Abstract
Cerium oxide nanoparticles (CeO2 NPs) have received immense interest due to their wide application in environmental remediation, photocatalytic dye degradation, biological applications, and industrial fields. The environmental concerns and energy issues encourage the development of a green protocol to synthesize CeO2 NPs. The greener approach proffers environment-friendly, non-toxic, economically affordable, and efficient. This review describes: (1) the plant extracts, microbes, biological product mediated synthesis of CeO2 NPs, and the mechanism involved in manufacturing it, (2) photocatalytic dye degradation ability of CeO2 NPs; and (3) potential biological application as an antibacterial agent, antifungal activity, antioxidant activity, antidiabetic, and anticancer property. We believe this review will provide a handy guide to the researcher interested in the biosynthesis of CeO2 NPs and their possible applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the modern era, nanotechnology got enormous interest from the scientific community due to its synthesis, design, analysis, and applications, making human life safer and more comfortable (Gawande et al. 2016; Dutta et al. 2021; Cuong et al. 2022). Application of the nanomaterials for water treatment (Raizada et al. 2020), environmental remediation (Khin et al. 2012), semiconductor (Dabhane et al. 2021b), gas sensor (Das et al. 2022), energy storage (Pomerantseva et al. 2019), catalysis (Pansambal et al. 2019; Bardapurkar et al. 2021; Dabhane et al. 2021a), agriculture (Peters et al. 2016), food technology (Frewer et al. 2014), pharmaceuticals (Sharma and Hussain 2020), textile industry (Makvandi et al. 2021), and biomedicine (Teradal and Jelinek 2017; Ryabchikova 2021) was well documented in the literature. Numerous physical, chemical, and biological methods have been employed for the synthesis of nanoparticles till now (Shah et al. 2015; Nikam et al. 2018). However, chemical methods use harmful chemicals and toxic reagents, influencing human health and environmental pollution (Kundu et al. 2020). To reduce this impact, the biological or green method was used to synthesize nanoparticles that proffer eclectic advantages (Nasrollahzadeh et al. 2021). Additionally, the biological or green method is simple, environmentally benign, economically affordable, and easy to scale up (Dabhane et al. 2021c; Ghotekar et al. 2021).
In the recent decade, nanoparticles synthesis of metals such as Silver, Gold, Copper, Zinc, Selenium, Aluminum, Cadmium, Titanium, Nickel, Magnesium, Iron, and Cerium employing bacteria, fungus, enzymes, proteins carbohydrates, and plant material has got the massive attraction from the scientific community (Shah et al. 2015; Hernández-Díaz et al. 2021; Mohammadzadeh et al. 2022). Cerium is the most abundant rare earth element of the lanthanide series. In a previous report, Arumugam et al. stated that CeO2 nanoparticles (NPs) synthesized using Gloriosa superba L. leaf extract show spherical morphology with 5 nm size and displayed good antibacterial activity (Arumugam et al. 2015). Also, Sisubalan et al. described the synthesis of CeO2 NPs utilizing Rubia cordifolia L. leaf extract and highlighted its anticancer potential on MG-63 human osteosarcoma cells (Sisubalan et al. 2017).
Moreover, CeO2 NPs shows diverse application in optical devices (Ghanashyam Krishna et al. 1998), solar cell (Corma et al. 2004), fuel oxidation catalysis (Jung et al. 2005), antioxidant (Dutta et al. 2016), cytotoxic activity (Irshad et al. 2019), catalysis (Zamani et al. 2018), wound-healing activity (Ahmed et al. 2021), antibacterial (Arumugam et al. 2015), anticancer activity (Sisubalan et al. 2017), angiogenesis induction (Das et al. 2012), and radical scavenging activity (Korotkova et al. 2019).
The manufacturing of nanoparticles can be accomplished in one of the two ways: “top-down” or “bottom-up” (Fig. 1) (Cuong et al. 2022). In the top-down approach, bulk materials are broken down into tiny particles via size reduction utilizing various lithographic techniques, including milling, grinding, laser ablation, sputtering, and so on. On the other hand, in the bottom-up approach, nanoparticles were manufactured via chemical, physical, and biological techniques (plant material, microbes, biological products, and so on). The biological methods are effectively beneficial from the environmental and economic points of view (Iravani 2011). Hence, numerous reports have been found on the synthesis of metal oxide NPs using the biological method nowadays. Likewise, there are numerous other advantages and disadvantages of diverse synthetic approaches, which have been summarized and tabulated in Table 1.
This review summarizes recent progress in the investigation and advancement of plant extract, microbes, and biological molecules mediated biosynthesis of CeO2 NPs-based on the available scientific literature, which was searched using several keywords, i.e., plant extract, green synthesis, biosynthesis, plant extract, CeO2 NPs, photocatalytic applications) on the Scopus database. While conducting literature reviews from 2003 to June 2022, publications published by the publishers like Elsevier, Springer, RSC, and Wiley were used. In addition, the keyword used for the literature search is "Bioengineered cerium oxide nanoparticles". Using above mentioned keyword, about 464 articles were obtained. The graphical representation of obtained literature is shown in Fig. 2. This study has also described the methods for plant extract, microbes, and biological molecule-mediated synthesis of CeO2 NPs and possible mechanisms for synthesis. Besides this, the multifunctional application of CeO2 NPs and biological application were explant in detail. The present review will help the researcher to understand the opportunities and challenges in the biogenic synthesis of CeO2 NPs.
Protocol for the synthesis of CeO2 NPs
Using plant extracts
A simple method is used for the biogenic production of CeO2 NPs using plant extracts. Initially, 10 gm of fresh plant material of the chosen plant was added into a 250 mL beaker containing 100 mL double-distilled water and heated beaker at 100 °C for 10 min. Next, filter the extract through Whatman No. 1 filter paper. Afterward, the aqueous solution of selected cerium salt was prepared using double-distilled water. The prepared cerium salt solution is mixed with aqueous plant extract and stirred the mixture at 80 °C for 4–8 h. After a certain period, a yellowish precipitate of CeO2 NPs was formed. Next, the synthesized CeO2 NPs were separated using centrifugation, washed with double-distilled water, and the precipitate was calcined at 500 °C for 2 h. Thus, yellow-colored CeO2 NPs were carefully collected and packed for characterization purposes. Table 2 offers numerous examples of plant extract-mediated synthesized CeO2 NPs.
Using microbial biomass
A selected fungus was inoculated in Czapek-Dox-Broth (CDB) medium, and the flask was incubated at 37–50 °C, at 120 rpm for 72–120 h. The fungi grown on CDB medium were filtered using Whatman No. 1 filter paper amd were stored at 4 °C for further use. Afterward, the selected cerium salt was added to 100 mL fungal filtrate under vigorous stirring. The stirring was continued for 4–6 h at 27–80 °C. A color change indicates completion of the reaction, and a precipitate was formed. The formed precipitate was collected, washed with double-distilled water several times, and the precipitate was calcined at 300–400 °C for 2 h to obtain CeO2 NPs.
Using biological products
Cerium nitrate was dissolved in 50 mL double-distilled water to prepare cerium salt solution and stirred for 5 min. In the meantime, the selected biological product was dissolved in 50 mL double-distilled water and he cerium salt solution was added. The mixture was heated to 60–70 °C with stirring for 3–8 h. Next, the dark color solution was centrifuged, the precipitate was washed with double-distilled water to remove impurities, and the precipitate was calcined at 300–400 °C for 2 h to obtain CeO2 NPs. Table 3 presents several examples of using different biological products and fungi source-assisted synthesized CeO2 NPs.
Green synthesis of CeO2 nanoparticles
From biological products
Biological products used to synthesize nanoparticles can tailor the properties such as morphology, polymorphism, and size of nanoparticles (Kargar et al. 2015b). Moreover, the bio-products are easily available, economical, biodegradable, non-toxic, and simple to use. Darroudi et al. reported the starch-mediated synthesis of CeO2 NPs using cerium nitrate hexahydrate as a precursor (Darroudi et al. 2014b). How the starch molecules are responsible for synthesizing CeO2 NPs, the detailed mechanism is shown in Fig. 2. Patil et al. highlighted the use of pectin for the synthesis of CeO2 NPs, which act as a reducing agent. First, the pectin on hydrolysis yields d-galacturonic acid, the functional group present in d-galacturonic acids such as carboxyl, hydroxyl, and keto carry out the formation of CeO2 NPs (Patil et al. 2016). Similarly, the synthesis of CeO2 NPs has been reported by other researchers and was tabulated in Table 3.
From microbial biomass
The metabolites found in the extracellular membrane of fungal cells, such as enzymes, proteins, and heterocyclic derivatives, can be used to make reducing and capping agents along with decent biocatalytic performance (Gopinath et al. 2015). Riddin et al. reported that using certain fungal species secreted proteins and enzymes could efficiently synthesize metal nanoparticles extracellularly, stabilizing the particles and allowing for a higher yield than intracellular production (Riddin et al. 2006). Moreover, fungi are easily available, easy to scale up on mass level, economical, and highly tolerant of heavy metals (Gade et al. 2010). Fusarium solani mediated synthesis of CeO2 NPs was carried out at pH 6.8, and within 5 h shows spherical morphology of synthesized CeO2 NPs was described by Venkatesh et al. (Venkatesh et al. 2016). Aspergillus niger-mediated synthesis of CeO2 NPs was achieved at 80 °C in 6 h with cubic and spherical morphology (Gopinath et al. 2015). The fungi-mediated synthesis is superior in economic and environmental viewpoints; hence, more attestation is needed. Figure 3 depicts the different green approaches for synthesizing CeO2 NPs.
From plants extract
Plants are the most common, readily available, and cost-effective source for the synthesis of nanoparticles. Plant-facilitated nanoparticle synthesis has several advantages over the fungi, bacteria, and algae reported by Tran et al. (Tran et al. 2022). Abelmoschus esculentus fruits-mediated synthesis of CeO2 NPs shows spherical morphology having 35–40 nm size with antioxidant, anticancer, antibacterial, and wound-healing activities (Ahmed et al. 2021). Leaf extract of Ceratonia siliqua utilized in the bio-fabrication of CeO2 NPs displays good antioxidant and cytotoxic activity. The synthesized particles were spherical with a size of 22 nm (Javadi et al. 2019). Irshad et al. synthesized CeO2 NPs applying Orange Peel and studied cytotoxicity, antioxidant, and photocatalytic activity (Irshad et al. 2019). Flower extract of Calotropis procera used to manufacture CeO2 NPs exhibited spherical morphology with 21 nm size besides, has a band gap of 3.29 eV, and studied their photocatalytic and antibacterial activity (Muthuvel et al. 2020). Similarly, there are several reports listed in Table 2.
Characterization techniques
X-ray diffraction analysis (XRD)
In materials science, a technique for identifying a material’s crystallographic structure is XRD. Miri and Sarani explain the Prosopis farcta leaf extract abetted synthesis of CeO2 NPs (Miri and Sarani 2018). The authors studied the effect of calcination temperature on the synthesis of CeO2 NPs (Fig. 4). The XRD pattern is in good agreement with JCPDS card number 00–043-1002, and the structure is fluorite cubic. The miller indices for the pattern are (111), (200), (220), (311), (222), (400), (331), and (420). Similarly, Singh et al. reported the fluorite cubic structure of CeO2 NPs synthesized utilizing Elaeagnus angustifolia leaves. The (2θ) angles 69.02°, 56.47°, 47.21°, 33.11°, and 28.51° are observed in the XRD peaks of CeO2 NPs (Singh et al. 2019).
XRD pattern of synthesized CeO2 NPs at three different calcination temperature including 300, 400, 500 °C. License No. 5246481286341 Adopted from Miri and Sarani (2018) with permission from Elsevier
Fourier transforms infrared spectroscopy (FTIR)
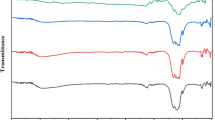
The technique used to recognize organic, polymeric, and inorganic materials is called FTIR. Dutta et al. described Aloe vera extract-facilitated synthesis of CeO2 NPs. They have explained how Aloe vera extract carries out the synthesis of CeO2 NPs (Fig. 5). The FTIR spectrum Aloe vera extract shows the band at 3410.23, 2926.03, 1610.88, 1493.83, 1244.27, 1060.69, 876.93, 772.72 and 621.90 cm−1 due to –OH gr, C–H bond, C=O stretching (symmetric and asymmetric), C–O, C–O–C, and C-H group respectively (Dutta et al. 2016). In CeO2 NPs, spectra show decreasing wavenumbers in a few peaks, which appear at 67.86, 747.98, 848.60, 1075.62, and 3384.14 cm−1, implying the participation of the C–H, C–N, and –OH group. Besides, the peaks at 1244.27, 1493.83, and 1610.88 cm−1 disappeared due to Ce ions interaction with the carbonyl group, and the new peak at 1390.94, 1560.86 cm−1 –C–H and C–C stretching, respectively (Dutta et al. 2016). The spectra of a mixture of Aloe vera extract and Ce(NO3)3 differed slightly from CeO2 NPs, implying that Aloe vera extract's distinct components were not solely involved with CeO2 NPs instead, they attached and stabilized the surface of CeO2 NPs (Dutta et al. 2016).
FTIR spectrum of CeO2 NPs, mixture of cerium nitrate and Aloe vera extract and Aloe vera extract. License No. 5246481489136 Adopted from Dutta et al. (2016) with permission from Elsevier
Transmission electron microscopy (TEM)
To evaluate the particle size, the TEM technique is used commonly. For example, Balaji et al. reported that E. globulus leaves extract assisted the synthesis of CeO2 NPs (Balaji et al. 2020). HRTEM micrograph showing particle size is in the range of 15–20 nm (Fig. 6).
TEM images of CeO2 NPs at different magnifications: a 50 nm, b 20 nm, c 10 nm, d 5 nm; e Particle size histogram, f SAED pattern with hkl values. Open access; Reproduced from Balaji et al. (2020) with permission from MDPI
Aseyd Nezhad et al. synthesized spherical CeO2 NPs using O. majorana leaf extract the size of the synthesized CeO2 NPs 20 nm (Aseyd Nezhad et al. 2020b). The Ceratonia siliqua leaf extract-mediated Javadi and co-worker reported CeO2 NPs synthesis NPs to possess spherical morphology with a size of 22 nm (Javadi et al. 2019). Similarly, more reports were summarized in Tables 2 and 3.
Thermogravimetric analysis and differential thermal analysis (TGA–DTA)
Darroudi et al. (Darroudi et al. 2014a) reported the honey-mediated synthesis of CeO2 NPs described in the TGA analysis. An initial temperature of 20 °C was used for the heating procedure, with a 10 °C/min rise to 800 °C. Adsorbed water and crystal water are the primary causes of the 37.4% mass loss between 20 °C and 160 °C (see Fig. 7. Ed1 and Ed2) because of the slow dehydration of cerium hydroxide occurs in this temperature range. According to Ed3 and Ed4 (see Fig. 7), the breakdown of chemically bonded groups is responsible for the second stage, which occurs around 160–355 1C (48.5%). As cerium components continue to oxidize, a further 8.2% (see Fig. 7 Ed5) of mass is lost between 355 and 400 °C. The sample has lost 94.1% of its original bulk. The TGA curve shows no weight loss between 400 and 800 °C, confirming the synthesis of CeO2 NPs as a stable product (Darroudi et al. 2014a).
TGA-DTA spectrum of honey-mediated synthesis of CeO2 NPs. License No. 5320870350678 Adopted from Darroudi et al. (2014a) with permission from Elsevier
UV–Vis spectrum of CeO2 NPs
Darroudi et al. (Darroudi et al. 2014a) presented the typical UV–vis spectrum of honey-mediated synthesized CeO2 NPs. The spectrum shows CeO2 NPs have an inherent bandgap absorption peak at 314 nm, which may be ascribed to electron transitions from the valence band to the conduction band. The band at 300 nm is produced by the absorption of the charge transfer transition from O2p to Ce4f in CeO2 NPs (Orel and Orel 1994). CeO2 NPs have a bandgap of 3.5 eV, as seen in the inset of Fig. 8 (Darroudi et al. 2014a).
UV–vis spectrum and bandgap estimation(inset) spectrum of honey-mediated synthesis of CeO2 NPs. License No. 532087035 0678 Adopted from Darroudi et al. (2014a) with permission from Elsevier
X-ray photoelectron spectroscopy (XPS)
Iqbal et al. (Iqbal et al. 2021) Reported the XPS spectra of plant extract-mediated synthesis of CeO2 NPs (See Fig. 9 (a–d)). Fig (a) shows the survey scan that demonstrates the absence of any pollutants other than carbon species produced from a plant extract or organic contaminants absorbed by the sample during handling. Figure 9(b) Ce (3d) spectra contain eight peaks with binding energy ranges between 880 and 925 eV, which matches the literature data well. The XPS peaks between 891.12 and 900.09 and 900.09 to 925.47 are linked to Ce (3d5/2) and Ce (3d3/2), respectively, according to previous reports (Zhang et al. 2012; Krawczyk et al. 2015; Dutta et al. 2016). There are two distinct peaks in the binding energy of CeO2 NPs at 527.5 and 529.5 energy levels, shown in Fig. 9(c) and (d) are attributed to O2− ion and O–H or COO− from plant extract used for the synthesis of CeO2 NPs, respectively (Iqbal et al. 2021).
XPS spectrum a Survey spectrum b Fitted XPS spectrum of Ce(3d) c XPS spectra of O (1 s) and d Fitted XPS spectrum of O (1 s) for CeO2 NPs. License No. 5322460343240 Adopted from Iqbal et al. (2021) with permission from Elsevier
Factors considered for the synthesis of CeO2 NPs
Various factors are responsible for altering the properties of synthesized nanoparticles, such as size, shape, surface area, etc. (Khan et al. 2019). Altering the synthesis condition, specifically metal ion concentration, pH, reaction temperature, reaction time, calcination temperature, etc. As a result, obtaining magnificent CeO2 NPs within a short reaction time, at moderate reaction conditions, which possesses manifold applications, is possible. Table 4 offers several examples of various factors used in synthesizing CeO2 NPs.
A proposed mechanism for plant extract-mediated synthesis of CeO2 NPs
Plant material contains phenolics, flavonoids, carotenoids, anthocyanins, hydroxytyrosol, tocopherols, etc. (Altemimi et al. 2017). These compounds were utilized to convert/reduce cerium salt into CeO2 NPs. Qaisar Maqbool et al. elaborated that these compounds reduced cerium salt into CeO2 NPs (Maqbool et al. 2016).
Darroudi et al. reported that in the starch-mediated synthesis of CeO2 NPs, the starch was hydrolyzed to α-glucose, and the hydroxyl group of α-glucose attacks the metal cation. Then, the nitrate associated with the metal precursor gets decomposed on heating to nitrogen dioxide and oxygen. Besides this form Ce(OH)3, which oxidized to Ce(OH)4 by adjusting the pH, the formation of Ce(OH)4 was identified by a change in solution from colorless to light yellow. The sol–gel process and calcination converts Ce(OH)4 to CeO2 (Darroudi et al. 2014b). The detailed mechanism is shown in Scheme 1.
Plausible mechanism for the synthesis of CeO2 NPs. License No. 5245400066113: Reproduce from Darroudi et al. (2014b) with permission from Elsevier
Similarly, Patil et al. highlighted the use of pectin for the synthesis of CeO2 NPs. Oxidizing destruction, depolymerization, and alkaline hydrolysis occur in an alkaline medium and convert pectin into galacturonic acid. The oxygen in the acid attacks the Cerium metal, and the nitrate associated with the metal gets decomposed into nitrogen dioxide and oxygen. The addition of ammonia converted Ce3+ into Ce(OH)3 and further oxidized to Ce(OH)4. Then, the calcination converted Ce(OH)4 into CeO2 NPs (Patil et al. 2016).
E. globulus leaves extract-facilitated synthesis of CeO2 NPs was reported by Balaji et al. The leaves extract encompasses 9, 12 octadeca trienoic acid chains the hydroxyl group present in the acid react with the cerium metal to form a complex structure. In a later step, CeO2 NPs were obtained on calcination (Balaji et al. 2020). Scheme 2 depicts the mechanism in further depth.
Proposed mechanism of green synthesis of CeO2 NPs using E. globulus leaf aqueous extract. Open access; Reproduced from Balaji et al. (2020) with permission from MDPI
Multifunctional applications of the CeO2 NPs
This paper focuses on recent advances in the biosynthesis of CeO2 NPs as building blocks for applications in various disciplines. These fields include environmental sensing, environmental remediation (adsorption, catalysis, and photocatalysis), antibacterial, antioxidant, and anticancer activities, organic synthesis, and emerging applications (nanofluid and energy storage) addressed as below. In addition, it is anticipated that CeO2 NPs possess potential applications in diverse areas, especially medicinal applications such as cosmetics, sun-screen protectors, and as antiseptics in ointments (Darroudi et al. 2014a).
Photocatalytical applications
Photocatalysis is considered an environmentally accepted method for strengthening the green economy. This technique is one of the most useful, cost-efficient, and non-toxic strategies for successfully degrading a wide spectrum of environmental pollutants at ambient temperature and pressure. On scrutiny, the literature uncovers that in the photocatalytic degradation of organic dyes, hydroxyl radicals (•OH), superoxide radicals (O2•−), and holes (h+) are the main active species (Elahi et al. 2019). Recently, some researchers concentrating on the green synthesis of CeO2 NPs for photocatalytic environmental remediation have received substantial consideration in this perspective. Different types of toxic chemical and environmental pollutant, such as Rhodamine B (Sharma et al. 2017) (Malleshappa et al. 2015), Methylene blue (Reddy Yadav et al. 2016), Methyl orange (Muthuvel et al. 2020), 4-nitrophenol, Acetaldehyde (Magudieshwaran et al. 2019), Crystal Violet (Surendra and Roopan 2016) has been degraded using biologically synthesized CeO2 NPs.
Surendra and Roopan (Surendra and Roopan 2016) reported the photocatalytic ability of CeO2 NPs toward Crystal Violet dye degradation. The degradation was done using Heber multi-lamp photoreactor with a UV lamp at 365 nm. It is observed that the 97.5% dye was degraded within 1 h following first-order kinetics, indicating the rate of degradation of crystal violet dye at 0.88 per min (Surendra and Roopan 2016). Elahi et al. (Elahi et al. 2019) illustrated the degradation of Rhodamine B dye by applying CeO2 NPs synthesized using different amounts of Salvia Macrosiphon Boiss seeds extract, suggesting that the degradation follows pseudo-first-order kinetics. The results revealed that the CNPs-30 shows superior degradation ability than the CNPs-10 and CNPs-20, i.e., 100% degradation in 12 h. Moreover, the enhanced degradation ability of CNPs-30 over the others is the smaller particle size that provides a large surface area for the RhB dye for faster degradation (Elahi et al. 2019).
Azadirachta indica leaf extract-mediated synthesis of CeO2 NPs was reported by Sharma and co-workers (Sharma et al. 2017), in which they described the efficiency of CeO2 NPs towards RhB dye degradation. The results suggest that 96% of the RhB dye was degraded within 2-h duration using a Xenon arc lamp of 300 W (Sharma et al. 2017). Malleshappa et al. (Malleshappa et al. 2015) stated the synthesis of CeO2 NPs utilizing leaves of Leucas aspera. They studied their photocatalytic ability for the RhB dye degradation using UV light and sunlight. The CeO2 NPs with varying amounts (i.e., 5, 10, 15, and 20 mL) of Leucas aspera leaf extract were designated as S1, S2, S3, and S4. The XRD results show that as the amount of leaf extract increases from 5, 10, 15, to 20 mL, the CeO2 NPs decrease and were found to be 11, 8, 5 to 5 nm for S1, S2, S3, and S4, respectively. However, the photocatalytic activity shows 73% efficiency (90 min) in UV light; whereas, in sunlight, the efficiency was further increased as was reached up to 80% (90 mint.) for the S4 sample. The highest efficiency of the S4 was attributed to the small crystalline size, which has a high surface defect and increases the concentration of both electron and hole traps. The catalyst also shows recyclability up to the 4th cycle without significant decrees in the catalytic efficiency (Malleshappa et al. 2015).
Reddy Yadav et al. (Reddy Yadav et al. 2016) studied the photocatalytic ability of CeO2 NPs synthesized from watermelon juice toward methylene blue (MB) dye degradation in UV and sunlight. The findings show that 98% of the MB dye was degraded in 3 h in UV light, whereas 93% in sunlight under the same conditions. The higher degradation rate in the UV light was observed because of the high intensity of light, which facilitates the easy penetration of light that results in a larger number of radicals and hence increases the degradation rate. Reddy Yadav et al. (Reddy Yadav et al. 2016) described the mechanism of dye degradation using CeO2 NPs synthesized from watermelon juice, as shown in Fig. 10. Green synthesis of CeO2 NPs was achieved using Calotropis procera flower extract by Muthuvel et al. and highlighted the photocatalytic degradation for methyl orange (MO) dye under sunlight irradiation (Muthuvel et al. 2020). The results show that 98% of the MO dye was degraded within 50 min of time interval under sunlight irradiation. Muthuvel et al. described the detailed mechanism (Fig. 11) of MO dye degradation was reproduced with License No. 5282230449234 Adopted from (Muthuvel et al. 2020) with permission from Elsevier.
Balaji et al. (Balaji et al. 2020) prepared CeO2 NPs using E. globulus leaf extract. They studied their photocatalytic activity for the sunset yellow (SY) dye degradation using Heber multi-lamp photo reactor HML MP 88 with an 8 W mercury lamp as a UV source. The results discovered that 97.3% of dye was degraded in 90 min under UV irradiation. In addition, the CeO2 NPs show threefold recyclability without significantly decreasing catalytic efficiency (Balaji et al. 2020).
We have summarized the photocatalytic application of green-synthesized CeO2 NPs by scrutiny the literature (Table 5).
Biological applications
Studies on the biomedical properties of green-synthesized CeO2 NPs have opened a new medical biology and pharmacology era. Recent reports have highlighted the negative effects of CeO2 on various microbes, larvae, and oxidative stress with potential photocatalytic activity. Some of the crucial biological activities of CeO2 NPs are summarized below.
Antibacterial agent
Bacteria are one of the microorganisms present everywhere, and they can have positive and negative effects on the surrounding life. The bacterial diseases encompass an array ranging from mild illnesses like the common cold to deadly pneumonia and tuberculosis. In such cases, antibacterial therapy comes handy besides giving symptomatic relief from the disease. The process of controlling and reducing bacterial counts in the body by tapping every step of life like sustenance, metabolism, respiration, and reproduction of bacteria is known as antibacterial activity. However, conventional antibacterial drugs usually possess short-term activity with potential environmentally toxic effects. On the other hand, antibacterial nanoparticles are thought to be minimally toxic with prolonged effects on drug-resistant bacterial strains.
Cerium dioxide, a rare earth metal oxide, is of biological interest due to its electronic, optical properties, unique surface chemistry, and biocompatible nature with high stability. These properties are due to the stable tetravalent valence state of the metal. It is a cubic fluorite-type metal oxide (Farias et al. 2018). In the quest for the green synthesis of CeO2 NPs, researchers have used various biological materials like plants (whole or parts of it), microbes, bio-products, etc. Several plants have been used for the biogenesis of CeO2 NPs. The leaf extracts of Acalypha indica (Kannan and Sundrarajan 2014b), Olei europaea (Maqbool et al. 2016), Gloriosa superba (Gopinath et al. 2015), Sida acuta (Senthilkumar et al. 2017), Prosofis julifora (Arunachalam et al. 2017), Moringa oleifera L (Eka Putri et al. 2021), flower extracts of Hibiscus sabdarifa (Thovhogi et al. 2015) and Calotropis procera (Muthuvel et al. 2020), Aloe vera (Sai Priya et al. 2014), etc. Further investigations on the biological activities of these bio-generated CeO2 NPs of varied sizes and shapes revealed their prominent antibacterial activities. The CeO2 NPs were found to possess anti-activities against many Gram-positive and Gram-negative bacteria, including a few multidrug-resistant pathogenic species. Gram-positive bacteria were detected to be more susceptible to the adverse effects of CeO2 NPs (Fig. 12) (Kannan and Sundrarajan 2014c; Maqbool et al. 2016; Arunachalam et al. 2017; Kumar et al. 2018).
Size of the zone of inhibition formed around each disc, loaded with test samples, indicating the antibacterial activity CeO2 NPs using G. superba leaf extract License No. 5254790351005 Adopted from Arumugam et al. (2015) with permission from Elsevier
A study on antibacterial activity of CeO2 NPs, synthesized by the green approach, on E. coli (Gram negative) and Staphylococcus aureus (Gram positive) demonstrated Staphylococcus aureus to be more sensitive to CeO2 NPs treatment. The antibacterial activity of these NPs was observed to be dose dependent, i.e., higher antibacterial activity at higher concentrations of the nanoparticles was reported (Surendra and Roopan 2016). Another group of researchers synthesized CeO2 NPs using Calotropis procera flower extract that showed significant antibacterial activity against Gram-negative bacteria—Escherichia coli and Pseudomonas aeruginosa (Muthuvel et al. 2020). CeO2 NPs synthesized using Pedalium murex L extract (Pandiyan et al. 2019), Moringa oleifera L (Surendra and Roopan 2016), Gloriosa Superba (Arumugam et al. 2015), and Oleo europaea (Maqbool et al. 2016) demonstrated potential antimicrobial activities (Fig. 13). The biogenic synthesis of CeO2 NPs using Moringa oleifera L exhibited good antibacterial activity against Staphylococcus aureus (Gram-positive), Pseudomonas aeruginosa (Gram-negative), and E. coli (Gram-negative) bacteria. Again, Gram-positive bacteria are more susceptible to CeO2 NPs than their Gram-negative counterparts (Eka Putri et al. 2021). Green-synthesized CeO2 NPs using Oleo europaea reported antibacterial activity against Gram-positive and Gram-negative bacterial strains (Maqbool et al. 2016). In a recent study by Altaf and his group (2021), the CeO2 NPs (22.03 nm) synthesized using an aqueous extract of Acorus calamus showed more than 75% antibiofilm activity against Gram-positive and Gram-negative bacteria, both in a dose-dependent manner. This study again highlighted the higher susceptibility of Gram-positive bacteria to the CeO2 NPs. (Altaf et al. 2021). The antibacterial effects of the CeO2 NPs generated using Abelmoschus esculentus extracts were tested using standard agar diffusion experiments. The results obtained in the study were following earlier studies exhibiting a more profound antibacterial effect on S. aureus (Gram-positive) bacteria as compared to K. pneumonia (Gram-negative) bacteria, with a higher zone of inhibition at increasing concentrations of NPs in both cases (Ahmed et al. 2021).
Schematic illustration of CeO2 NPs antibacterial activity. Open access Reproduced from Maqbool et al. (2016) with permission from Dove Press
In this way, different studies carried out in various laboratories unanimously exhibited significant antibacterial activity of green-synthesized CeO2 NPs against Gram-positive and Gram-negative bacteria, with the greatest susceptibility of Gram-positive bacteria to these NPs. Till now, the antibacterial activity of CeO2 NPs has been explained through many mechanisms. One of the proposed mechanisms is via Peptidoglycans, a linear polysaccharide chain with short peptides, forming a thick layer of Gram-positive bacteria. On the other hand, Gram-negative bacteria have a thin layer of peptidoglycans and lipopolysaccharides. Moreover, the peptidoglycan layer has teichoic acid, which interacts well with the CeO2 NPs, thus inducing more toxicity of the NPs in the Gram-positive bacteria (Farias et al. 2018).
The other proposed mechanism for the antibacterial activity of CeO2 NPs is attributed to the strong electrostatic properties and unique morphologies of the CeO2 NPs. Due to these, the CeO2 NPs interact with the thiol groups of the bacterial cell membrane proteins. The interaction with the thiol groups denatures the cell membrane proteins and turns them impermeable to outside material resulting in the death of the bacteria (Nadeem et al. 2020a).
Another, widely accepted mechanism suggests that the reactive oxygen species (ROS) in the bacterial cells are elevated due to the electrostatic attractions of the CeO2 NPs. As a result, the bacterial cells cannot cope with the high levels of ROS and oxidative stress, thus showing a decline in growth (Nadeem et al. 2020a). In this way, the biogenic CeO2 NPs have been proved to be one of the best baits for antibacterial therapy in various studies and with medical implications in bacterial diseases.
Nadeem et al. reported the antibacterial activity of CeO2 NPs. The precise process by which bacteria are killed is still a mystery. Despite this, it is believed that CeO2 NPs destroy microorganisms by causing cells to produce a large amount of reactive oxygen species (ROS) (Nadeem et al. 2020b). Fig. 14 represents the antibacterial activity of CeO2 nanoparticles.
a MTT cell viability assay of synthesized CeO2 NPs on HT-29 cancer cell line were measured at 24 h. Morphology of cells b Before treatment and c After treatment with 800 μg/mL of synthesized CeO2 NPs. License number 5246190583995; Reproduced from Miri et al. (2020) with permission from John Wiley and Sons
Antifungal activity
Fungal infection has become a public health issue. Amongst many infectious fungal species, Candida albicans is the most prevalent or dominant species worldwide, mainly in hospitalized and immune-compromised patients (Bassetti and Righi 2015). The most widely used antifungal agents come under flucytosine, polyenes, echinocandins, or azoles class of drugs (Mukherjee et al. 2005). Similar to many pathogenic bacteria, many infectious fungal species have developed multidrug resistance. Therefore, besides the antibacterial potential, researchers have also investigated the antifungal activities of the green-synthesized CeO2 NPs as one of the potential antifungal medications in the future.
Recent studies have put forth CeO2 NPs to be a better antimicrobial agent in the case of multidrug-resistant microbes as it is heat resistant with very low cytotoxic effects, as proposed by Puri and his group (Putri et al. 2019). A study on investigations of antifungal potential of biogenic CeO2 NPs synthesized using Moringa oleifera leaf extract was tested on Candida albicans and Aspergillus fumigatus. These fungi were cultured for 24 h at 37 °C on Sabouraud Dextrose Agar (SDA) plates, and the CeO2 NP solution was applied at certain sites to observe the zone of clearance, if any. Surprisingly, the antifungal potential of these CeO2 NPs was found to be very strong as compared to its bactericidal activity. The NPs could effectively inhibit the growth of both the fungi, and the zone of clearance was calculated to be 20 mm for Candida albicans and 26 mm for Aspergillus fumigatus. A drug/test substance inducing a zone of inhibition of more than 20 mm is classified as a very strong antimicrobial agent (Sebastiammal et al. 2019). In another study, Maqbool and his group (2016) tested the antifungal activity of biogenic CeO2 NPs on Mucor species, Aspergillus flavus, Aspergillus niger and Fusarium solani by disk diffusion method. This study reported the highest zone of clearance for Mucor species (22 mm), followed by Aspergillus flavus, and Aspergillus niger (19 mm for both), and the least zone of clearance for Fusarium solani (10 mm). In this way, CeO2 NPs exhibited a great ability to act as antifungal agents (Maqbool et al. 2016).
The antifungal potential of green-synthesized CeO2 NPs needs to be further addressed against various pathogenic fungi to analyze its potential in the biomedical field, especially against the multidrug-resistant fungal species. Currently, the mechanism by which CeO2 NPs elicit antifungal activity is not well understood. However, some of the reports point to the formation of excess ROS, electromagnetic interactions (Xia et al. 2008), and cell membrane protein denaturation (Howlett and Avery 1997) as the route cause of the antimicrobial activity of CeO2 NPs (Estes et al. 2021). Therefore, more rigorous studies on antifungal activities of bio-mediated CeO2 NPs are essential to explore the canopy of fungal species sensitive to the CeO2 NPs.
As mentioned earlier, bacteria and fungi are the most diverse organisms on this earth. Some of the species of these groups can have beneficial and adverse impacts on life. However, these organisms adapt quickly to the changing environment and, thus, develop resistance to antibiotics/antifungal medicines in a short time. Thus, developing novel efficient therapies to treat these pathogenic organisms is essential. Green-synthesized nanoparticles of many metals have been shown to possess antibacterial and antifungal potential against a wide variety of species. Compared to these metals, cerium has a unique advantage of biocompatible nature with small size and specific morphological features, which imparts it more efficient in killing a vast range of pathogenic microorganisms (Nadeem et al. 2020a).
Antioxidant activity
The process of limiting or inhibiting the oxidation of biomolecules, especially lipids and proteins, and restraining oxidative chain reactions is known as antioxidant activity. Endogenous antioxidant activity checks the production of reactive oxygen and/or nitrogen species (ROS/RNS), which are highly unstable and responsible for the oxidation of lipids, proteins, DNA, etc. Thus, to avoid physiological problems, the body must tackle and maintain the balance between producing and utilizating ROS/RNS through endogenous antioxidant enzymes. Nevertheless, on the other hand, higher production of reactive oxygen species results in oxidative stress in an organism that forms the base for several pathological conditions like cellular death, cancer, diabetes, etc. (Perona et al. 2021).
Antioxidants minimize oxidative stress by reacting with free radicals and inhibiting the downstream chain of reactions and producing harmful byproducts. However, natural and synthetic antioxidants exhibit poor absorption with limited cell permeability and biodegradation till it reaches the activity site. In this scenario, the antioxidants coupled with metals in metal oxide nanoparticles serve as an alternative to natural and synthetic antioxidant supplements (Khalil et al. 2019). To date, nanoparticles of various metals like silver (Ansar et al. 2020), zinc (Daoud et al. 2021 123AD), etc., are known to possess antioxidant potential. In the crystalline structure, cerium oxide exhibits a deficiency of oxygen atoms and is available in Ce3+ and Ce4+ oxidation states. Due to its unique structural property, it mimics endogenous antioxidant enzymes like phosphatase, oxidase, catalase, superoxide dismutase, and peroxidase in their activity and, thus, shows high antioxidant potential (Turin-Moleavin et al.).
Custom-synthesized CeO2 NPs by the wet synthetic process have been shown to possess antioxidant and neuroprotective activities. However, the chemically synthesized NPs may have toxic effects at higher concentrations. CeO2 NPs induced antioxidant activity and declined the damage caused due to oxidation and lipid peroxidation in the rat brain (Najafi et al. 2017; Estevez et al. 2019) CeO2 NPs have also exhibited radioprotective capability due to there free radical scavenging activity (Popov et al. 2016). In this way, the neuroprotective and radioprotective activities of CeO2 NPs are attributed to their antioxidant potential. A fructose polysaccharide-Levan is a bio-product produced by many plants, bacteria, and other animals. Levan has been extensively explored for its antioxidant and antibacterial potential (Hertadi et al. 2021). The CeO2 NPs, coated with Levan polysaccharide, significantly inhibited the oxidation of biomolecules in NIH 3T3 cells treated with hydrogen peroxide. The study also reported enhanced water solubility and stability of Levan coated CeO2 NPs. Further, CeO2 NPs were shown to interfere with hydrogen peroxide activity, reduce the production of ROS/RNS and increase the life expectancy of human epithelial cells (Rubio et al. 2015).
In another study, CeO2 NPs stabilized with Aloe vera leaf extracts were shown to neutralize the adverse effects of hydrogen peroxide and protect mouse brain cells from an overproduction of ROS, cell death, and loss of connectivity between neural cells (Dutta et al. 2016). Biogenically, CeO2 NPs synthesized using Origanum majorana L. leaf extract. Synthesized CeO2 NPs increased catalase and superoxide dismutase expression levels and exhibited higher antioxidant activity in human umbilical vein endothelial cells (Aseyd Nezhad et al. 2020b). CeO2 NPs generated using Ceratonia siliqua extracts exhibited antioxidant activities in a dose-dependent manner with high antioxidant potential at high concentrations. Surprisingly, these CeO2 NPs demonstrated better antioxidant potential than the industrial antioxidant—BHA (Javadi et al. 2019). Cerium oxide NPs generated with Aloe barbadensis, a medicinally important plant, resulted in the generation of spherical nanoparticles of approximately 63.6 nm with high antioxidant potential (Sai Priya et al. 2014). Earlier studies suggest that biosynthesized CeO2 NPs could eradicate free radicals more efficiently, resulting in better cellular functions.
Vitamin C (ascorbic acid) is a conventional natural antioxidant used by humankind for years for its highest antioxidant potential. However, a recent study wherein the antioxidant potential of ascorbic acid was compared with CeO2 NPs, generated using Abelmoschus esculentus extract, exhibited higher antioxidant activity in CeO2 NPs compared to ascorbic acid in a standard DPPH assay. In this way, green-synthesized CeO2 NPs are proposed to be a better candidate for antioxidant therapy in contrast to conventional natural antioxidants (Ahmed et al. 2021) and commercial synthetic antioxidants (Aseyd Nezhad et al. 2020b). Hence, the green-synthesized CeO2 NPs exhibit effective scavenging the free radicals and possessed remarkable antioxidant potential with significant biomedical applications.
Antidiabetic activity
Diabetes is a chronic health condition where the pancreas cannot produce sufficient amounts of insulin, or the body cannot use the insulin produced by the pancreas effectively. As a result, patients with unattended diabetes usually experience hyperglycemia and oxidative stress induced by high sugar levels. Oxidative stress is the cause of pathogenesis and complications of diabetes. The free radicals formed under hyperglycemic conditions lead to glucose oxidation, increased lipid peroxidation and oxidation of biomolecules resulting in their degradation (Maritim et al. 2003).
Since being green-synthesized, CeO2 NPs have displayed a significant antioxidant potential, even better than the conventional natural and synthetic antioxidants. The studies on the probable use of these NPs for combating the complications of diabetes are in progress. A group of researchers looked at the effect of biogenic CeO2 NPs on the L6 (rat myoblast) cells which exhibit insulin-driven movement of glucose across the cell membrane, i.e., show glucose uptake by cells in response to insulin (Shamprasad et al. 2019). In diabetic conditions, cells usually fail or show a very low capacity to respond to insulin signals and uptake glucose from circulating blood (Cerf 2013). Thus, the L6 cell line is thought to be one of the best models to investigate the antidiabetic activity of various agents, as detected by glucose uptake by the cells in response to these agents. The CeO2 NPs synthesized using the fruit extract of Morus nigra, when used to treat the L6 cell line, showed significantly higher uptake of glucose (more than 65% at 100 ug/mL concentration of NPs), suggesting the excellent antidiabetic potential of the NPs. The L6 cells could show higher glucose uptake in response to higher doses of CeO2 NPs. The efficient antidiabetic activity of the CeO2 NPs may be because of the very small dimensions of the NPs facilitating their easy penetration into the cells (Rajan et al. 2019).
In an experiment, the diabetic rats (streptozotocin induced) were treated with either CeO2 NPs and sodium selenite, alone or in combination, which resulted in higher expression levels of antioxidant enzymes and reduced cholesterol levels triglycerides oxidative stress that is elevated under diabetic conditions in treated rats. Again, this study again clearly hints at the probable antidiabetic activity of the CeO2 NPs (Pourkhalili et al. 2011). In another set of experiments, a combination of CeO2 NPs and sodium selenium led to lower levels of ROS and higher levels of secreted insulin. These studies hint at the potential use of CeO2 NPs to treat physiological disorders like diabetes (Singh et al. 2020). In a recent study, CeO2 NPs synthesized using the Stachys japonica Miq plant extract and posed no toxicity to NIH3T3 (immortalized mouse embryonic fibroblast cell line) and HepG2 (human hepatoma) cells. Simultaneously these biogenic CeO2 NPs reverted the insulin resistance of HepG2 cells and, thus, elevated the glucose uptake by these cells hinting at the antidiabetic activity of the NPs (Saravanakumar et al. 2021).
Because of these studies, it would be interesting to prepare and explore the antidiabetic potential of CeO2 NPs, synthesized using either Rhus hirta, Picea glauca, Juniperus communis, or Solidago Canadensis, plants with high antioxidant capacity (LM McCune 2013).
Anticancer activity
ROS has been implicated in normal cell–cell signaling and related cellular processes. Organisms have an antioxidant defense mechanism to check the ROS levels. Either way, higher or lower, uncontrolled modulation in the ROS amounts can impair cellular functioning and cause diseased conditions. Cancer is one such critical illness that is related to cellular ROS levels. Cancer is characterized as the uncontrolled growth of abnormal cells that can infiltrate, spread to other parts of the body and destroy surrounding normal tissues. It is one of the leading clinical causes of death worldwide. ROS is thought to possess pro-tumorigenic activity that promotes cell survival and proliferation. The metabolic rate is elevated in tumor cells, producing higher ROS amounts. Moderately elevated levels of ROS cause oxidative stress, gene mutations, and DNA instability in cells leading to cancer progression. On the other hand, very high levels of ROS can be toxic to the cancer cells as they trigger programmed cell death (apoptosis). Cancer cells usually have their mechanism to maintain ROS levels to avoid apoptotic death. Because of these conditions, researchers find ROS a potential and crucial target for cancer therapies as it can be used as both pro- and anti-tumorigenic agents (Perillo et al. 2020; Arfin et al. 2021).
Conventional cancer treatments include the use of alkylating agents, biological agents, and antimetabolites, which have serious side effects like drug resistance, loss of hair, and obliteration of normal cells, as these drugs cannot differentiate between cancerous and normal cells. Nanoparticles of various metal oxides like zinc, copper, iron, cobalt, etc., drew scientists’ attention as the NPs were found to differentiate between cancer cells and normal cells and induce cytotoxic effects selectively in cancer cells (Anjum et al. 2021). Metal oxide NPs (zinc oxide) have been shown to induce ROS formation, destroy mitochondrial membrane potential and activate apoptotic pathways via caspase cascade in cancerous cells (Anjum et al. 2021). Thus, the antitumor mechanism of metal oxide NPs is thought to be mainly through the generation of excess ROS and apoptosis, among other possibilities. Compared to synthesizing metal oxide NPs chemically, green-synthesized NPs have proved to be better in medical therapies, mainly due to their non-toxic nature (Marouzi et al. 2021).
The CeO2 NPs, synthesized with leaf extract of E. globulus, resulted in the formation of NPs of about 13.7 nm. Human adenocarcinomic alveolar epithelial cells (A549) were more susceptible than another cancer cell line (HCT 116) developed from human colorectal carcinoma. The study further revealed that the toxic effects of these NPs were mediated through ROS generation (Balaji et al. 2020). In another study, CeO2 NPs (26 nm), synthesized using the Rubia cordifolia leaf fusions, exhibited remarkable anticancer activity against the MG-63 cell line (human osteosarcoma cells). However, the treated cells lost the membrane integrity and suffered oxidative stress-mediated apoptosis (Sisubalan et al. 2017).
Orange peel extract was used to synthesize CeO2 NPs of about 23 nm by Irshad and group (2019). These biogenic CeO2 NPs exhibited significant ROS and cytotoxic activities in human cervical cancer cells (HeLa) (Irshad et al. 2019). Ahmed and his group prepared CeO2 NPs using Abelmoschus esculentus extract and tested its cytotoxicity on HeLa cells. The results obtained in this study followed the earlier studies on CeO2 NPs generated with orange peel by Irshad et al. (2019). HeLa cells exhibited mortality in a dose-dependent manner in response to the CeO2 NPs generated with Abelmoschus esculentus extract (Ahmed et al. 2021). Remarkable growth inhibition of breast cancer cells is reported in CeO2 NPs prepared with Ceratonia siliqua extracts (Javadi et al. 2019). In an another study, the cytotoxic effects of green-synthesized CeO2 NPs (10–70 nm) using Origanum majorana L. leaf extract on MDA-MB-231 (human breast cancer cells) showed higher adverse effects as compared to the HUVEC cells (human vein endothelial); normal cell line that was used as the control (Aseyd Nezhad et al. 2020b). CeO2 NPs, synthesized using the Prosopis farcta extract, exhibited cytotoxic effects on HT-29 (human colorectal adenocarcinoma) cells (Miri and Sarani 2018). Miri et al. reported that the cytotoxic activity of Salvadora persica extract-mediated synthesis of CeO2 NPs was determined through 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay against a colon (HT-29) cancer cell line (Fig. 15) (Miri et al. 2020). The number of WEHI 164 (mouse fibrosarcoma) cells was significantly lowered, in a dose-dependent fashion, when treated with CeO2 NPs generated using carrageenan (Nourmohammadi et al. 2018b).
Schematic representation of antibacterial activity of CeO2 nanoparticles. Open access Reproduced from Nadeem et al. (2020b) with permission from Dove Press
In this way, research on the anticancer activity of green-synthesized CeO2 NPs, carried out in various laboratories across the world, hints at the cytotoxic activity of these NPs specifically against cancerous cells and does not affect normal cells. Therefore, as indicated in earlier studies, the strong anticancer potential of these biogenic NPs should be explored further in detail to approve the NPs as a safer or better chemotherapeutic agent with minimum toxicity on normal cells.
Commercialization of CeO2 nanoparticles with polymers
To improve the properties of both ceria and polymers, the fabrication of CeO2-based polymer composites appears to be a beneficial tool for Biomedical Applications (Shcherbakov et al. 2021). CeO2 NPs therapeutic antioxidant potential has been recently verified by advances in nanomedicine(Davoodbasha et al. 2019). Davoodbasha et al. synthesized the cellulose-coated CeO2 NPs and studied DPPH, superior hydroxyl, superoxide, and hydrogen peroxide radicals scavenging activity in a pH-depended manner (Davoodbasha et al. 2019). Besides, Davoodbasha et al. also stated that cellulose-coated CeO2 NPs, as a possible antioxidant material, might be used in targeted treatment to reduce ROS and oxidative stress in the cells. Furthermore, according to Cheng et al. impressively high electrical characteristics, the CeO2@G modified separator has the potential to be used in the development of high-performance Li–S batteries (Cheng et al. 2021).
The wound-healing activity of biocompatible poly (acrylamide) hydrogel (PAGE)-based dressing material encompassing CeO2 NPs and curcumin was tested by Bhattacharya et al. (Bhattacharya et al. 2019). Moreover, the combination of curcumin and CeO2 NPs improved cell proliferation, higher collagen content, accelerated wound maturity, re-epithelialization, and granulation tissue formation (Bhattacharya et al. 2019). According to Huang et al., adding two weight percent CeO2 NPs to poly(ether–ester) gave it the best mechanical and low-temperature elastic recovery properties (Huang et al. 2014). Electrospun polycaprolactone-based tissue engineering scaffolds loaded with CeO2 NPs have been developed and tested as an innovative approach for tissue engineering(Augustine et al. 2018). CeO2 NPs are an essential component of scaffolds used in situ tissue engineering for enhancing angiogenesis, cell adhesion, and cell proliferation(Augustine et al. 2018).
CeO2 NPs can be delivered via biodegradable PLGA (lactide: glycolide), which provides a long-term release of the CeO2 NPs, as reported by Singh et al. (Singh et al. 2012). Furthermore, the synthesized PLGA (lactide: glycolide) encapsulated CeO2 NPs were studied for tissue engineering, such as bone remodeling, regeneration, and protection from neurological illnesses (Singh et al. 2012).
Challenges and opportunities
The green chemistry of CeO2 NPs synthesis has been studied, and some important difficulties and opportunities have been found. Almost 90% of the waste from nanoparticle syntheses is generated by precipitation, and separations raise solvent waste even more. The elimination of size sorting and other post-processing of the product, as well as developing syntheses that reduce solvent consumption, maybe through wholly new methodologies, is crucial to decreasing waste.
Because green and non-toxic reducing agents are too weak to create high-quality CeO2 NPs, which require faster kinetics, the toxicity of reducing agents was highlighted as a critical concern. To manufacture high-quality CeO2 NPs without harmful ingredients, either stronger green reducing agents must be developed, or more effective reaction conditions for weaker reducing agents must be discovered; this remains an open and critical challenge.
Furthermore, achieving high-yield synthesis of CeO2 NPs with little or no solvent employing benign reducing and capping agents is also a challenging but worthwhile goal. To work toward this aim and secure a greener future for CeO2 NPs in research, industry, and the marketplace, it is evident that larger debates on sustainability lead to a greater appreciation among nanoparticle chemists of the benefits of green synthetic techniques are required.
Conclusion and future prospects
In conclusion, we lucidly emphasized and considered the current advancement in the fabrication and application of biosynthesized CeO2 NPs as an effective nanomaterial for numerous applications. In recent years green or biosynthesis of CeO2 NPs has fascinated the research community and is at an exponential pace. Biosynthesis implicated plant extract, nutrients, bacteria, fungai, biological products, etc., mediated the synthesis of CeO2 NPs, which is environment-friendly, non-toxic, economically affordable, and effective. Furthermore, green or biosynthesis protocols have various advantages in the eco-friendly synthesis of CeO2 NPs. Some of the crucial points are listed below.
-
Prevention—The green or biosynthesis of CeO2 NPs prevents waste generation.
-
Less hazardous chemical—The non-toxic and eco-friendly chemicals were used in the green or biosynthesis of CeO2 NPs.
-
Designing safer chemicals—Plant extract, nutrients, bacteria, fungi, and biological product encompasses biomolecules that act as reducing and/or stabilizing agents in the manufacture of CeO2 NPs. Hence, there is no need to employ harmful compounds like sodium borohydride, hydrazine hydrate, or ethylene glycol, which raises toxicity risk.
-
Solvents—The green or biosynthesis approaches involve water as a solvent in preparing CeO2 NPs, which is inexpensive, environmentally benign, non-flammable, non-toxic, and pollution-free.
-
Energy Efficiency—The ambient temperature, pressure, and energy is required for the green or biosynthesis of CeO2 NPs. which reduces production expense. As a result of the lower production costs, the process becomes more cost-effective.
-
Renewable raw material—The green or biosynthesis of CeO2 NPs utilizes renewable materials such as plant extract, nutrients, bacteria, fungi, and biological products that are abundant and readily available in nature.
-
Degradation—In the green or biosynthesis of CeO2 NPs, biodegradable material is used. So, there is no issue of environmental hazard, and the method is eco-friendly.
To make versatile CeO2 NPs, a variety of plants have been used. However, this technique impedes practical uses of biosynthesis-assisted CeO2 NPs that need enhancement. Various reaction parameters such as precursor concentration, reaction temperature, reaction time, pH, and calcination temperature, can play a vital role in regulating the size, shape, and morphology of CeO2 NPs. To achieve desired CeO2 NPs, the parameters should be optimized. In addition, researchers need to explore the fungi-mediated CeO2 NPs as very few reports present on the fungi-mediated synthesis of CeO2 NPs. Moreover, the underlying mechanism by which microorganisms and medicinal plant extracts are examined for the biosynthesis of CeO2 NPs is still unknown. Also, the stoichiometric ratios of microorganisms, plant extracts, and metal precursors are needed to describe the fabrication and morphological control.
Furthermore, the researcher has not explored the application of CeO2 NPs as a catalyst in organic transformations. After all, discovering unique features for plants, microbes, and biological product extract-assisted CeO2 NPs is essential. Plant extract, microbes, and biological product directed CeO2 NPs will undoubtedly gain significance and use in catalysis, biomedicine, bio-sensing, energy, agriculture, and the environment in the subsequent years and will become more powerful and reliable.
Abbreviations
- NPs:
-
Nanoparticles
- CeO2:
-
Cerium oxide
- CDB:
-
Czapek-Dox-Broth
- XRD:
-
X-ray diffraction analysis
- JCPDS:
-
Joint Committee on Powder Diffraction Standards
- FTIR:
-
Fourier transform infrared spectroscopy
- TEM:
-
Transmission electron microscopy
- HR-TE:
-
High-resolution transmission electron microscopy
- TGA:
-
Thermogravimetric analysis
- DTA:
-
Differential thermal analysis
- UV:
-
Ultra violet
- XPS:
-
X-ray photoelectron spectroscopy
- ROS:
-
Reactive oxygen species
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl-hydrate
- PAGE:
-
Polyacrilamide gel electrophoresis
- BHA:
-
Butylated hydroxyanisole
References
Ahmed HE, Iqbal Y, Aziz MH et al (2021) Green synthesis of CeO2 nanoparticles from the abelmoschus esculentus extract: evaluation of antioxidant, anticancer, antibacterial, and wound-healing activities. Molecules 26:4659. https://doi.org/10.3390/MOLECULES26154659
Alpaslan E, Yazici H, Golshan NH et al (2015) PH-dependent activity of dextran-coated cerium oxide nanoparticles on prohibiting osteosarcoma cell proliferation. ACS Biomater Sci Eng 1:1096–1103. https://doi.org/10.1021/ACSBIOMATERIALS.5B00194/SUPPL_FILE/AB5B00194_SI_001.PDF
Altaf M, Manoharadas S, Zeyad MT (2021) Green synthesis of cerium oxide nanoparticles using Acorus calamus extract and their antibiofilm activity against bacterial pathogens. Microsc Res Tech 84:1638–1648. https://doi.org/10.1002/JEMT.23724
Altemimi A, Lakhssassi N, Baharlouei A et al (2017) Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. https://doi.org/10.3390/plants6040042
Anjum S, Hashim M, Malik SA et al (2021) Cancers recent advances in Zinc Oxide Nanoparticles (ZnO NPs) for cancer diagnosis, target drug delivery, and treatment. Cancers (basel) 13:4570. https://doi.org/10.3390/cancers13184570
Ansar S, Tabassum HM, Aladwan NS et al (2020) Eco friendly silver nanoparticles synthesis by Brassica oleracea and its antibacterial, anticancer and antioxidant properties. Sci Rep. https://doi.org/10.1038/s41598-020-74371-8
Arfin S, Kumar Jha N, Kumar Jha S et al (2021) Antioxidants oxidative stress in cancer cell metabolism. Antioxidants. https://doi.org/10.3390/antiox10050642
Arumugam A, Karthikeyan C, Haja Hameed AS et al (2015) Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Mater Sci Eng, C 49:408–415. https://doi.org/10.1016/J.MSEC.2015.01.042
Arunachalam T, Karpagasundaram U, Rajarathinam N (2017) Ultrasound assisted green synthesis of cerium oxide nanoparticles using Prosopis juliflora leaf extract and their structural, optical and antibacterial properties. Mat Sci Poland 35:791–798. https://doi.org/10.1515/MSP-2017-0104
Aseyd Nezhad S, Es-haghi A, Tabrizi MH (2020a) Green synthesis of cerium oxide nanoparticle using Origanum majorana L leaf extract, its characterization and biological activities. Appl Organometal Chem. https://doi.org/10.1002/aoc.5314
Augustine R, Dalvi YB, Dan P et al (2018) Nanoceria can act as the cues for angiogenesis in tissue-engineering scaffolds: toward next-generation in situ tissue engineering. ACS Biomater Sci Eng 4:4338–4353. https://doi.org/10.1021/ACSBIOMATERIALS.8B01102/SUPPL_FILE/AB8B01102_SI_001.PDF
Balaji S, Mandal BK, Vinod Kumar Reddy L, Sen D (2020) Biogenic cezria nanoparticles (CeO2 NPs) for effective photocatalytic and cytotoxic activity. Bioengineering 7:26. https://doi.org/10.3390/BIOENGINEERING7010026
Bardapurkar PP, Shewale SS, Arote SA et al (2021) Effect of precursor pH on structural, magnetic and catalytic properties of CoFe2O4@SiO2 green nanocatalyst. Res Chem Intermedi 47(5):1919–1939. https://doi.org/10.1007/S11164-020-04366-7
Bassetti M, Righi E (2015) Overview of fungal infections—the italian experience. Semin Respir Crit Care Med 36:796–806. https://doi.org/10.1055/S-0035-1562890/ID/JR01142-43
Bhattacharya D, Tiwari R, Bhatia T et al (2019) Accelerated and scarless wound repair by a multicomponent hydrogel through simultaneous activation of multiple pathways. Drug Delivery Trans Res 9(6):1143–1158. https://doi.org/10.1007/S13346-019-00660-Z
Cerf ME (2013) Beta cell dysfunction and insulin resistance. Front Endocrinol 4:37. https://doi.org/10.3389/FENDO.2013.00037/BIBTEX
Cheng P, Guo P, Sun K et al (2021) CeO2 decorated graphene as separator modification material for capture and boost conversion of polysulfide in lithium-sulfur batteries. J Membr Sci 619:118780. https://doi.org/10.1016/J.MEMSCI.2020.118780
Corma A, Atienzar P, García H, Chane-Ching JY (2004) Hierarchically mesostructured doped CeO2 with potential for solar-cell use. Nat Mater 3(6):394–397. https://doi.org/10.1038/nmat1129
Cuong HN, Pansambal S, Ghotekar S et al (2022) New frontiers in the plant extract mediated biosynthesis of copper oxide (CuO) nanoparticles and their potential applications: a review. Environ Res 203:111858. https://doi.org/10.1016/J.ENVRES.2021.111858
Dabhane H, Dabhane H, Ghotekar S et al (2021a) MgO nanoparticles: Synthesis, characterization, and applications as a catalyst for organic transformations. Eur J Chem 12:86–108. https://doi.org/10.5155/eurjchem.12.1.86-108.2060
Dabhane H, Ghotekar S, Tambade P et al (2021b) A review on environmentally benevolent synthesis of CdS nanoparticle and their applications. Envir Chem Ecotoxicol 3:209–219. https://doi.org/10.1016/J.ENCECO.2021.06.002
Daoud NM, Aly MS, Ezzo OH, Ali NA (2021) Zinc oxide nanoparticles improve testicular steroidogenesis machinery dysfunction in benzo[α] pyrene-challenged rats. Sci Rep 11:11675. https://doi.org/10.1038/s41598-021-91226-y
Darroudi M, Hoseini SJ, Kazemi Oskuee R et al (2014a) Food-directed synthesis of cerium oxide nanoparticles and their neurotoxicity effects. Ceram Int 40:7425–7430. https://doi.org/10.1016/J.CERAMINT.2013.12.089
Darroudi M, Sarani M, Kazemi Oskuee R et al (2014b) Green synthesis and evaluation of metabolic activity of starch mediated nanoceria. Ceram Int 40:2041–2045. https://doi.org/10.1016/J.CERAMINT.2013.07.116
Das S, Singh S, Dowding JM et al (2012) The induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environments. Biomaterials 33:7746. https://doi.org/10.1016/J.BIOMATERIALS.2012.07.019
Das S, Mojumder S, Saha D, Pal M (2022) Influence of major parameters on the sensing mechanism of semiconductor metal oxide based chemiresistive gas sensors: a review focused on personalized healthcare. Sens Actuators, B Chem 352:131066. https://doi.org/10.1016/J.SNB.2021.131066
Davoodbasha MA, Saravanakumar K, Abdulkader AM et al (2019) Synthesis of biocompatible cellulose-coated nanoceria with pH-dependent antioxidant property. ACS Appl Bio Mater 2:1792–1801. https://doi.org/10.1021/ACSABM.8B00647/SUPPL_FILE/MT8B00647_SI_002.PDF
Dutta D, Mukherjee R, Patra M et al (2016) Green synthesized cerium oxide nanoparticle: a prospective drug against oxidative harm. Colloids Surf, B 147:45–53. https://doi.org/10.1016/J.COLSURFB.2016.07.045
Dutta V, Sharma S, Raizada P et al (2021) Recent advances and emerging trends in (BiO)2CO3 based photocatalysts for environmental remediation: a review. Surf Interf 25:101273. https://doi.org/10.1016/J.SURFIN.2021.101273
Eka Putri G, Rilda Y, Syukri S et al (2021) Highly antimicrobial activity of cerium oxide nanoparticles synthesized using Moringa oleifera leaf extract by a rapid green precipitation method. J Market Res 15:2355–2364. https://doi.org/10.1016/J.JMRT.2021.09.075
Elahi B, Mirzaee M, Darroudi M et al (2019) Preparation of cerium oxide nanoparticles in Salvia Macrosiphon Boiss seeds extract and investigation of their photo-catalytic activities. Ceram Int 45:4790–4797. https://doi.org/10.1016/J.CERAMINT.2018.11.173
Elahi B, Mirzaee M, Darroudi M et al (2020) Role of oxygen vacancies on photo-catalytic activities of green synthesized ceria nanoparticles in Cydonia oblonga miller seeds extract and evaluation of its cytotoxicity effects. J Alloy Compd 816:152553. https://doi.org/10.1016/J.JALLCOM.2019.152553
Estes LM, Singha P, Singh S et al (2021) Characterization of a nitric oxide (NO) donor molecule and cerium oxide nanoparticle (CNP) interactions and their synergistic antimicrobial potential for biomedical applications. J Colloid Interface Sci 586:163–177. https://doi.org/10.1016/J.JCIS.2020.10.081
Estevez AY, Ganesana M, Trentini JF et al (2019) Antioxidant enzyme-mimetic activity and neuroprotective effects of cerium oxide nanoparticles stabilized with various ratios of citric acid and EDTA. Biomolecules 9:562. https://doi.org/10.3390/BIOM9100562
Farias IAP, dos Santos CCL, Sampaio FC (2018) Antimicrobial activity of cerium oxide nanoparticles on opportunistic microorganisms: a systematic review. BioMed Res Intern. https://doi.org/10.1155/2018/1923606
Frewer LJ, Gupta N, George S et al (2014) Consumer attitudes towards nanotechnologies applied to food production. Trends Food Sci Technol 40:211–225. https://doi.org/10.1016/J.TIFS.2014.06.005
Gade A, Ingle A, Whiteley C, Rai M (2010) Mycogenic metal nanoparticles: progress and applications. Biotechnol Lett 32(5):593–600. https://doi.org/10.1007/S10529-009-0197-9
Gawande MB, Goswami A, Felpin FO-X et al (2016) Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem Rev 116(6):3722–3811. https://doi.org/10.1021/acs.chemrev.5b00482
Ghanashyam Krishna M, Hartridge A, Bhattacharya AK (1998) Temperature and ionic size dependence of the properties of ceria based optionic thin films. Mater Sci Eng, B 55:14–20. https://doi.org/10.1016/S0921-5107(98)00203-7
Ghotekar S, Pansambal S, Bilal M et al (2021) Environmentally friendly synthesis of Cr2O3 nanoparticles: Characterization, applications and future perspective—a review. Case Stud Chem Envir Eng 3:100089. https://doi.org/10.1016/J.CSCEE.2021.100089
Gopinath K, Karthika V, Sundaravadivelan C et al (2015) Mycogenesis of cerium oxide nanoparticles using Aspergillus niger culture filtrate and their applications for antibacterial and larvicidal activities. J Nanostruct Chem. https://doi.org/10.1007/s40097-015-0161-2
Gopinath K, Karthika V, Sundaravadivelan C et al (2015) Mycogenesis of cerium oxide nanoparticles using Aspergillus niger culture filtrate and their applications for antibacterial and larvicidal activities. J Nanostruct Chem 5(3):295–303. https://doi.org/10.1007/S40097-015-0161-2
Hernández-Díaz JA, Garza-García JJO, Zamudio-Ojeda A et al (2021) Plant-mediated synthesis of nanoparticles and their antimicrobial activity against phytopathogens. J Sci Food Agric 101:1270–1287. https://doi.org/10.1002/JSFA.10767
Hertadi R, Umriani Permatasari N, Ratnaningsih E (2021) Box-Wilson design for optimization of in vitro levan production and levan application as antioxidant and antibacterial agents. Iranian Biomed J 25:202–212
Howlett NG, Avery S, v. (1997) Induction of lipid peroxidation during heavy metal stress in Saccharomyces cerevisiae and influence of plasma membrane fatty acid unsaturation. Appl Environ Microbiol 63:2971–2976. https://doi.org/10.1128/AEM.63.8.2971-2976.1997
Huang X, Lin S, Shang J et al (2014) Mechanical, thermal, and ultraviolet resistance properties of poly(ether–ester)/cerium oxide (CeO2) composite fibers. J Reinf Plast Compos 33:1207–1215. https://doi.org/10.1177/0731684413518826
Iqbal A, Ahmed AS, Ahmad N et al (2021) Biogenic synthesis of CeO2 nanoparticles and its potential application as an efficient photocatalyst for the degradation of toxic amido black dye. Envir Nanotechnol Monit Manage 16:100505. https://doi.org/10.1016/J.ENMM.2021.100505
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:2638–2650. https://doi.org/10.1039/C1GC15386B
Irshad MS, Aziz MH, Fatima M et al (2019) Green synthesis, cytotoxicity, antioxidant and photocatalytic activity of CeO2 nanoparticles mediated via orange peel extract (OPE). Mat Res Exp. https://doi.org/10.1088/2053-1591/ab3326
Jan H, Khan MA, Usman H et al (2020) The Aquilegia pubiflora (Himalayan columbine) mediated synthesis of nanoceria for diverse biomedical applications. RSC Adv. https://doi.org/10.1039/d0ra01971b
Javadi F, Taghavizadeh Yazdi ME, Baghani M, Es-Haghi A (2019) Biosynthesis, characterization of cerium oxide nanoparticles using Ceratonia siliqua and evaluation of antioxidant and cytotoxicity activities. Mat Res Exp 6:065408. https://doi.org/10.1088/2053-1591/AB08FF
Jung H, Kittelson DB, Zachariah MR (2005) The influence of a cerium additive on ultrafine diesel particle emissions and kinetics of oxidation. Combust Flame 142:276–288. https://doi.org/10.1016/J.COMBUSTFLAME.2004.11.015
Kannan SK, Sundrarajan M (2014a) A green approach for the synthesis of a cerium oxide nanoparticle: characterization and antibacterial activity. Int J Nanosci. https://doi.org/10.1142/S0219581X14500185
Kargar H, Ghasemi F, Darroudi M (2015a) Bioorganic polymer-based synthesis of cerium oxide nanoparticles and their cell viability assays. Ceram Int 41:1589–1594. https://doi.org/10.1016/J.CERAMINT.2014.09.095
Kargar H, Ghazavi H, Darroudi M (2015b) Size-controlled and bio-directed synthesis of ceria nanopowders and their in vitro cytotoxicity effects. Ceram Int 41:4123–4128. https://doi.org/10.1016/J.CERAMINT.2014.11.108
Khalil I, Yehye WA, Etxabide Etxeberria A et al (2019) Nanoantioxidants: recent trends in antioxidant delivery applications. Antioxidants 9(1):1–30. https://doi.org/10.3390/antiox9010024
Khan SA, Ahmad A (2013) Fungus mediated synthesis of biomedically important cerium oxide nanoparticles. Mater Res Bull 48:4134–4138. https://doi.org/10.1016/J.MATERRESBULL.2013.06.038
Khan I, Saeed K, Khan I (2019) Nanoparticles: Properties, applications and toxicities. Arab J Chem 12:908–931. https://doi.org/10.1016/J.ARABJC.2017.05.011
Khatami M, Sarani M, Mosazadeh F et al (2019) Nickel-doped cerium oxide nanoparticles: green synthesis using stevia and protective effect against harmful ultraviolet rays. Molecules 24:4424. https://doi.org/10.3390/MOLECULES24244424
Khin MM, Nair AS, Babu VJ et al (2012) A review on nanomaterials for environmental remediation. Energy Environ Sci 5:8075–8109. https://doi.org/10.1039/C2EE21818F
Korotkova AM, Borisovna PO, Aleksandrovna GI et al (2019) “Green” synthesis of cerium oxide particles in water extracts petroselinum crispum. Current Nanomaterials 4:176–190. https://doi.org/10.2174/2405461504666190911155421
Krawczyk M, Holdynski M, Lisowski W et al (2015) Electron inelastic mean free paths in cerium dioxide. Appl Surf Sci 341:196–202. https://doi.org/10.1016/J.APSUSC.2015.02.177
Kumar KM, Mahendhiran M, Diaz MC et al (2018) Green synthesis of Ce3+ rich CeO2 nanoparticles and its antimicrobial studies. Mater Lett 214:15–19. https://doi.org/10.1016/J.MATLET.2017.11.097
Kundu P, Sharma P, Mahato R et al (2020) A brief review for the development of bio-nanoparticles using some important Indian ethnomedicinal plants. J Med Plants Stud 8:26–33
Maensiri S, Labuayai S, Laokul P et al (2014) Structure and optical properties of CeO2 nanoparticles prepared by using lemongrass plant extract. Jap J Appl Phy. https://doi.org/10.7567/JJAP.53.06JG14/XML
Magudieshwaran R, Ishii J, Raja KCN et al (2019) Green and chemical synthesized CeO2 nanoparticles for photocatalytic indoor air pollutant degradation. Mater Lett 239:40–44. https://doi.org/10.1016/J.MATLET.2018.11.172
Makvandi P, Iftekhar S, Pizzetti F et al (2021) Functionalization of polymers and nanomaterials for water treatment, food packaging, textile and biomedical applications: a review. Environ Chem Lett 19:583–611. https://doi.org/10.1007/s10311-020-01089-4
Malleshappa J, Nagabhushana H, Prashantha SC et al (2014) Eco-friendly green synthesis, structural and photoluminescent studies of CeO2:Eu3+ nanophosphors using E. tirucalli plant latex. J Alloy Compd 612:425–434. https://doi.org/10.1016/J.JALLCOM.2014.05.101
Malleshappa J, Nagabhushana H, Sharma SC et al (2015) Leucas aspera mediated multifunctional CeO2 nanoparticles: Structural, photoluminescent, photocatalytic and antibacterial properties. Spectrochim Acta Part A Mol Biomol Spectrosc 149:452–462. https://doi.org/10.1016/J.SAA.2015.04.073
Maqbool Q, Nazar M, Naz S et al (2016) Antimicrobial potential of green synthesized CeO2 nanoparticles from Olea europaea leaf extract. Int J Nanomed 11:5015. https://doi.org/10.2147/IJN.S113508
Maritim AC, Sanders RA, Watkins JB (2003) Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17:24–38. https://doi.org/10.1002/JBT.10058
Marouzi S, Sabouri Z, Darroudi M (2021) Greener synthesis and medical applications of metal oxide nanoparticles. Ceram Int 47:19632–19650. https://doi.org/10.1016/J.CERAMINT.2021.03.301
McCune LM (2013) Traditional medicinal plants of indigenous peoples of Canada and their antioxidant activity in relation to treatment of diabetes. In: Watson RR, Preedy VR (eds) Bioactive Food as Dietary Interventions for Diabetes. Elsevier, Netherlands
Miri A, Sarani M (2018) Biosynthesis, characterization and cytotoxic activity of CeO2 nanoparticles. Ceram Int 44:12642–12647. https://doi.org/10.1016/J.CERAMINT.2018.04.063
Miri A, Darroudi M, Sarani M (2020) Biosynthesis of cerium oxide nanoparticles and its cytotoxicity survey against colon cancer cell line. Appl Organomet Chem. https://doi.org/10.1002/aoc.5308
Mohamed HEA, Afridi S, Khalil AT et al (2020) Promising antiviral, antimicrobial and therapeutic properties of green nanoceria. Nanomedicine 15(5):467–488. https://doi.org/10.2217/NNM-2019-0368
Mohammadzadeh V, Barani M, Amiri MS et al (2022) Applications of plant-based nanoparticles in nanomedicine: a review. Sust Chemistry Pharm 25:100606. https://doi.org/10.1016/J.SCP.2022.100606
Mukherjee PK, Sheehan DJ, Hitchcock CA, Ghannoum MA (2005) Combination treatment of invasive fungal infections. Clin Microbiol Rev 18:163–194. https://doi.org/10.1128/CMR.18.1.163-194.2005/ASSET/87274E1B-3B52-4429-B3F1-FA15B8C074BB/ASSETS/GRAPHIC/ZCM0010521160004.JPEG
Murugesan A, Prakash R, Srinithi S et al (2020) Preparation and characterization of green synthesized cerium oxide nano particle doped with biodiesel blends. IOP Conf Series: Mat Sci Eng 912:042035. https://doi.org/10.1088/1757-899X/912/4/042035
Muthuvel A, Jothibas M, Mohana V, Manoharan C (2020) Green synthesis of cerium oxide nanoparticles using Calotropis procera flower extract and their photocatalytic degradation and antibacterial activity. Inorg Chem Commun 119:108086. https://doi.org/10.1016/J.INOCHE.2020.108086
Nadaroglu H, Onem H, Gungor AA (2017) Green synthesis of Ce2 O3 NPs and determination of its antioxidant activity. IET Nanobiotechnol 11:411–419. https://doi.org/10.1049/IET-NBT.2016.0138
Nadeem M, Khan R, Afridi K et al (2020a) Green Synthesis of Cerium Oxide Nanoparticles (CeO2 NPs) and their antimicrobial applications: a Review. Int J Nanomed 15:5951. https://doi.org/10.2147/IJN.S255784
Najafi R, Hosseini A, Ghaznavi H et al (2017) Neuroprotective effect of cerium oxide nanoparticles in a rat model of experimental diabetic neuropathy. Brain Res Bull 131:117–122. https://doi.org/10.1016/J.BRAINRESBULL.2017.03.013
Nasrollahzadeh M, Sajjadi M, Iravani S, Varma RS (2021) Green-synthesized nanocatalysts and nanomaterials for water treatment: current challenges and future perspectives. J Hazard Mater 401:123401. https://doi.org/10.1016/J.JHAZMAT.2020.123401
Nikam AV, Prasad BLV, Kulkarni AA (2018) Wet chemical synthesis of metal oxide nanoparticles: a review. CrystEngComm 20:5091–5107. https://doi.org/10.1039/C8CE00487K
Nourmohammadi E, Kazemi Oskuee R, Hasanzadeh L et al (2018) Cytotoxic activity of greener synthesis of cerium oxide nanoparticles using carrageenan towards a WEHI 164 cancer cell line. Ceram Int 44:19570–19575. https://doi.org/10.1016/J.CERAMINT.2018.07.201
Orel ZC, Orel B (1994) Optical Properties of Pure CeO2 and Mixed CeO2/SnO2 Thin Film Coatings. Phys Stat Sol (b) 186:K33–K36. https://doi.org/10.1002/PSSB.2221860135
Pandiyan N, Murugesan B, Sonamuthu J et al (2019) [BMIM] PF6 ionic liquid mediated green synthesis of ceramic SrO/CeO2 nanostructure using Pedalium murex leaf extract and their antioxidant and antibacterial activities. Ceram Int 45:12138–12148. https://doi.org/10.1016/J.CERAMINT.2019.03.116
Pansambal SS, Ghotekar SK, Oza R, Deshmukh KK (2019) Biosynthesis of CuO nanoparticles using aqueous extract of Ziziphus mauritiana L. leaves and their Catalytic performance for the 5-aryl-1, 2, 4-triazolidine-3-thione derivatives synthesis. Int J Sci Res Sci Technol 5:122–128
Patil SN, Paradeshi JS, Chaudhari PB et al (2016) Bio-therapeutic potential and cytotoxicity assessment of pectin-mediated synthesized nanostructured cerium oxide. Appl Biochem Biotechnol 180(4):638–654. https://doi.org/10.1007/S12010-016-2121-9
Perillo B, di Donato M, Pezone A et al (2020) ROS in cancer therapy: the bright side of the moon. Exp Mol Med 52(2):192–203. https://doi.org/10.1038/s12276-020-0384-2
Perona JS, Alonso A, Martínez-González MA, Ruiz-Gutiérrez V (2021) Olives and olive oil in health and disease prevention, second. Elsevier Inc., Netherlands
Peters RJB, Bouwmeester H, Gottardo S et al (2016) Nanomaterials for products and application in agriculture, feed and food. Trends Food Sci Technol 54:155–164. https://doi.org/10.1016/J.TIFS.2016.06.008
Pomerantseva E, Bonaccorso F, Feng X et al (2019) Energy storage: the future enabled by nanomaterials. Science. https://doi.org/10.1126/SCIENCE.AAN8285/ASSET/87ACF1BD-33BB-46EE-BB4E-A8FD33DDBE2F/ASSETS/GRAPHIC/366_AAN8285_FA.JPEG
Pon S, Sri S, George M (2020) Spherical CeO2 nanoparticles encapsulated with Nelumbo nucifera Gaertn. flower extract and its in vitro anticancer activity against HCT 116 human colon cancer cell line. IJCT 27:153–160
Popov AL, Zaichkina SI, Popova NR et al (2016) Radioprotective effects of ultra-small citrate-stabilized cerium oxide nanoparticles in vitro and in vivo. RSC Adv 6:106141–106149. https://doi.org/10.1039/C6RA18566E
Pourkhalili N, Hosseini A, Nili-Ahmadabadi A et al (2011) Biochemical and cellular evidence of the benefit of a combination of cerium oxide nanoparticles and selenium to diabetic rats. Available from: World J Diabetes 2:204–210. https://doi.org/10.4239/wjd.v2.i11.204
Putri GE, Arief S, Jamarun N et al (2019) Microstructural analysis and optical properties of nanocrystalline cerium oxides synthesized by precipitation method. Rasayan J Chem 12(1):85–90. https://doi.org/10.31788/RJC.2019.1215029
Qi L, Fresnais J, Muller P et al (2012) Interfacial activity of phosphonated-peg functionalized cerium oxide nanoparticles. Langmuir 28:11448–11456. https://doi.org/10.1021/LA302173G/SUPPL_FILE/LA302173G_SI_001.PDF
Qian J, Chen F, Zhao X, Chen Z (2011) China rose petal as biotemplate to produce two-dimensional ceria nanosheets. J Nanoparticle Res 13(12):7149–7158. https://doi.org/10.1007/S11051-011-0626-2
Raizada P, Sudhaik A, Patial S et al (2020) Engineering nanostructures of CuO-based photocatalysts for water treatment: Current progress and future challenges. Arab J Chem 13:8424–8457. https://doi.org/10.1016/J.ARABJC.2020.06.031
Rajan AR, Rajan A, John A, Philip D (2019) Green synthesis of CeO2 nanostructures by using Morus nigra fruit extract and its antidiabetic activity. AIP Conf Proc 2105:020008. https://doi.org/10.1063/1.5100693
Rajan AR, Vilas V, Rajan A et al (2020) Synthesis of nanostructured CeO2 by chemical and biogenic methods: optical properties and bioactivity. Ceram Int 46:14048–14055. https://doi.org/10.1016/J.CERAMINT.2020.02.204
Reddy Yadav LS, Manjunath K, Archana B et al (2016) Fruit juice extract mediated synthesis of CeO2 nanoparticles for antibacterial and photocatalytic activities. Eur Phy J plus 131(5):1–10. https://doi.org/10.1140/EPJP/I2016-16154-Y
Riddin TL, Gericke M, Whiteley CG (2006) Analysis of the inter- and extracellular formation of platinum nanoparticles by Fusarium oxysporum f sp lycopersici using response surface methodology. Nanotechnology. https://doi.org/10.1088/0957-4484/17/14/021
Rubio L, Annangi B, Vila L et al (2015) Antioxidant and anti-genotoxic properties of cerium oxide nanoparticles in a pulmonary-like cell system. Arch Toxicol 90(2):269–278. https://doi.org/10.1007/S00204-015-1468-Y
Ryabchikova E (2021) Nanomaterials editorial advances in nanomaterials in biomedicine. Nanomaterials 11(1):1–5. https://doi.org/10.3390/nano11010118
Sai Priya G, Kanneganti A, Anil Kumar K et al (2014) Bio Synthesis of Cerium oxide nanoparticles using aloe barbadensis miller gel. Int J Sci Res Publ 4(6):199–224
Sankar V, Salinraj P, Athira R et al (2015) Cerium nanoparticles synthesized using aqueous extract of Centella asiatica: characterization, determination of free radical scavenging activity and evaluation of efficacy against cardiomyoblast hypertrophy. RSC Adv 5:21074–21083. https://doi.org/10.1039/C4RA16893C
Saravanakumar K, Sathiyaseelan A, Mariadoss AVA, Wang MH (2021) Antioxidant and antidiabetic properties of biocompatible ceria oxide (CeO2) nanoparticles in mouse fibroblast NIH3T3 and insulin resistant HepG2 cells. Ceram Int 47:8618–8626. https://doi.org/10.1016/J.CERAMINT.2020.11.230
Sebastiammal S, Mariappan A, Neyvasagam K, Lesly Fathima A (2019) Annona muricatainspired synthesis of CeO2 nanoparticles and their antimicrobial activity. Mat Today: Proc 9:627–632. https://doi.org/10.1016/J.MATPR.2018.10.385
Senthilkumar RP, Bhuvaneshwari V, Ranjithkumar R et al (2017) Synthesis, characterization and antibacterial activity of hybrid chitosan-cerium oxide nanoparticles: as a bionanomaterials. Int J Biol Macromol 104:1746–1752. https://doi.org/10.1016/J.IJBIOMAC.2017.03.139
Shah M, Fawcett D, Sharma S et al (2015) Green synthesis of metallic nanoparticles via biological entities. Materials 8(11):7278–7308. https://doi.org/10.3390/ma8115377
Shamprasad BR, Keerthana S, Megarajan S et al (2019) Photosynthesized escin stabilized gold nanoparticles exhibit antidiabetic activity in L6 rat skeletal muscle cells. Mater Lett 241:198–201. https://doi.org/10.1016/J.MATLET.2019.01.086
Sharma D, Hussain CM (2020) Smart nanomaterials in pharmaceutical analysis. Arab J Chem 13:3319–3343. https://doi.org/10.1016/J.ARABJC.2018.11.007
Sharma JK, Srivastava P, Ameen S et al (2017) Phytoconstituents assisted green synthesis of cerium oxide nanoparticles for thermal decomposition and dye remediation. Mater Res Bull 91:98–107. https://doi.org/10.1016/J.MATERRESBULL.2017.03.034
Sharmila G, Muthukumaran C, Saraswathi H et al (2019) Green synthesis, characterization and biological activities of nanoceria. Ceram Int 45:12382–12386. https://doi.org/10.1016/J.CERAMINT.2019.03.164
Shcherbakov AB, Reukov VV, Yakimansky AV et al (2021) CeO2 nanoparticle-containing polymers for biomedical applications: a review. Polymers 13:924. https://doi.org/10.3390/POLYM13060924
Singh V, Singh S, Das S et al (2012) A facile synthesis of PLGA encapsulated cerium oxide nanoparticles: release kinetics and biological activity. Nanoscale 4:2597–2605. https://doi.org/10.1039/C2NR12131J
Singh A, Hussain I, Singh NB, Singh H (2019) Uptake, translocation and impact of green synthesized nanoceria on growth and antioxidant enzymes activity of Solanum lycopersicum L. Ecotoxicol Environ Saf 182:109410. https://doi.org/10.1016/J.ECOENV.2019.109410
Singh KRB, Nayak V, Sarkar T, Singh RP (2020) Cerium oxide nanoparticles: properties, biosynthesis and biomedical application. RSC Adv 10:27194–27214. https://doi.org/10.1039/D0RA04736H
Sisubalan N, Ramkumar VS, Pugazhendhi A et al (2017) ROS-mediated cytotoxic activity of ZnO and CeO2 nanoparticles synthesized using the Rubia cordifolia L leaf extract on MG-63 human osteosarcoma cell lines. Envir Sci Poll Res 25(11):10482–10492. https://doi.org/10.1007/S11356-017-0003-5
Surendra TV, Roopan SM (2016) Photocatalytic and antibacterial properties of phytosynthesized CeO2 NPs using Moringa oleifera peel extract. J Photochem Photobiol, B 161:122–128. https://doi.org/10.1016/J.JPHOTOBIOL.2016.05.019
Teradal NL, Jelinek R (2017) Carbon Nanomaterials in biological studies and biomedicine. Adv Healthcare Mater 6:1700574. https://doi.org/10.1002/ADHM.201700574
Thovhogi N, Diallo A, Gurib-Fakim A, Maaza M (2015) Nanoparticles green synthesis by Hibiscus Sabdariffa flower extract: Main physical properties. J Alloy Compd 647:392–396. https://doi.org/10.1016/J.JALLCOM.2015.06.076
Turin-Moleavin I-A, Fifere A, Lungoci A-L et al (2019) In Vitro and In Vivo antioxidant activity of the new magnetic-cerium oxide nanoconjugates. Nanomaterials 9(11):1–21. https://doi.org/10.3390/nano9111565
van Tran T, Nguyen DTC, Kumar PS et al (2021) (2022) Green synthesis of ZrO2 nanoparticles and nanocomposites for biomedical and environmental applications: a review. Environ Chem Lett 1:1–23. https://doi.org/10.1007/S10311-021-01367-9
Venkatesh KS, Gopinath K, Palani NS et al (2016) Plant pathogenic fungus F. solani mediated biosynthesis of nanoceria: antibacterial and antibiofilm activity. RSC Adv 6:42720–42729. https://doi.org/10.1039/C6RA05003D
Xia T, Kovochich M, Liong M et al (2008) Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2:2121–2134. https://doi.org/10.1021/NN800511K/SUPPL_FILE/NN800511K_SI_001.PDF
Yulizar Y, Kusrini E, Apriandanu DOB, Nurdini N (2020) Datura metel L Leaves extract mediated CeO2 nanoparticles: synthesis, characterizations, and degradation activity of DPPH radical. Surfaces Interfaces 19:100437. https://doi.org/10.1016/J.SURFIN.2020.100437
Zamani A, Marjani AP, Alimoradlu K (2018) Walnut shell-templated ceria nanoparticles: green synthesis characterization and catalytic application. Int J Nanosci. https://doi.org/10.1142/S0219581X18500084
Zhang Y, Hou F, Tan Y (2012) CeO2 nanoplates with a hexagonal structure and their catalytic applications in highly selective hydrogenation of substituted nitroaromatics. Chem Commun 48:2391–2393. https://doi.org/10.1039/C1CC16983A
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pansambal, S., Oza, R., Borgave, S. et al. Bioengineered cerium oxide (CeO2) nanoparticles and their diverse applications: a review. Appl Nanosci 13, 6067–6092 (2023). https://doi.org/10.1007/s13204-022-02574-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-022-02574-8