200 g/l, the corresponding productivity rates achieved for both the dilution rates of 0.02, and 0.046 1/h were 0.56 g/l h and 0.58 g/l h. High cell density culture system containing high concentration of PHB leads to rapid decrease of DO during fermentations. It seems likely that using higher concentration of sucrose (table sugar) cause rapid accumulation of substrate in the fermenter during fed-batch process, and further increase of table sugar concentration beyond 200 g/l causes cell growth inhibition, cell lysis, and excess foam production. Such higher concentration of sucrose in feed solution decreases the final production of PHB. Using similar feeding strategy of comparatively high substrate concentration of fructose and the same microbial strain, PHB concentration and productivity achieved by Khanna and Srivastava (2008b) were comparatively lesser with respect to our observation. The detail results of batch and fed-batch cultivation process achieved in our research are summarized in Table 5.
Microorganism like A. australica (previously known as A. latus) has been extensively investigated for large-scale production of PHB from sucrose-based resources because of its ability to accumulate up to 80% of dry biomass, and PHB synthesis is microbial growth associated (van Wegen et al. 2001; Khanna and Srivastava 2005a). At the same time, A. eutrophus is very well known for large-scale production of PHB and their co polymers are supported with better quality and suitability for medical applications (Khanna and Srivastava 2005a). In our research, for the first time this particular strain has been exploited for large scale PHB production from table sugar-based cheap resource. While working with maximum concentration of sucrose, i.e., 200 g/l, the microorganism showed the ability to accumulate PHB up to 64% of its dry cell weight. To improve the overall effectiveness of the process continuous and membrane cell recycle fed-batch system also have been investigated by few researchers (Khanna and Srivastava 2008a; Koller and Muhr 2014; Ienczak et al. 2016). Maintaining adequate oxygen concentration and proper sterility during cell recycle phase are the two biggest challenges which hinder the application of membrane technology in PHB production process (Cui and Muralidhara 2010; Koller and Muhr 2014). In terms of biomass concentration and PHB concentration, the values of produced biomass concentration of 37.56 g/l and PHB concentration of 23.07 g/l through constant feeding strategy in our present research are highly comparable with the existing studies of similar sucrose-based media (Kulpreecha et al. 2009; García et al. 2014). Through our research efforts, we have also emphasized on the development of an ideal method for large-scale isolation of PHB from PHB containing cells. Among the three adopted methodologies (surfactant, sodium hypochlorite, and surfactant—sodium hypochlorite) for the extraction of PHB, surfactant—sodium hypochlorite method turned out to be most efficient method with maximum 71% extraction yield and 98% purity of PHB. This method is well known for rapid recovery of high-quality PHB with lowest operating cost (Kunasundari and Sudesh 2011) and maximum yield of 87% has already achieved by Dong and Sun (2000) by using the same method. In our case, the method was largely effective for the recovery of PHB with high purity and high molecular weight, which are essential parameters for its medical application. On the other hand high molecular weight of PHB is also necessary for the thermal processing of the PHA during various applications (Dong and Sun 2000). In our case, molecular weights of the PHB samples after surfactant—sodium hypochlorite digestion method were analyzed between 480,000 and 740,000 Da with a polydispersity index (PDI) of 2.15 on average. Such results clearly indicate the high purity of PHB, close to medical grade (Kaplan 1998). SEM image, EDS spectra, and XRD peak of such highly pure PHB, produced after fed-batch fermentation in our research, are represented in Fig. 8. SEM image in Fig. 8a clearly signifies larger granular particles of pure PHB with high molar mass whereas EDS spectrum in Fig. 8b of PHB signifies the upper surface of such granular PHB molecules basically consists of C, H and O2 atom. During EDS analysis, there were no detectable impurities in the form of Na or N in any of the samples. The XRD pattern which was recorded between 0 and 70 of 2θ in Fig. 8c confirms the presence of significant PHB fraction in crystalline form. Similar EDS spectrum and XRD pattern of pure PHB molecules were recorded by Ke et al. (2014) and Panayotidou et al. (2014). The sharp peaks and high peak intensities signify high crystallinity of PHB molecules in our study.
Conclusions
A novel process for production of high-purity PHB was developed by means of continuous feeding strategy in fed-batch fermentation under highly optimized conditions.The overall PHB production process remained simple and cost-efficient through continuous feeding strategies with different dilution rates. The success of the process is evident in high productivity (0.58 g/l h) and purity (98%). The adopted feeding strategy is sensitive, robust, inexpensive, and it helped to convert overall production process of PHB economically attractive. Such high cell density-optimized fermentation strategy was developed from sucrose-based cheap carbon source using C. necator (MTCC 1472). surfactant—sodium hypochlorite-based digestion methodology has resulted in high purity of the product.
References
Ahn WS, Park SJ, Lee SY (2001) Production of poly(3-hydroxybutyrate) from whey by cell recycle fed-batch culture of recombinant Escherichia coli. Biotechnol Lett 23:235–240. doi:10.1023/A:1005633418161
Arifin Y, Sabri S, Sugiarto H et al (2010) Deletion of cscR in Escherichia coli W improves growth and poly-3-hydroxybutyrate (PHB) production from sucrose in fed batch culture. J Biotechnol 156:275–278. doi:10.1016/j.jbiotec.2011.07.003
Bengtsson S, Werker A, Christensson M, Welander T (2008) Production of polyhydroxyalkanoates by activated sludge treating a paper mill wastewater. Bioresour Technol 99:509–516. doi:10.1016/j.biortech.2007.01.020
Bengtsson S, Pisco AR, Reis MAM, Lemos PC (2010) Production of polyhydroxyalkanoates from fermented sugar cane molasses by a mixed culture enriched in glycogen accumulating organisms. J Biotechnol 145:253–263. doi:10.1016/j.jbiotec.2009.11.016
Choi J, Lee SY (1999) Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl Microbiol Biotechnol 51:13–21
Cui ZF, Muralidhara HS (eds) (2010) Membrane technology a practical guide to membrane technology and applications in food and bioprocessing. Elsevier, Amsterdam
Dong Z, Sun X (2000) A new method of recovering polyhydroxyalkanoate from Azotobacter chroococcum. Chin Sci Bull 45:252–256. doi:10.1007/BF02884685
Doran PM (1995) An impart of Elsevier
Du G, Chen J, Yu J, Lun S (2001) Continuous production of poly-3-hydroxybutyrate by Ralstonia eutropha in a two-stage culture system. J Biotechnol 88:59–65. doi:10.1016/S0168-1656(01)00266-8
Fahmy R, Kona R, Dandu R et al (2012) Quality by design I: application of failure mode effect analysis (FMEA) and Plackett–Burman design of experiments in the identification of “main factors” in the formulation and process design space for roller-compacted ciprofloxacin hydrochloride immediate-release tablets. AAPS PharmSciTech 13:1243–1254. doi:10.1208/s12249-012-9844-x
Gahlawat G, Sengupta B, Srivastava AK (2012) Enhanced production of poly(3-hydroxybutyrate) in a novel airlift reactor with in situ cell retention using Azohydromonas australica. J Ind Microbiol Biotechnol 39:1377–1384. doi:10.1007/s10295-012-1138-5
García A, Segura D, Espín G et al (2014) High production of poly-β-hydroxybutyrate (PHB) by an Azotobacter vinelandii mutant altered in PHB regulation using a fed-batch fermentation process. Biochem Eng J 82:117–123. doi:10.1016/j.bej.2013.10.020
Grothe E, Moo-Young M, Chisti Y (1999) Fermentation optimization for the production of poly(β-hydroxybutyric acid) microbial thermoplastic. Enzyme Microb Technol 25:132–141. doi:10.1016/S0141-0229(99)00023-X
Hafuka A, Sakaida K, Satoh H et al (2011) Effect of feeding regimens on polyhydroxybutyrate production from food wastes by Cupriavidus necator. Bioresour Technol 102:3551–3553. doi:10.1016/j.biortech.2010.09.018
Ienczak JL, Schmidt M, Quines LK et al (2016) Poly(3-hydroxybutyrate) production in repeated fed-batch with cell recycle using a medium with low carbon source concentration. Appl Biochem Biotechnol 178:408–417. doi:10.1007/s12010-015-1883-9
Horwitz W (ed) (1980) Official methods of analysis of the Association of Official Analytical Chemists. Association of Official Analytical Chemists, Washington
Kaplan DL (1998) Introduction to biopolymers from renewable resources. In: Kaplan DL (ed) Biopolymers from renewable resources. Macromolecular systems — Materials approach. Springer, Berlin
Ke Y, Wu G, Wang Y (2014) PHBV/PAM scaffolds with local oriented structure through UV polymerization for tissue engineering. Biomed Res Int 2014:1–9. doi:10.1155/2014/157987
Khanna S, Srivastava AK (2005a) Recent advances in microbial polyhydroxyalkanoates. Process Biochem 40:607–619
Khanna S, Srivastava AK (2005b) Statistical media optimization studies for growth and PHB production by Ralstonia eutropha. Process Biochem 40:2173–2182. doi:10.1016/j.procbio.2004.08.011
Khanna S, Srivastava AK (2008a) Continuous production of poly-β-hydroxybutyrate by high-cell-density cultivation of Wautersia eutropha. J Chem Technol Biotechnol 83:799–805. doi:10.1002/jctb.1868
Khanna S, Srivastava AK (2008b) Productivity enhancement of poly-(β-hydroxybutyrate) by fed-batch cultivation of nutrients using variable (decreasing) nutrient rate by Wautersia eutropha. Chem Eng Commun 195:1424–1436. doi:10.1080/00986440801964087
Koller M, Muhr A (2014) Continuous production mode as a viable process-engineering tool for efficient poly(hydroxyalkanoate) (PHA) bio-production. Chem Biochem Eng Q 28:65–77
Koller M, Bona R, Hermann C et al (2005) Biotechnological production of poly(3-hydroxybutyrate) with Wautersia eutropha by application of green grass juice and silage juice as additional complex substrates. Biocatal Biotransform 23:329–337. doi:10.1080/10242420500292252
Kulpreecha S, Boonruangthavorn A, Meksiriporn B, Thongchul N (2009) Inexpensive fed-batch cultivation for high poly(3-hydroxybutyrate) production by a new isolate of Bacillus megaterium. J Biosci Bioeng 107:240–245. doi:10.1016/j.jbiosc.2008.10.006
Kunasundari B, Sudesh K (2011) Isolation and recovery of microbial polyhydroxyalkanoates. Express Polym Lett 5:620–634
Lee J, Lee SY, Park S, Middelberg AP (1999) Control of fed-batch fermentations. Biotechnol Adv 17:29–48
Lee SH, Oh DH, Ahn WS et al (2000) Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by high-cell-density cultivation of Aeromonas hydrophila. Biotechnol Bioeng 67:240–244. doi:10.1002/(SICI)1097-0290(20000120)67:2<240:AID-BIT14>3.0.CO;2-F
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. doi:10.1021/ac60147a030
Mozumder MSI, De Wever H, Volcke EIP, Garcia-Gonzalez L (2014) A robust fed-batch feeding strategy independent of the carbon source for optimal polyhydroxybutyrate production. Process Biochem 49:365–373. doi:10.1016/j.procbio.2013.12.004
Narayanan A, Ramana KV (2012) Polyhydroxybutyrate production in Bacillus mycoides DFC1 using response surface optimization for physico-chemical process parameters. 3Biotech 2:287–296. doi:10.1007/s13205-012-0054-8
Osman Y, Elrazak AA, Khater W (2016) Bioprocess optimization of microbial biopolymer production. J Biobased Mater Bioenergy 10:119–128. doi:10.1166/jbmb.2016.1582
Panayotidou E, Kroustalli A, Baklavaridis A, Deligianni D (2014) XRD diffraction patterns of pure PHB and the PHB/3 wt% C 18 MMT hybrid material—Figure 1 of 10. J Appl Polym Sci 132(11). https://www.researchgate.net/figure/268280877_fig1_Figure-2-XRD-diffraction-patterns-of-pure-PHB-and-the-PHB-3-wt-C-18-MMT-hybrid. Accessed 1 May 2017
Pohlmann A, Fricke WF, Reinecke F et al (2006) Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat Biotechnol 24:1257–1262. doi:10.1038/nbt1244
Ramsay JA, Berger E, Ramsay BA, Chavarie C (1990) Recovery of poly-3-hydroxyalkanoic acid granules by a surfactant-hypochlorite treatment. Biotechnol Tech 4:221–226. doi:10.1007/BF00158833
Rohini D, Phadnis S, Rawal SK (2006) Synthesis and characterization of poly-β-hydroxybutyrate from Bacillus thuringiensis R1. Indian J Biotechnol 5:276–283
Sabra W, Zeng AP, Lünsdorf H, Deckwer WD (2000) Effect of oxygen on formation and structure of Azotobacter vinelandii alginate and its role in protecting nitrogenase. Appl Environ Microbiol 66:4037–4044
Schmidt M, Ienczak JL, Quines LK et al (2016) Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production in a system with external cell recycle and limited nitrogen feeding during the production phase. Biochem Eng J 112:130–135. doi:10.1016/j.bej.2016.04.013
Valappil SP, Misra SK, Boccaccini AR et al (2007) Large-scale production and efficient recovery of PHB with desirable material properties, from the newly characterised Bacillus cereus SPV. J Biotechnol 132:251–258. doi:10.1016/j.jbiotec.2007.03.013
van Wegen RJ, Lee SY, Middelberg AP (2001) Metabolic and kinetic analysis of poly(3-hydroxybutyrate) production by recombinant Escherichia coli. Biotechnol Bioeng 74:70–80
Vijayendra SVN, Rastogi NK, Shamala TR et al (2007) Optimization of polyhydroxybutyrate production by Bacillus sp. CFR 256 with corn steep liquor as a nitrogen source. Indian J Microbiol 47:170–175. doi:10.1007/s12088-007-0033-7
Acknowledgements
The authors are thankful to the Government of India for financial support under the Technical Education Quality Improvement Programme (TEQIP) Phase‐II.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Dey, P., Rangarajan, V. Improved fed-batch production of high-purity PHB (poly-3 hydroxy butyrate) by Cupriavidus necator (MTCC 1472) from sucrose-based cheap substrates under response surface-optimized conditions. 3 Biotech 7, 310 (2017). https://doi.org/10.1007/s13205-017-0948-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0948-6