Abstract
In this study, the influence of different physicochemical parameters on the yield of polyhydroxyalkanoates (PHAs) produced by Bacillus cereus FA11 is investigated. The physicochemical factors include pH, temperature, time, inoculum size and its age, agitation speed and composition of the glucose rich peptone deficient (GRPD) medium. During two-stage fermentation, B. cereus FA11 produced a significantly high (p < 0.05) yield (80.59% w/w) of PHAs copolymer using GRPD medium containing glucose (15 g/L) and peptone (2 g/L) at pH 7, 30 °C and 150 rpm after 48 h of incubation. On the other hand, the presence of olive oil (1% v/v) and peptone (2 g/L) in the GRPD medium resulted in biosynthesis of tercopolymer during two-stage fermentation and the yield of tercopolymer was 60.31% (w/w). The purified PHAs was characterized by Fourier transform infrared spectroscopy and proton resonance magnetic analysis. Proton resonance magnetic analysis confirmed that the tercopolymer was comprised of three different monomeric subunits, i.e., 3-hydroxybutyrate, 3-hydroxyvalerate and 6-hydroxyhexanoate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The polyhydroxyalkanoates (PHAs) are the natural polyesters and are synthesized by a variety of microorganisms under the conditions of nutrient stress (Masood et al. 2015a; Muhammadi et al. 2015; Kumar et al. 2014). The PHAs act as carbon and energy reserves (Quelas et al. 2016; Masood et al. 2015a; Kumar et al. 2009). The PHAs are biodegradable, biocompatible and non-toxic in nature. About 150 different monomeric subunits of PHAs are identified as homopolymer [e.g. poly-3-hydroxybutyrate (PHB)], copolymer [e.g. poly-3-hydroxybutyrate-co-3-hydroxyvalerate (PHBV) and tercopolymer [e.g. poly-3-hydroxybutyrate-co-3-hydroxyvalerate-co-6-hydroxyhexanoate (PHBVHHx)]. This huge diversity of monomeric subunits is mainly dependent upon a type of microorganism, the type of substrate used, the substrate specificity of PHA synthases and the physico-chemical parameters used for PHA production (Tariq et al. 2015; Foong et al. 2014). The incorporation of diverse monomeric subunits on the main backbone of polymer has deeply influenced the physical properties of PHAs synthesized by microorganisms.
The PHAs have a wide range of applications in biomaterials, packaging, agriculture and food industries depending upon their monomeric compositions (Chen et al. 2016; Muhammadi et al. 2015; Bugnicourt et al. 2014). It is expected that demand of PHAs would reach to 34,000 MT by 2018 (Masood et al. 2015a, b). However, the key impediment in the commercialization of PHAs is their high production cost. The price of carbon source accounts for 80% of the overall production cost of PHAs. Carbon sources such as glucose and fructose along with fatty acids are successfully used to synthesize PHAs copolymer and tercopolymer by various bacterial strains (Ray and Kalia 2017; Kumar et al. 2016; Masood et al. 2011, 2015b ). On the other hand, plant oil is considered as a cheap and viable source for the biosynthesis of PHAs. There are several reports on the production of PHA tercopolymer from Gram negative bacteria using waste glycerol (Cavalheiro et al. 2012), mixtures of fructose and sodium heptanoate (Rathi et al. 2013), oleic acid (Cheema et al. 2012), and mixtures of crude palm kernel oil (Bhubalan et al. 2010). However, the absence of the toxic lipo-polysaccharides secretion (Singh et al. 2009) in member of the genus Bacillus made them an ideal candidate to produce PHAs homo-, co- and ter-copolymers intended to be used as biomaterials.
The two-stage fermentation is considered as an ideal approach on bio-industrial scale to achieve a high cell density in bioreactor and overcomes the problems associated with inhibition by substrate or induction, which ultimately declines the product yield. The pH (Mohd Zahari et al. 2012; Venkata Mohan et al. 2010), temperature (Gomaa 2014; Wei et al. 2011), dissolved oxygen (Kshirsagar et al. 2013; Mohd Zahari et al. 2012), carbon source (Patel et al. 2016; Tomizawa et al. 2014), nutrient limitations via nitrogen (Gowda and Shivakumar 2014; Singh et al. 2013) and phosphorus (Grousseau et al. 2014) are considered as critical variables for the enhancement of PHAs yield.
This study reports for the first time, the potential of Bacillus cereus FA11 to consume olive oil to produce a high yield of PHA tercopolymer under the nitrogen deficient environment using two-stage fermentation. In this study, various sets of experimental conditions were optimized to obtain a maximum yield of PHAs by B. cereus FA11 on the small scale. We had separately used both the short chain fatty acids (propionic acid, butyric acid, valeric acid, hexanoic acid) and a mixture of long chain fatty acids (olive oil) along with the glucose in the GRPD medium (considered as an optimal medium). Batch and two-stage fermentation were carried out using glucose (as a sole carbon source) on the large scale. Additionally, the glucose was used to obtain a high density of bacterial cells and then olive oil was added during the second stage to find out its influence on the chemical composition of PHAs. Fourier transform infrared spectroscopy and proton resonance magnetic analysis techniques were used for the structural characterization of the PHAs. The molecular weights of the PHAs copolymer and tercopolymer were determined.
Materials and methods
Bacterial strain
A gram-positive rod-shaped bacterium Bacillus cereus FA11 (GenBank Accession number JN593008) was used in the present study. This strain was isolated from trinitrotoluene (TNT)-contaminated soil (Masood et al. 2011). The bacterial strain was maintained on a nutrient agar slant at 4 °C and sub-cultured every month.
Inoculum preparation and PHAs fermentation
Inoculum preparation of B. cereus FA11 was done using nutrient broth. Four different media, i.e., nutrient broth, PHA-detection broth (PDB) (Lee and Choi 1999) Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) broth (Chaijamrus and Udpuay 2008) and glucose rich peptone deficient (GRPD) were used to screen out the best medium for obtaining a high yield of PHAs by B. cereus FA11. The composition of all media used in this study is given in Supplementary file (Table I). About 200 mL of each of the above-mentioned media were prepared in 500 mL Erlenmeyer flasks. Each of medium was inoculated with 2% (v/v) of 24 h old inoculum and was incubated at 37 °C and 150 rpm in a shaking incubator for 48 h.
PHAs extraction
Sodium hypochlorite (NaOCl) digestion method was used for PHAs extraction (Arnold et al. 1999). Centrifugation was done at 10,000 rpm for 20 min to collect bacterial cells. A biomass of 200 mg was suspended in 5 mL of 0.4% (v/v) NaOCl solution and the mixture was incubated for 1 h at 37 °C. The PHAs pellet was separated by centrifugation at 10,000 rpm for 20 min. The pellet was washed with acetone followed by water. Chloroform was used for purification of the PHAs pellet. Then, chloroform was evaporated and the PHAs were weighed. The PHAs yield was defined as a mass fraction of PHAs (g/L) in biomass (g/L).
Optimization of physico-chemical parameters
The effect of physical factors, including pH (6.0, 6.5, 7.0, 7.5 and 8.0), temperature (25, 30, 35 and 40 °C), time (12, 24, 36, 48, 60, 72, 84 and 96 h), inoculum size (1, 2, 3, 4 and 5% v/v), age (24, 48 and 72 h) and agitation speed (0, 50, 100, 150 and 200 rpm) on the PHAs production by B. cereus FA11 were studied. Moreover, the optimization of the contents of the GRPD medium, i.e., carbon source (glucose, lactose, fructose and molasses), its concentration (0, 5, 10, 15 and 20 g/L), nitrogen source [no nitrogen source (control), tryptone, peptone from meat, KNO3 and (NH4)2SO4] and its concentration (2, 4, 6, 8 and 10 g/L) was done. The effect of volatile fatty acids (VFAs), i.e., propionic acid, butyric acid, valeric acid, hexanoic acid and olive oil (1% v/v solution) on PHAs yield was also evaluated.
Fermentation on the large scale
To carry out the PHAs fermentation on the large scale, two sets of experiments were done using: (1) glucose as a sole carbon source, (2) glucose and olive oil as carbon sources. The batch and two-stage fermentation were carried out using 2800 mL Fernbach-style culture flasks using shaking incubator. About 1500 mL of the optimized GRPD medium [yeast extract (2.5 g/L), peptone (4.0 g/L), NaCl (1.25 g/L) and glucose (15 g/L)] was prepared and batch fermentation was carried out for 48 h at pH 7, 30 °C and 150 rpm. While, for a two-stage fermentation, about 1000 mL of the GRPD medium A [yeast extract (2.5 g/L), peptone (4.0 g/L), NaCl (1.25 g/L) and glucose (10 g/L)] was inoculated with 3% (v/v) of 24 h old inoculum and incubated for 18 h during first stage (biomass production). The bacterial cells were then harvested by centrifugation at 10,000 rpm for 20 min and washed with sterilized distilled water. During second stage, the washed cells were resuspended in 1500 mL GRPD medium B [yeast extract (2.5 g/L), peptone (2.0 g/L), NaCl (1.25 g/L) and glucose (15 g/L)] and incubated for 48 h at pH 7, 30 °C and 150 rpm for PHA production. Similarly, in another experiment the bacterial cells were initially grown in GRPD medium containing glucose for 18 h during first stage (biomass production). During second stage, the separated cells were resuspended into GRPD medium containing olive oil (1% v/v) for PHA accumulation.
Characterization of PHAs
Fourier transform infrared (FTIR) spectroscopy
The structural analysis of the PHAs sample was performed using the FTIR spectrophotometer (Nicolet model 6700; Thermo Electron Crop, Marietta, OH). The sample was placed on the sample holder and the spectrum was recorded using an attenuated reflectance technique having a diamond crystal. The spectrum was scanned from 4000–400 cm−1 at a resolution of 6.0 cm−1.
Proton nuclear magnetic resonance (1H-NMR) analysis
The 1H-NMR of PHAs sample was done using deuterated chloroform at a room temperature with AVANCE 300 B spectrometer (Bruker Daltonics, Billerica, MA). The mole fraction of the 3-HV monomeric subunit in the PHAs copolymer was calculated by an equation described by Liu et al. (2010). The mole fractions of monomeric subunits were determined from the peak of proton resonance of 3-HB, 3-HV and 6-HHx in the PHAs tercopolymer (Labuzek and Radecka 2001).
Determination of molecular weight
About 20 mg of PHAs was dissolved in 15 mL of chloroform and the average molecular weight of sample solution was determined using following the Mark-Houwink equation:
where η was the viscosity (Pa s), K was the consistency index 1.18 × 10−4, Mw was the average molecular weight and α was the flow behavior index 0.78 (Brandrup et al. 1990).
Statistical analysis
All fermentation experiments were performed in triplicate. All the data with repeated measurements were statistically compared and evaluated by employing the analysis of variance (ANOVA). The comparison between means was calculated by the Student-Newman–Keuls test.
Results and discussion
Optimization of physico-chemical parameters
Optimization of physico-chemical parameters is of vital importance for obtaining a high yield of PHAs on the commercial scale. Four different media, i.e., nutrient broth, GRPD, PDB and DSMZ broth were screened for PHAs production and the GRPD medium produced significantly a high (p < 0.05) yield of PHAs (22.66%) (Fig. 1a). Therefore, this medium was selected for further optimization studies. Additionally, a decrease in the final pH of the medium was found at the end of the fermentation process due to entry of bacterial cells from exponential phase to stationary phase. Philip et al. (2009) stated that low pH conditions prevent PHAs degradation during un-buffered Bacillus fermentation. During optimization of temperature, the GRPD medium was incubated at various temperatures ranging from 25–40 °C and the optimum yield of PHAs (22.28%) was found at 30 °C (Fig. 1b). Cupriavidus taiwanensis produced a high PHB yield at pH 7 and 28–30 °C temperature and an alteration in temperature variable reduced the dissolved oxygen levels, mass transfer efficiency and ultimately resulted in a decrease in PHAs synthesis (Wei et al. 2011). The effect of different initial pH (6, 6.5, 7, 7.5 and 8) on PHAs production was evaluated. B. cereus FA11 produced a maximum yield of PHAs (29.20%) using the GRPD medium at an initial pH 7 and 30 °C (Fig. 1c). The Bacillus mycoides DFC1 strain produced a high PHAs yield at pH 7.3 (Narayanan and Ramana 2012). The PHAs production was carried out at 0, 50, 100, 150 and 200 rpm using the GRPD medium to determine the influence of agitation speed. The significantly high (p < 0.05) yield of PHAs (42.38%) was obtained at 150 rpm and 30 °C (Fig. 1d).
The PHAs production was carried out by B. cereus FA11 for 96 h to determine a suitable harvesting time. The significantly high (p < 0.05) yield (44.59%) of PHAs was found after 48 h of incubation at pH 7, 30 °C and 150 rpm (Fig. 2a). B. thuringiensis EGU45 produced optimal quantities of PHAs over an incubation period of 48 h using medium containing 1% crude glycerol medium supplemented with nutrient broth (Kumar et al. 2015). The PHAs production was carried out with an inoculum of different ages to obtain its optimum yield. The optimum yield of PHAs (44%) was obtained with 2% (v/v) of 24 h old inoculum of B. cereus FA11 at pH 7.0, 30 °C and 150 rpm (Fig. 2b). An inoculum of various sizes (1, 2, 3, 4, 5% v/v) was also added to the GRPD medium to achieve a high yield of PHAs. The maximum yield of PHAs (48.81%) was obtained using 3% (v/v) of 24 h old inoculum at pH 7.0, 30 °C and 150 rpm (Fig. 2c). An inocula containing higher cell density resulted in higher PHAs yield (Kulkarni et al. 2010). The volatile fatty acids (propionic, butyric, valeric, hexanoic acid) and olive oil were separately supplied in the optimized GRPD medium to evaluate their effect on PHAs yield and its composition. A significantly high (p < 0.05) yield of PHAs (28.10%) was obtained by Bacillus cereus FA11 due to the presence of olive oil in GRPD medium after 48 h of incubation at pH 7, 30 °C and 150 rpm (Fig. 2d).
Various carbon sources, i.e., fructose, lactose, glucose and molasses at 1 g/L concentration were added to the GRPD medium to determine their effect on PHAs yield (Fig. 3a). A significantly high (p < 0.05) yield of PHAs (49.20%) was obtained by B. cereus FA11 due to the presence of glucose in the GRPD medium in comparison to the other carbon sources. Different concentrations (0, 5, 10, 15 and 20 g/L) of glucose were added to the GRPD medium and the significantly high (p < 0.05) yield of PHAs (53.48%) was obtained in the presence of 15 g/L glucose in the GRPD medium after 48 h of incubation at pH 7.0, 30 °C and 150 rpm as compared to others (Fig. 3b). Different nitrogen sources, i.e., control (no nitrogen source), tryptone, peptone, KNO3 and (NH4)2SO4 were added to the GRPD medium to find out the most suitable nitrogen source for obtaining a high yield of PHAs. The maximum yield of PHAs (56.21%) was obtained in the presence of peptone as a nitrogen source in the GRPD medium at pH 7, 30 °C and 150 rpm after 48 h of incubation (Fig. 3c). Then, different concentrations of peptone (0, 2, 4, 6, 8 and 10 g/L) were provided in the GRPD medium. The significantly high (p < 0.05) yield of PHAs (62.03%) was obtained in the presence of peptone (2 g/L) and glucose (15 g/L) in the GRPD medium at pH 7, 30 °C and 150 rpm after 48 h of incubation (Fig. 3d). This behavior is consistent with the previous finding that Bacillus mycoides DFC1 gave a high yield of PHB in the presence of glucose and peptone in the growth medium (Narayanan and Ramana 2012). Thus, the selection of the maximum amount of carbon source and minimal quantity of nitrogen source (C/N ratio) is necessary to obtain a high yield of PHAs.
Fermentation on the large scale
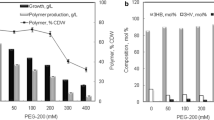
Different fermentation strategies, i.e., batch, fed batch, continuous and two-stage fermentation were used to obtain high yields of PHAs from microorganisms on a large scale. The effect of batch and two-stage fermentation on the PHAs production by Bacillus cereus FA11 was evaluated. Bacillus cereus FA11 produced significantly a high (p < 0.05) yield of PHAs (80.59%) using glucose as a sole carbon source in the GRPD medium at pH 7, 30 °C and 150 rpm during two-stage fermentation (Fig. 4a). The members of the genus Bacillus harbor the type IV PHA synthases (PhaRC) (Mizuno et al. 2017). The PhaRCYB4 from Bacillus cereus YB4 demonstrates a boarder substrate specificity than Bacillus megaterium and synthesized PHA copolymers from sugar and plant oils during fermentation (Tsuge et al. 2015). An olive oil is a mixture of mainly triacylglycerols and various long chain fatty acids, i.e., palmitic acid (C16:0), palmitoleic acid (16:1), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2) and linolenic acid (C18:3) (Monfreda et al. 2014). The PHAs fermentation was carried out in the presence of renewable carbon substrates (glucose and olive oil) to evaluate the substrate specificity of the type IV PHAs synthases of Bacillus cereus FA11. The yield of PHAs produced by B. cereus FA11 was 60.31% using the GRPD medium containing olive oil after 48 h of incubation at pH 7, 30 °C and 150 rpm during two-stage fermentation (Fig. 4b). The consumption of olive oil by Bacillus cereus FA11 may provide an evidence that a lager inter-atomic distance between the sulfhydryl group of cysteine and amino group of histidine of a phaC subunit of the type IV PHA synthase is responsible for the utilization of long-chain fatty acid as claimed by Tariq et al. (2015) during in silico studies.
Characterization of PHAs
FTIR analysis
The FTIR analysis of the PHAs sample obtained from B. cereus FA11 via two-stage fermentation in the presence of glucose and olive oil as a carbon source in the GRPD medium is given in Fig. 5. The absorption band at 1275 cm−1 was corresponding to the C-O stretching. The absorption peaks at 1375 and 1456 cm−1 were attributed to the C-H bending vibrations form -CH2, -CH3 and -CH bonds. A characteristic band at 1739 cm−1 was corresponding to C=O stretching vibration and is associated with the amorphous nature of PHAs. The band appeared at 2935 cm−1 was representing the CH2 anti-symmetric stretching. The absorption peaks appeared in region 3200–3300 cm−1 were corresponding to the CH3 asymmetric stretching. The presence of a band above 3000 cm−1 (CH3 asymmetric stretching) was a result of the C-H–O hydrogen bond (Patel et al. 2015; Sato et al. 2004). The absorption bands in the regions at 3015–2960, 2945–2925, 2855–2875, 1739 and 1275 cm−1 indicated the presence of the asymmetrical CH3 stretching, anti-symmetrical CH2 stretching, symmetrical CH3 stretching modes, C=O stretching and C-O stretching, respectively (Kumar et al. 2015; Patel et al. 2015; López-Cuellar et al. 2011).
1H-NMR analysis
B. cereus FA11 synthesized a PHAs copolymer using glucose as a sole carbon source in the GRPD medium. This copolymer was made up of two monomeric subunits, i.e., 85 mol % of 3-HB and 15 mol % of 3-HV, as reported previously (Masood et al. 2011). B. cereus FA11 also produced variations in the monomeric composition of the copolymer during its growth on GRPD medium containing both glucose and VFAs as carbon source. The result is given in the supplementary file (Table II). B. cereus FA11 produced a PHAs tercopolymer in the presences of glucose and olive oil as carbon sources in the GRPD medium during two-stage fermentation. The characteristic signals observed at 0.87 (4) and 1.24 (1) ppm were corresponding to protons of methyl (CH3) group of 3-HV and 3-HB, respectively. Two signals (2, 6) appeared in a region between 5.25–5.45 ppm and were attributed to the protons of methine (CH) groups of 3-HB and 3-HV, respectively. The signal (8) obtained in a region between 4.1 and 4.4 pm was corresponding to protons of methylene (CH2) group of 6-HHx. This tercopolymer was comprised of 45.66 mol % of 3-HB, 9.28 mol % of 3-HV and 45.35 mol % of 6-HHx.
Molecular weight determination
The average molecular weights of copolymer and tercopolymer were 91.60 and 1.50 kDa, respectively. The characteristic weight average molecular weight of PHBV produced by Halomonas campisalis ranged from 2.08 × 104 Da (Kshirsagar et al. 2013).
Conclusion
In this study, the optimization of physicochemical parameters was done to obtain a high yield of PHAs by B. cereus FA11. During batch fermentation, the yield of PHAs was increased from 22.66 to 62.03% of its biomass (w/w) using GRPD medium containing peptone (2 g/L) and glucose (15 g/L) at pH 7, 30 °C and 150 rpm after 48 h of incubation. During two-stage fermentation, the PHAs yield was increased to 80.58% of its biomass (w/w) using glucose as a sole carbon source. While, a high yield of PHAs tercopolymer was obtained due to the presence of olive oil in the GRPD medium during two-stage fermentation. The yield of tercopolymer was 60.31% of its biomass (w/w). Thus, B. cereus FA11 was capable to produce both PHAs copolymer and tercopolymer depending upon the type of substrates used during fermentation.
References
Arnold L, Demain J, Davis E (1999) Polyhydroxyalkanoates. Manual of microbiology and biotechnology. Am Soc Microbiol, Washington, pp 616–627
Bhubalan K, Rathi D-N, Abe H et al (2010) Improved synthesis of P(3HB-co-3HV-co-3HHx) terpolymers by mutant Cupriavidus necator using the PHA synthase gene of Chromobacterium sp. USM2 with high affinity towards 3HV. Polym Degrad Stab 95:1436–1442. doi:10.1016/j.polymdegradstab.2009.12.018
Brandrup J, Immergut E, Grulke EA (1990) Polymer handbook. Wiley, New York, vol 12, pp 265. doi:10.1016/0168-3659(90)90108-6
Bugnicourt E, Cinelli P, Lazzeri A, Alvarez V (2014) Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. eXPRESS Polym Lett 8:791–808
Cavalheiro JMBT, Raposo RS, de Almeida MCMD et al (2012) Effect of cultivation parameters on the production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) and poly (3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by Cupriavidus necator using waste glycerol. Bioresour Technol 111:391–397. doi:10.1016/j.biortech.2012.01.176
Chaijamrus S, Udpuay N (2008) Production and Characterization of Polyhydroxybutyrate from Molasses and Corn Steep Liquor produced by Bacillus megaterium ATCC 6748. Agric Eng X:1–12
Cheema S, Bassas-Galia M, Sarma PM et al (2012) Exploiting metagenomic diversity for novel polyhydroxyalkanoate synthases: production of a terpolymer poly (3-hydroxybutyrate-co-3-hydroxyhexanoate-co-3-hydroxyoctanoate) with a recombinant Pseudomonas putida strain. Bioresour Technol 103:322–328. doi:10.1016/j.biortech.2011.09.098
Chen G-Q, Jiang X-R, Guo Y (2016) Synthetic biology of microbes synthesizing polyhydroxyalkanoates (PHA). Synth Syst Biotechnol 1:236–242. doi:10.1016/j.synbio.2016.09.006
Foong CP, Lau N-S, Deguchi S et al (2014) Whole genome amplification approach reveals novel polyhydroxyalkanoate synthases (PhaCs) from Japan Trench and Nankai Trough seawater. BMC Microbiol 14:318. doi:10.1186/s12866-014-0318-z
Gomaa EZ (2014) Production of polyhydroxyalkanoates (PHAs) by Bacillus subtilis and Escherichia coli grown on cane molasses fortified with ethanol. Braz Arch Biol Technol 57:145–154
Gowda V, Shivakumar S (2014) Agrowaste-based Polyhydroxyalkanoate (PHA) production using hydrolytic potential of Bacillus thuringiensis IAM 12077. Braz Arch Biol Technol 57:55–61
Grousseau E, Blanchet E, Déléris S et al (2014) Phosphorus limitation strategy to increase propionic acid flux towards 3-hydroxyvaleric acid monomers in Cupriavidus necator. Bioresour Technol 153:206–215. doi:10.1016/j.biortech.2013.11.072
Kshirsagar PR, Suttar R, Nilegaonkar SS et al (2013) Scale up production of polyhydroxyalkanoate (PHA) at different aeration, agitation and controlled dissolved oxygen levels in fermenter using Halomonas campisalis MCM B-1027. J Biochem Technol 4:512–517
Kulkarni SO, Kanekar PP, Nilegaonkar SS et al (2010) Production and characterization of a biodegradable poly (hydroxybutyrate-co-hydroxyvalerate) (PHB-co-PHV) copolymer by moderately haloalkalitolerant Halomonas campisalis MCM B-1027 isolated from Lonar Lake, India. Bioresour Technol 101:9765–9771. doi:10.1016/j.biortech.2010.07.089
Kumar T, Singh M, Purohit HJ, Kalia VC (2009) Potential of Bacillus sp. to produce polyhydroxybutyrate from biowaste. J Appl Microbiol 106:2017–2023. doi:10.1111/j.1365-2672.2009.04160.x
Kumar P, Singh M, Mehariya S et al (2014) Ecobiotechnological approach for exploiting the abilities of Bacillus to produce co-polymer of polyhydroxyalkanoate. Indian J Microbiol 54:151–157. doi:10.1007/s12088-014-0457-9
Kumar P, Ray S, Patel SKS et al (2015) Bioconversion of crude glycerol to polyhydroxyalkanoate by Bacillus thuringiensis under non-limiting nitrogen conditions. Int J Biol Macromol 78:9–16. doi:10.1016/j.ijbiomac.2015.03.046
Kumar P, Ray S, Kalia VC (2016) Production of co-polymers of polyhydroxyalkanoates by regulating the hydrolysis of biowastes. Bioresour Technol 200:413–419. doi:10.1016/j.biortech.2015.10.045
Labuzek S, Radecka I (2001) Biosynthesis of PHB tercopolymer by Bacillus cereus UW85. J Appl Microbiol 90:353–357
Lee SY, Choi JIL (1999) Polyhydroxyalkanoates: biodegradable polymer. In: Demain AL, Davies JE (eds) Manual of industrial microbiology and biotechnology, 2nd edn. ASM, Washington, DC, pp 616–627
Liu H, Pancholi M, Stubbs J, Raghavan D (2010) Influence of hydroxyvalerate composition of polyhydroxy butyrate valerate (PHBV) copolymer on bone cell viability and in vitro degradation. J Appl Polym Sci 116:3225–3231. doi:10.1002/app.31915
López-Cuellar MR, Alba-Flores J, Rodríguez JNG, Pérez-Guevara F (2011) Production of polyhydroxyalkanoates (PHAs) with canola oil as carbon source. Int J Biol Macromol 48:74–80. doi:10.1016/j.ijbiomac.2010.09.016
Masood F, Hasan F, Ahmed S, Hameed A (2011) Biosynthesis and characterization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) from Bacillus cereus FA11 isolated from TNT-contaminated soil. Ann Microbiol 62:1377–1384. doi:10.1007/s13213-011-0386-3
Masood F, Yasin T, Hameed A (2015a) Polyhydroxyalkanoates—what are the uses? Current challenges and perspectives. Crit Rev Biotechnol 35:514–521. doi:10.3109/07388551.2014.913548
Masood F, Yasin T, Hameed A (2015b) Production and characterization of Tailor- made polyhydroxyalkanoates by Bacillus cereus FC11. Pak J Zool 47:491–503
Mizuno K, Kihara T, Tsuge T et al (2017) Cloning and heterologous expression of a novel subgroup of class IV polyhydroxyalkanoate synthase genes from the genus Bacillus. Biosci Biotechnol Biochem 81:194–196. doi:10.1080/09168451.2016.1230006
Mohd Zahari MAK, Ariffin H, Mokhtar MN et al (2012) Factors affecting Poly (3-hydroxybutyrate) production from Oil Palm Frond Juice by Cupriavidus necator (CCUG52238(T)). J Biomed Biotechnol 2012:125865. doi:10.1155/2012/125865
Monfreda M, Gobbi L, Grippa A (2014) Blends of olive oil and seeds oils: characterisation and olive oil quantification using fatty acids composition and chemometric tools. Part II. Food Chem 145:584–592. doi:10.1016/j.foodchem.2013.07.141
Muhammadi Shabina, Afzal M, Hameed S (2015) Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: production, biocompatibility, biodegradation, physical properties and applications. Green Chem Lett Rev 8:56–77. doi:10.1080/17518253.2015.1109715
Narayanan A, Ramana KV (2012) Polyhydroxybutyrate production in Bacillus mycoides DFC1 using response surface optimization for physico–chemical process parameters. 3 Biotech 2:287–296. doi:10.1007/s13205-012-0054-8
Patel SKS, Kumar P, Singh M et al (2015) Integrative approach to produce hydrogen and polyhydroxybutyrate from biowaste using defined bacterial cultures. Bioresour Technol 176:136–141. doi:10.1016/j.biortech.2014.11.029
Patel SKS, Lee J-K, Kalia VC (2016) Integrative approach for producing hydrogen and polyhydroxyalkanoate from mixed wastes of biological origin. Indian J Microbiol 56:293–300. doi:10.1007/s12088-016-0595-3
Philip S, Sengupta S, Keshavarz T, Roy I (2009) Effect of impeller speed and pH on the production of poly (3-hydroxybutyrate) using Bacillus cereus SPV. Biomacromol 10:691–699. doi:10.1021/bm801395p
Quelas JI, Mesa S, Mongiardini EJ et al (2016) Regulation of polyhydroxybutyrate synthesis in the soil bacterium Bradyrhizobium diazoefficiens. Appl Environ Microbiol 82:4299–4308. doi:10.1128/AEM.00757-16
Rathi D-N, Persson EJ, Maurer FHJ, Sudesh K (2013) Biosynthesis of P(3HB-co-3HV-co-3HHp) terpolymer by Cupriavidus necator PHB-4 transformant harboring the highly active PHA synthase gene of Chromobacterium sp. USM2. Malays J Microbiol 9:140–146
Ray S, Kalia VC (2017) Co-metabolism of substrates by Bacillus thuringiensis regulates polyhydroxyalkanoate co-polymer composition. Bioresour Technol 224:743–747. doi:10.1016/j.biortech.2016.11.089
Sato H, Murakami R, Padermshoke A et al (2004) Infrared spectroscopy studies of CH···O hydrogen bondings and thermal behavior of biodegradable poly (hydroxyalkanoate). Macromolecules 37:7203–7213. doi:10.1021/ma049117o
Singh M, Patel SK, Kalia VC (2009) Bacillus subtilis as potential producer for polyhydroxyalkanoates. Microb Cell Fact 8:38. doi:10.1186/1475-2859-8-38
Singh G, Kumari A, Mittal A et al (2013) Poly β-hydroxybutyrate production by Bacillus subtilis NG220 using sugar industry waste water. Biomed Res Int. doi:10.1155/2013/952641
Tariq A, Hameed A, Bukhari H, Masood F (2015) Is atomic rearrangement of type IV PHA synthases responsible for increased PHA production? J Biomol Struct Dyn 33:1225–1238
Tomizawa S, Chuah J-A, Matsumoto K et al (2014) Understanding the limitations in the biosynthesis of polyhydroxyalkanoate (PHA) from Lignin derivatives. ACS Sustain Chem Eng 2:1106–1113. doi:10.1021/sc500066f
Tsuge T, Hyakutake M, Mizuno K (2015) Class IV polyhydroxyalkanoate (PHA) synthases and PHA-producing Bacillus. Appl Microbiol Biotechnol 99:6231–6240. doi:10.1007/s00253-015-6777-9
Venkata Mohan S, Venkateswar Reddy M, Venkata Subhash G, Sarma PN (2010) Fermentative effluents from hydrogen producing bioreactor as substrate for poly (β-OH) butyrate production with simultaneous treatment: an integrated approach. Bioresour Technol 101:9382–9386. doi:10.1016/j.biortech.2010.06.109
Wei YH, Chen WC, Huang CK et al (2011) Screening and evaluation of polyhydroxybutyrate-producing strains from indigenous isolate Cupriavidus taiwanensis strains. Int J Mol Sci 12:252–265. doi:10.3390/ijms12010252
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
We thank Higher Education Commission (HEC) Pakistan for providing financial assistance under the Indigenous 5000 Ph.D. Fellowship Program to complete this research work.
Conflict of interest
Authors report no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Masood, F., Abdul-Salam, M., Yasin, T. et al. Effect of glucose and olive oil as potential carbon sources on production of PHAs copolymer and tercopolymer by Bacillus cereus FA11. 3 Biotech 7, 87 (2017). https://doi.org/10.1007/s13205-017-0712-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0712-y