Abstract
The utilization of green matrix on the manufacturing of nanocomposites has gained enormous interest in researchers to optimize an eco-friendly, cost-effective protocol in silver nanoparticles (AgNPs) production. AgNPs are among the most potent diagnostic and therapeutic representatives in the treatment of many diseases in nanomedicine field. In this research work, we focused on characterizations, antimicrobial, cytotoxic activity and apoptosis assessment against Pa-1 (Human ovarian teratocarcinoma) cell line via flow cytometry studies of biomodulated AgNPs using Cucumis sativus var. hardwickii fruit extract. The UV-Visible spectrum result revealed a maximum surface plasmon resonance at 408 nm. The characteristic FTIR analysis showed probable bioactive constituents subjected to reduction of silver ions. The morphology of Cs-AgNPs was spherical, polydispersed and size of the particles found to be 11.12 to 39 nm in range. In addition, the antimicrobial potential of Cs-AgNPs has shown moderate result against tested pathogens. Later, the biosynthesized Cs-AgNPs were tested for in vitro cytotoxic effect against Pa-1 cell line. The results revealed that the IC50 value of Cs-AgNPs against Pa-1 cells was observed to be 49.71 μg/mL. In addition, apoptotic property was analyzed using Annexin V/Propidium iodide (PI) via flow cytometry technique. The obtained results were displayed significant early apoptosis cell population of 12.91% and late apoptosis cell population of 45.03% towards Pa-1 cell line, respectively. Thus, it can be ascertained that the biomodulated Cs-AgNPs possess significant cytotoxic effect against tested cell line. So, it will be useful aspect in medical field in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is emerging by leaps and bounds due to utilization of nano composites with very specialized functions and size dependent physicochemical functions altering remarkably from bulk counterpart. Nanotechnology is the most vital sector in research field of material science and the nanomaterials synthesis process is significantly picking up all around the globe (Muhammad et al. 2017). The potentiality of inorganic nanoparticles has been explored globally in many fields such as drug delivery, cosmetics, nanomedicine, biomedical devices, environmental protection and energy sector. Nanotechnology has gigantic potential to transform research in the field of biomedicine by developing advanced and improved products for clinical therapy and diagnosis. Among nanoparticles, various noble metal nanoparticles from silver, platinum, palladium, gold and copper were extensively synthesized by implementing different procedures (Chintamani et al. 2018).
Nanotechnology led revolutions are also being used in genomic transformation and plant improvement programs. However, like other synthetic agrochemicals, indiscreet use of nanomaterials may have massive impacts on the global environment. Hence, the research-based, knowledgeable application of nanotechnology is required for sustainability of crop production systems and for environment (Veerasamy et al. 2011). The most commercialized metal used in nanotechnology is silver and nearly around 5 hundred tons of AgNPs are synthesized every year (Larue et al. 2014). Physicochemical properties of AgNPs include high thermal along with electrical conductivity, nontoxicity, durability at surrounding temperature, low-price of metal, catalytic activity, nonlinear optical behavior, chemical stability and wide absorption in far and visible light region, optical frequency, which make the uses of AgNPs more advantageous and desirable over other metallic nanoparticles (Krutyakov et al. 2008; Ivanova et al. 2019). AgNPs also exhibited broad spectrum of biological properties like bactericidal and fungicidal activity with extraordinary defense mechanism against broad range of microbes and also due to the appearance of drug resistance against frequently used antibiotics (Sharma et al. 2009; Thakur et al. 2018).
The synthesis process of nanoparticles is involved in physical, chemical and biogenic methods. Each methodology has some advantages and disadvantages with recurrent obstacle of uniform particle size, cost, size-distribution and scalability (Chouhan, 2018). When compared with physical and chemical synthesis methods, green synthesis have more advantages because it requires less chemicals, lacking of lengthy process for culture and maintenance the cell, requirement of more energy and less contaminants that avoid high-cost purifications (Awwad et al. 2013). Natural resources, such as plant extracts, microorganisms and enzymes have been found attention for biosynthesis of metallic nanoparticles in recent years. In this regard, utilizing green materials has many advantages which include less use of toxic chemicals and low energy consumption (Guzel and Erdal 2018; Devanesan et al. 2021). Plant mediated synthesis of nanoparticles is a needful approach of nanotechnology and the usefulness of such nanoparticles in many fields. After identifying biomolecules that are responsible for synthesizing the nanoparticles for quick single step protocol to conquer the problems in several sectors (Mohammadlou et al. 2016).
Cucumis sativus var. hardwickii belongs to the family Cucurbitaceae and commonly known as Cucumis hardwickii or Melo sativus. The plant is slender climber with rough stem, tendril simple. Leaves were broad and 12 cm across, entire with five lobed, rough, leaf stalks measures up to 15 cm. Fruit is medium sized, oval or spherical in appearance, spiny, greenish yellow with white stripes, with many seeds and compressed (Bisht et al. 2004). It is a medicinal plant with much unsweetened fruit. The plant is rich in many phytochemicals, like alkaloids, flavonoids, terpenoids, phenolics etc. These constituents have anti-diabetic, anticarcinogenic, anti-inflammatory and hepatoprotective properties (Cardoso-Taketa et al. 2008).
Cancer causes various changes in metabolic and cellular pathways in the organisms which develop through wide signaling pathway mechanisms including cell proliferation, apoptosis, metastasis and angiogenesis (Seigneuric et al. 2010; Abdel-Fattah and Ali 2018). The metabolic and cellular activities like alteration in genomic expression, aerobic glycolysis, damaged mitochondrial DNA and respiratory chain is caused by the abnormality of cancer cells (Burns and Manda 2017). According to the global estimates, 225,000 new cancer cases are detected each year, and 140,000 people die annually due to this. Ovarian cancer is one of the five causes of cancer-related deaths in women and comprises a histological and genetic broad range of tumors. Ovarian cancer is the most gynecological malignancy and is often fatal, primarily due to the late detection in most of the patients because the symptoms are asymptomatic. Hence the treatment methods during the early stages are highly critical (Karnezis et al. 2017). Sandhya et al. (2020) synthesized silver nanoparticles using Boerhavia erecta was very effective and cytotoxic to ovarian cancer Pa-1 cell line at the concentration of 25 μg/mL confirmed by performing MTT assay. Similarly, the in vitro cytotoxicity of biogenic gold nanoparticles against Pa-1 cell lines recorded half maximal inhibitory concentration IC50 of 45.88 μg/mL, respectively (Balashanmugam et al. 2018).
Therefore, the present research work was focused on the biomimetic synthesis of AgNPs using Cucumis sativus var. hardwickii fruit extract was conducted to evaluate its antimicrobial, anticancer activity and apoptosis via flow cytomtery against Pa-1 (Human ovarian teratocarcinoma) cancer cell line.
Materials and methods
Collection of materials and chemicals
Cucumis sativus var. hardwickii medicinal plant was collected in Karnatak University, Dharwad campus (15°26′09.5′′ N, 74°58′54.5′′ E) and the authenticity was confirmed by comparing with the herbarium collection of plants at Departmental Museum, Karnatak University, Dharwad, Karnataka, India. The herbarium of specimen was labeled KU/BOT/2018-19/CsH/126 and maintained for further reference. The chemicals, such as silver nitrate, nutrient broth, Muller Hinton broth, agar, Dulbecco's modified eagle's medium (DMEM), fetal bovine serum (FBS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), D-PBS, FITC Annenxin V, Propidium Iodide reagents were procured from Hi-media and BD Biosciences. Pathogen strains, such as Staphylococcus aureus (MTCC 6908), Escherichia coli (MTCC 40), Candida glabrata (MTCC 3019) and Candida albicans (MTCC 227), procured from Institute of Microbial Technology (IMTECH), Chandigarh.
Preparation of fruit extract and biosynthesis of silver nanoparticles
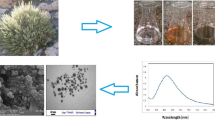
The fresh fruits of Cucumis sativus var. hardwickii (Fig. 1) were taken and washed several times with running tap water and later with Milli-Q water. About 100 g of chopped fruits were added to 500 mL distilled water, suspension was boiled and cooled down; subsequently suspension was sieved using Whatman No. 1 filter paper. Finally, the obtained fruit extract was kept aside in a refrigerator at 4 ℃ for further investigations (Ramesh et al. 2015).
Aqueous solution of 1 mM AgNO3 was mixed by dissolving 0.169 g of silver nitrate in 1000 mL of distilled water. The prepared fruit extract and AgNO3 solution were assorted in the fraction of 1:4 (v/v) in 1000 mL conical flask. The pH of the solution was set to 9.0, after 2 h of incubation period; suspension color was gradually switched from pale green to dark brown. This confirms the formation of AgNPs. Further the synthesized AgNPs were centrifuged at 10,000 rpm for 10 min to get Cs-AgNPs, accumulated Cs-AgNPs were air dried and stored in refrigerator for further investigations (Chand et al. 2020).
Characterizations of biosynthesized Cs-AgNPs
UV−visible spectrophotometeric analysis.
UV−visible spectra of biosynthesized Cs-AgNPs were performed by setting double beam UV−visible spectrophotometer (METASH UV-9600A) with 1 nm of resolution, and measured at wave length range of 300–700 nm, respectively (Ahmed et al. 2016).
Fourier transform infrared (FT-IR) spectroscopy analysis
Dried powder form of biosynthesized Cs-AgNPs from aqueous fruit extract was assorted with potassium bromide (Kbr) to get a pellet and analyzed to know the presence of possible bioactive constituents present on the surface of AgNPs (Ghramh et al. 2020). The spectrum data were recorded in the range of 400–4000 cm−1 resolution to decipher the functional groups present in the sample with the help of FT-IR instrument (NICOLET 6700, Thermo Fisher Scientific).
X- ray diffraction (XRD) analysis
Dried powder form of biosynthesized Cs-AgNPs were analyzed with the help of XRD to know the crystalline nature by placing AgNPs in a sample holder, then it was placed in analyzer (Rigaku Miniflex 600, SmartLab SE) to record the spectral patterns with angle of 2θ from 30° to 90° at 40 kV and 30 mA (Kambale et al. 2020).
Atomic force microscopy (AFM) analysis
AFM analysis was performed to know the size, distribution and aggregation of Cs-AgNPs. A thin film of the sample was prepared on a glass slide, which was allowed to oven dry for about 1 h (Gaddala and Nataru 2015) and then the slides were examined with AFM instrument (Nano surf Flex AFM).
Scanning electron microscopy analysis (SEM) and Energy dispersive X-ray spectroscopy (EDS) analysis
Scanning electron microscopy analysis of biosynthesized Cs-AgNPs were carried out using SEM instrument (JEOL JSM IT 500LA). Oven dried Cs-AgNPs were placed on the stub with the help of carbon tape then fixed and covered by sputtering with gold. Biosynthesized Cs-AgNPs was examined to know the morphological details and elemental analysis was also done by using EDS (Sreenivasa et al. 2020a, b).
High resolution transmission electron microscopy (HR-TEM) analysis
The size of the biosynthesized Cs-AgNPs can be analyzed using HR-TEM (FEI, TECNAI G2, F30) instrument. For the preparation of HR-TEM analysis, the following steps were carried out. The dried Cs-AgNPs were diluted with Milli-Q water, applied on carbon fiber paper-based copper TEM grids and dried in room temperature according to Wu et al. (2020) and then observed under HR-TEM.
Antimicrobial activity
The antimicrobial activity of Cs-AgNPs were determined against gram negative bacteria Escherichia coli (MTCC 40) and gram positive bacteria Staphylococcus aureus (MTCC 6908) and fungi like Candida albicans (MTCC 227) and Candida glabrata (MTCC 3019) by agar well diffusion method. Microorganisms were swabbed separately on the agar plates, After that 6 mm diameter wells were punched on 4 mm thick nutrient agar (pH 7.4) plates . Then 25, 50, 75 and 100 µg/µL concentrations of biosynthesized Cs-AgNPs were poured into separate wells. Sterile Milli-Q water was used as negative control and Streptomycin and Amphotericin B (50 µg/mL) were used as positive control for bacteria and fungi respectively. Then the plates were incubated overnight at 37 ℃. The diameter of clear zone was measured according to standard antibiotic zone scale (Aritonang et al. 2019).
In vitro anticancer activity of biosynthesized Cs-AgNPs
Cell viability assessment was determined using MTT assay according to process of Mosmann, (1983) on Pa-1 (Human ovarian teratocarcinoma) cancer cell lines. The cell line was procured from the NCCS (National Centre for Cell Science) Pune, India and was used for in vitro experimental purpose. The cells were cultured on DMEM with high glucose for cell proliferation at 37 ℃ and 5% CO2 atmosphere was maintained. After successful proliferation; the cells were seeded in a 96 well plate at density of 20,000 cells per well in 200 µL of medium. Several concentrations of Cs-AgNPs (6.25, 12.5, 25, 50 and 100 µL) were prepared in DMSO and the cancer cells were treated separately followed by incubation at 37 ℃ for 24 h. 12.5 µg/mL cisplatin was used as standard and cells without Cs-AgNPs were used as negative control for the completion of experiment. At the end of incubation period, freshly prepared 200 µL (0.5 mg/mL) of MTT was added to medium containing cells and incubated at 37 ℃ until formazan crystals were formed. After incubation, crystals were dissolved in 100 µL of dimethyl sulfoxide and viable cells were determined at 570 nm and the IC50 value was also determined at the end (Sreenivasa et al. 2020a).
Assessment of cell apoptosis via flow cytometry
Apoptosis and necrotic studies were determined via flow cytometric analysis of biosynthesized Cs-AgNPs. The control samples were stained with Annexin incorporated with fluorescein isothiocyanate (FITC) and propidium iodide (PI) using Annexin V-FITC apoptosis detection kit according to manufacturers protocol (BD Bioscience). Pa-1 (Human ovarian teratocarcinoma) cancer cells were seeded into 6 well plates and cells were treated with 49.71 µg/mL of Cs-AgNPs for 24 h at 37 ℃ in a 5% CO2 incubator. Cells were accumulated by trypsinization, and then cells were rinsed with cold PBS and re-suspended with Annexin V (5 μl) binding buffer. Cells were incubated for about 10 min at room temperature with Annexin V-FITC and PI stain in the dark room. Afterward, the samples were straight away analyzed using flow cytometer (BD FACS Calibur) according to the O’Brien and Bolton (1995) protocol. Finally, the results were measured using BD Cell Quest Pro Ver.6.0 software.
Statistical analysis
All the analyses were performed in triplicates and the data represented as mean ± standard deviation.
The statistical analysis was performed by using SPSS software.
Results and discussions
UV−visible analysis
In this research work, Cucumis sativus var. hardwickii aqueous fruit extract was used for synthesis of AgNPs. For biomodulated synthesis of AgNPs different optimization parameters were required which includes (i) extract concentration, (ii) precursor concentration and (iii) temperature and time. After the addition of fruit aqueous extract with 1 mM precursor solution (AgNO3), a change in color from pale green to dark brown after incubation in room temperature for 2 h at pH 9.0 (Fig. 2A, B). The adjustment of alkaline pH indicates synthesis of smaller size of AgNPs. At the higher pH level, the bulk concentration of H+ ions decreases, resulting in a higher surface charge on particles. Protonation and deprotonation surface reactions are used to obtain local surface charge, which depends on particle size and pH. At higher pH, the large numbers of phenolic functional groups become available and facilitate a higher number of Ag+ ions to bind and subsequently form a large number of nanoparticles with smaller diameters (Barisik et al. 2014; Ndikau et al. 2017). This result confirmed the vital role played by pH 9 in controlling the shape and size of the AgNPs. The UV absorption spectrum of prepared Cs-AgNPs was found at 408 nm in the range of 300–700 nm (Fig. 2C) and the single peak specified the size dependent synthesis of nanoscale sized AgNPs. This is due to the distinctive optical properties of AgNPs that enable them to intensely interact with particular wavelengths of UV−visible light spectrum. The absorption spectra showed that the surface plasmon resonance (SPR) peak rises with increasing extract concentration and time. Therefore, a high concentration of fruit extract avails the time-dependent optimum amount of bioactive constituents required to reduce the silver ions to silver nanoparticles. This is attributed to an increase in size and change in the shape of nanoparticles and this specifies that highly concentrated fruit extracts, synthesizes spherical and polydispersed nanoparticles. These observations were conceded with the results of Lee and El-Sayed, (2006); Ssekatawa et al. (2021).
FTIR analysis
FTIR analysis was performed to identify the bioactive chemical constituents comprising the functional groups, which were accountable for the reduction in Ag+ to Ag0 and capping of reduced AgNPs for stabilization. The IR spectrum of Cs-AgNPs revealed that there were marginal shifts in some peak positions of IR spectrum of AgNPs due to bioreduction (Fig. 3). A medium absorption spectrum at 3308.28 cm−1 represented the O–H stretching of alcohol groups and the peak at 2926.15 cm−1 corresponded to C–H stretching of alkane, a medium peak at 2360.09 cm−1 corresponds to O=C=O stretching of carbon dioxide, then a strong peak at 1593.66 cm−1 was assigned to N–H bending of amines. A strong peak at 1384.41 cm−1 represented to the S=O stretching of sulfate, a medium peak at 1080.85 cm−1 corresponded to C–N stretching of amine and finally a weak peak at 838.98 cm−1 corresponded to C=C bending of alkenes. Similar results of FTIR analysis suggested that, the AgNPs were capped by various groups of phyto-constituents, which act as stabilizing agents with the help of these bioactive binding sites (Algebaly et al. 2020; Femi-Adepoju et al. 2019). In a previous study, Gudikandula et al. (2017) found that these similar kinds of peaks confirmed the extracellular formation of AgNPs.
XRD analysis
XRD analysis of Cs-AgNPs (Fig. 4) exhibited intense peaks corresponded to the 2θ value ranging from 30° to 90°. The peak values obtained at 38.25°, 46.41°, 64.55° and 77.48° were corresponded to the (111, 222, 200 and 311) Bragg reflection based on the crystal faces of face-centered cubic structure of AgNPs. The results were corresponds to the standard silver card JCPDS pattern 04-0783. The XRD pattern also displayed some extra peaks which were unassigned. The extra peaks were due to the presence of bio-organic phase on the surface of particles. Although, the results show that the main phase is silver. However, there may be other crystalline phases belonging to the inorganic moieties of plant extracts which also accord to the XRD pattern as impurities. These peaks were attributed to the crystalline impurities of the left over plant extracts present on the surface of resultant nanoparticles (Awwad et al. 2013). Lakshmanan et al. (2017) also reported the same plane of crystalline nature of biosynthesized AgNPs using Cleome viscosa fruit extract.
AFM analysis
The surface morphological features and size distribution of Cs-AgNPs at different dimensions was examined by using AFM. The AFM images clearly appeared that the surface morphology of the nanoparticles were poly dispersed and spherical in shape without any agglomerations in topography structure, particle size ranging of 15–36 nm (Fig. 5A). Figure 5B represents the size distribution of nanoparticles, and Fig. 5C represents Cs-AgNPs in a three-dimensional plane. Our results are in accordance with earlier results of biosynthesized silver nanoparticles from Momordica cymbalaria fruit extract and different plant species (Swamy et al. 2015; Nayaka et al. 2020).
SEM and EDS analysis
The morphology of biosynthesized AgNPs were studied by SEM. The SEM image in Fig. 6A gave an overview of Cs-AgNPs. It showed nanoparticles were poly-dispersed with low agglomeration and spherical in nature. The size determined by SEM was correlated with size showed by AFM analysis. The chemical components were assessed using EDS analysis from 0 to 5 keV and showed a strong signal from silver metal and the presence of 37.29% this confirms the synthesis of Cs-AgNPs. Other than silver metal, few peaks represented elements like carbon, sodium, oxygen, potassium, etc. (Fig. 6B). Similarly, our results were in confirmation with earlier reports on morphology and elemental composition of AgNPs using SEM and EDS patterns with a prominent signal at 3 keV, which was characteristics to metallic silver due to surface plasmon resonance (Gnanajobitha et al. 2013; Isaac et al. 2013).
HR-TEM analysis
The morphological and size distribution of biosynthesized Cs-AgNPs was confirmed by doing HR-TEM analysis with particle size 11.12–39 nm range (Fig. 7A), the smallest size of the particle is 11.12 nm (Fig. 7B). Biosynthesized Cs-AgNPs were found to be poly-disperse and spherical in structures with minor aggregation. These findings are also reported by other researchers Gavamukulya et al. (2020) worked on the biogenic synthesis of AgNPs using Annona muricata fruit extracts. Previous studies have shown similar size variations of biosynthesized AgNPs from Terminalia chebula fruit extract (Ankegowda et al. 2020).
Antimicrobial activity
The biosynthesized Cs-AgNPs showed decent dose dependent antimicrobial activity against the tested pathogenic bacteria and fungal strains. The assessed inhibition zones (Fig. 8A–D) exhibited that E.coli and C. glabrata were more sensitive resulting 19.6 ± 0.5 and 20 ± 1 mm in 100 µl concentration of Cs-AgNPs, followed by S. aureus and C. albicans showed comparatively minimal sensitivity by showing 16 ± 1 and 14 ± 1 mm in 100 µl concentration towards the Cs-AgNPs. The antibacterial activity mechanism of silver nanoparticles on microbes is relatively known. AgNPs get attached to the cell membrane of the bacteria via linking with sulfur containing proteins, altering and disturbing permeability of the cell, as well as respiration functions. Finally at the end cell death occurs (Gavade et al. 2015). AgNPs can also enter inside the bacteria due to the interaction with functional groups found in the cell respiratory enzymes, thus inhibiting the respiration in bacteria (Li et al. 2010).
In vitro anticancer activity
The biosynthesized Cs-AgNPs exhibited direct dose dependent cytotoxic potential towards Pa-1 cells. MTT assay was rendered to figure out the anticancer activity of Cs-AgNPs. The negative and positive controls were shown in Fig. 9A, B, respectively. The cytotoxicity was gradually enhanced along gradual rising concentration of Cs-AgNPs from 6.25 μg/mL to 100 μg/mL. The morphological changes in cell treated with Cs-AgNPs are shown in Fig. 9C–G. The viability of cancer cells was 97.98%, 93.64%, 72.49%, 49.51% at 6.25, 12.5, 25, 50 μg/mL AgNPs, respectively, and it was reduced to a viability of 27.32% during the treatment with 100 μg/mL AgNPs. The IC50 value was calculated to be 49.71 μg/mL. Similarly our results were in accordance with previous studies on morphological changes occurs when Pa-1 cancer cell lines were treated with AgNPs synthesized using Cleome viscosa fruit extract with the lowest IC50 at 30 μg/mL (Lakshmanan et al. 2017).
Apoptosis and necrosis in cells
Apoptosis and necrosis test was done to evaluate the early and late apoptosis in Pa-1 cell lines treated with IC50 concentration of biosynthesized Cs-AgNPs, the cancer cells were stained with FITC Annexin V and PI and the statistical analysis of early and late apoptosis cells was confirmed by fluorescence activated cell sorting (FACS) via flow cytometry as shown in Fig. 10A, B. The assay revealed that the apoptosis in cancer cells treated to Cs-AgNPs (IC50 concentration 49.71 μg/mL) after 24 h, the cells displayed significant early apoptosis cell population of 12.91% and late apoptosis cell population of 45.03% and whereas, untreated cells did not displayed any significant apoptosis. Similarly, Han et al. (2017) reported the dual functions of AgNPs on F9 teratocarcinoma stem cells to evaluate the cytotoxicity in cancer therapy. Furthermore, many biochemical pathways that are involved in high cytotoxic potential induced from AgNPs.
Conclusion
In this research, an eco-friendly, cost-effective biosynthesis of AgNPs was accomplished using Cucumis sativus var. hardwickii aqueous fruit extract. The Cs-AgNPs were characterized through various characterization methods. The UV spectra demonstrated a peak at 408 nm and particle size was 11.12–39 nm. The FTIR spectra demonstrated various bioactive chemical constituents comprising many functional groups, which are mainly responsible for AgNPs synthesis. The biosynthesized Cs-AgNPs exhibited a significant antimicrobial potential inhibitory activity of pathogenic organisms, thus it may lead a way in advancement of treating bacterial and fungal diseases. Finally, the Cs-AgNPs revealed an efficient anticancer activity against Pa-1 (Human ovarian teratocarcinoma) cancer cell line by showing IC50 value of 49.71 μg/mL and evaluation of apoptosis and necrosis of cells via flow cytometry displayed significant early apoptosis cell population of 12.91% and late apoptosis cell population of 45.03% towards Pa-1 cell line, respectively. Since the last decade, the cancer diagnosis and treatment with AgNPs has become an interesting therapy owing to their potential to selectively bind and kill targeted cancer cells. Hence, it was suggested that the Cucumis sativus var. hardwickii aqueous fruit extract synthesized AgNPs could be used in treatment of ovarian cancer after further in vivo experiments. Therefore, future investigations are required to fully understand the mechanism and extensive in vivo characterization of antibacterial and anticancer efficiency and evaluation of apoptosis and necrosis of cells via flow cytometry associated with the nanoparticles.
Availability of data and material
Not Applicable.
References
Abdel-Fattah WI, Ali GW (2018) On the anti-cancer activities of silver nanoparticles. J Appl Biotech Bioeng 5:200116. https://doi.org/10.15406/jabb.2018.05.00116
Ahmed S, Saifullah AM, Swami BL (2016) Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J of Radia Res and Appl Sci 9:1–7. https://doi.org/10.1016/j.jrras.2015.06.006
Algebaly AS, Mohammed AE, Abutaha N, Elobeid MM (2020) Biogenic synthesis of silver nanoparticles: antibacterial and cytotoxic potential. Saudi J Biol Sci 27(5):1340–1351. https://doi.org/10.1016/j.sjbs.2019.12.014
Ankegowda VM, Kollur SP, Prasad SK, Pradeep S, Dhramashekara C, Jain AS, Prasad A, Srinivasa C, Sridhara Setty PB, Gopinath SM, Prasad RS, Bahkali AH, Syed A, Shivamallu C (2020) Phyto-mediated synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of its cytotoxic and antimicrobial potential. Molecules 25:5042. https://doi.org/10.3390/molecules25215042
Aritonang HF, Koleangan H, Wuntu AD (2019) Synthesis of silver nanoparticles using aqueous extract of medicinal plants (Impatiens balsamina and Lantana camara) fresh leaves and analysis of antimicrobial activity. Int J Micro. https://doi.org/10.1155/2019/8642303
Awwad AM, Salem NM, Abdeen AO (2013) Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int J Ind Chem 4:29. https://doi.org/10.1186/2228-5547-4-29
Balashanmugam P, Mosachristas K, Kowsalya E (2018) In vitro cytotoxicity and antioxidant evaluation of biogenic synthesized gold nanoparticles from Marsilea quadrifolia on lung and ovarian cancer cells. Int J Appl Pharma 10(5):153–158. https://doi.org/10.22159/ijap.2018v10i5.27999
Barisik M, Atalay S, Beskok A, Qian S (2014) Size dependent surface charge properties of silica nanoparticles. J Phys Chem C 118(4):1836–1842. https://doi.org/10.1021/jp410536n
Bisht IS, Bhat KV, Tanwar SPS, Bhandari DC, Kamal J, Sharma AK (2004) Distribution and genetic diversity of Cucumis sativus var. hardwickii (Royle) Alef. in India. The J of Horti Sci and Biotech 79:783–791. https://doi.org/10.1080/14620316.2004.11511843
Burns JS, Manda G (2017) Metabolic pathways of the Warburg effect in health and disease: Perspectives of choice, chain or chance. Int J Mol Sci 18(12):2755. https://doi.org/10.3390/ijms18122755
Cardoso-Taketa A, Pereda-Miranda R, Choi YH, Verpoorte R, Villarreal ML (2008) Metabolic profiling of the mexican anxiolytic and sedative plant Galphimia glauca using nuclear magnetic resonance spectroscopy and multivariate data analysis. Planta Med 74:1295–1301. https://doi.org/10.1055/s-2008-1074583
Chand K, Cao D, Eldin Fouad D, Hussain A, Qadeer A, Zhu K, Nazim M, Mehdi G (2020) Green synthesis, characterization and photocatalytic application of silver nanoparticles synthesized by various plant extracts. Arab J Chem 13:8248–8261. https://doi.org/10.1016/j.arabjc.2020.01.009
Chintamani RB, Salunkhe KS, Chavan M (2018) Emerging use of green synthesis metallic nanoparticles: an updated review. Int J Pharm Sci and Res 9(10):4029–4055. https://doi.org/10.13040/IJPSR.0975-8232
Chouhan N (2018) Silver Nanoparticles: Synthesis, characterization and applications. In: Maaz K (ed) Silver nanoparticles, fabrication, characterization and applications. Intech Open, pp 21–57. https://doi.org/10.5772/intechopen.75611
Devanesan S, Jayamala M, AlSalhi MS, Umamaheshwari S, Ranjitsingh AJA (2021) Antimicrobial and anticancer properties of Carica papaya leaves derived di-methyl flubendazole mediated silver nanoparticles. J Infect Public Health 14:577–587. https://doi.org/10.1016/j.jiph.2021.02.004
Femi-Adepoju AG, Dada AO, Otun KO, Adepoju AO, Fatoba OP (2019) Green synthesis of silver nanoparticles using terrestrial fern Gleichenia pectinata (Willd.) C. Presl characterization and antimicrobial studies. Heliyon 5:e01543. https://doi.org/10.1016/j.heliyon.2019.e01543
Gaddala B, Nataru S (2015) Synthesis, characterization and evaluation of silver nanoparticles through leaves of Abrus precatorius L.: an important medicinal plant. Appl Nanosci 5:99–104. https://doi.org/10.1007/s13204-014-0295-4
Gavade SJM, Nikam GH, Dhabbe RS, Sabale SR, Tamhankar BV, Mulik GN (2015) Green synthesis of silver nanoparticles by using carambola fruit extract and their antibacterial activity. Adv Nat Sci Nanosci Nanotechnol 6:045015. https://doi.org/10.1088/2043-6262/6/4/045015
Gavamukulya Y, Maina EN, Meroka AM, Madivoli ES, El-Shemy HA, Wamunyokoli F, Magoma G (2020) Green synthesis and characterization of highly stable silver nanoparticles from ethanolic extracts of fruits of Annona muricata. J Inorg Organomet Polym 30:1231–1242. https://doi.org/10.1007/s10904-019-01262-5
Ghramh HA, Ibrahim EH, Kilnay M, Ahmad Z, Alhag SK, Khan KA, Taha R, Asiri FM (2020) Silver nanoparticle production by Ruta graveolens and testing its safety, bioactivity, immune modulation, anticancer, and insecticidal potentials. Bioinorg Chem Appl. https://doi.org/10.1155/2020/5626382
Gnanajobitha G, Paulkumar K, Vanaja M, Shanmugam R, Chelladurai M, Gurusamy A, Cellapandian K (2013) Fruit-mediated synthesis of silver nanoparticles using Vitis vinifera and evaluation of their antimicrobial efficacy. J Nanostruct Chem 3:67. https://doi.org/10.1186/2193-8865-3-67
Gudikandula K, Vadapally P, Charya MS (2017) Biogenic synthesis of silver nanoparticles from white rot fungi: their characterization and antibacterial studies. Open Nano 2:64–78. https://doi.org/10.1016/j.onano.2017.07.002
Guzel R, Erdal G (2018) Synthesis of silver nanoparticles. In: Maaz K (ed) Silver nanoparticles: fabrication, characterization and applications. Intech Open, pp 1–20. https://doi.org/10.5772/intechopen.75363
Han JW, Gurunathan S, Choi YJ, Kim JH (2017) Dual functions of silver nanoparticles in F9 teratocarcinoma stem cells, a suitable model for evaluating cytotoxicity and differentiation mediated cancer therapy. Int J Nanomed 12:7529–7549. https://doi.org/10.2147/IJN.S145147
Isaac RSR, Sakthivel G, Murthy CH (2013) Green synthesis of gold and silver nanoparticles using Averrhoa bilimbi fruit extract. J Nanotech 2013:1–6. https://doi.org/10.1155/2013/906592
Ivanova N, Gugleva V, Dobreva M, Pehlivanov I, Stefanov S, Andonova V (2019) Silver nanoparticles as multi-functional drug delivery systems. In: Akhyar Farrukh M (Eds) Nanomedicines. Intech Open 71–92. https://doi.org/10.5772/intechopen.80238
Kambale EK, Nkanga CI, Mutonkole BPI, Bapolisi AM, Tassa DO, Liesse JMI, Krause RWM, Memvanga PB (2020) Green synthesis of antimicrobial silver nanoparticles using aqueous leaf extracts from three Congolese plant species (Brillantaisia patula, Crossopteryx febrifuga and Senna siamea). Heliyon 6:e04493. https://doi.org/10.1016/j.heliyon.2020.e04493
Karnezis AN, Cho KR, Gilks CB, Pearce CL, Huntsman DG (2017) The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nat Rev Cancer 17:65–74. https://doi.org/10.1038/nrc.2016.113
Krutyakov YA, Kudrinskiy AA, Olenin AY, Lisichkin GV (2008) Synthesis and properties of silver nanoparticles: advances and prospects. Russ Chem Rev 77:233–257. https://doi.org/10.1070/RC2008v077n03ABEH003751
Lakshmanan G, Sathiyaseelan A, Kalaichelva PT, Murugesan K (2017) Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: assessment of their antibacterial and anticancer activity. Karbala Int J Mod Sci 4:61–68. https://doi.org/10.1016/j.kijoms.2017.10.007
Larue C, Castillo-Michel H, Sobanska S, Cecillon L, Bureau S, Barthes V, Ouerdane L, Carriere M, Sarret G (2014) Foliar exposure of the crop Lactuca sativa to silver nanoparticles: Evidence for internalization and changes in Ag speciation. J Hazard Mater 264:98–106. https://doi.org/10.1016/j.jhazmat.2013.10.053
Lee K-S, El-Sayed MA (2006) Gold and Silver nanoparticles in sensing and imaging: Sensitivity of plasmon response to size, shape, and metal composition. J Phys Chem B 110(39):19220–19225. https://doi.org/10.1021/jp062536y
Li W-R, Xie X-B, Shi Q-S, Zeng H-Y, Sheng Y-U, Yang O-U, Chen Y-B (2010) Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol 85:1115–1122. https://doi.org/10.1007/s00253-009-2159-5
Mohammadlou M, Maghsoudi H, Jafarizadeh MH (2016) A review on green silver nanoparticles based on plants: synthesis, potential applications and eco-friendly approach. Inter Food Res J 23(2):446–463
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Muhammad R, Iqra S, Shahid RM, Bilal TM (2017) A review on green synthesis of nanoparticles and their applications. Artif Cells Nanomed Biotechnol 45(7):1272–1291. https://doi.org/10.1080/21691401.2016.1241792
Nayaka S, Chakraborty B, Bhat MP, Shashiraj KN, Dattatraya A, Pallavi SS, Muthuraj R, Halaswamy HM, Dhanyakuamara SB, Bharati K (2020) Biosynthesis, characterization, and in vitro assessment on cytotoxicity of actinomycete-synthesized silver nanoparticles on Allium cepa root tip cells. Beni-Suef Univ J Basic Appl Sci 9:51. https://doi.org/10.1186/s43088-020-00074-8
Ndikau M, Noah NM, Andala DM, Masika E (2017) Green synthesis and characterization of silver nanoparticles using Citrullus lanatus fruit rind extract. Int J Analytic Chem. https://doi.org/10.1155/2017/8108504
O’Brien MC, Bolton WE (1995) Comparison of cell viability probes compatible with fixation and permeabilization for combined surface and intracellular staining in flow cytometry. Cytometry 19(3):243–255. https://doi.org/10.1002/cyto.990190308
Ramesh PS, Kokila T, Geetha D (2015) Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochim Acta Part A Mol Biomol Spectrosc 142:339–343. https://doi.org/10.1016/j.saa.2015.01.062
Sandhya RP, Josthna P, Kousalya L, Susmila AG, Venkata SK, Varadarajulu NC (2020) Green synthesis of silver nanoparticles by leaf extracts of Boerhavia erecta and spectral characterization and their antimicrobial, antioxidant and cytotoxic studies on ovarian cancer cell lines. Lett Appl NanoBioSci 9:1165–1176. https://doi.org/10.33263/LIANBS93.11651176
Seigneuric R, Markey L, Nuyten DS, Dubernet C, Evelo CT (2010) From nanotechnology to nanomedicine: applications to cancer research. Curr Mol Med 10(7):640–652. https://doi.org/10.2174/156652410792630634
Sharma VK, Yngard RA, Lin Y (2009) Silver nanoparticles: green synthesis and their antimicrobial activities. Adv in Colloid and Interface Sci 145:83–96. https://doi.org/10.1016/j.cis.2008.09.002
Sreenivasa N, Bidhayak C, Pallavi SS, Bhat MP, Shashiraj KN, Ghasti B (2020a) Synthesis of biogenic silver nanoparticles using Zanthoxylum rhetsa (Roxb.) DC seed coat extract as reducing agent and in-vitro assessment of anticancer effect on A549 lung cancer cell line. Int J Pharm Res 12:302–314. https://doi.org/10.31838/ijpr/2020.12.03.024
Sreenivasa N, Meghashyama BP, Pallavi SS, Bidhayak C, Dattatraya A, Muthuraj R, Shashiraj KN, Halaswamy H, Dhanyakumara SB, Vaishnavi MD (2020b) Biogenic synthesis of silver nanoparticles using Paenibacillus sp. in-vitro and their antibacterial, anticancer activity assessment against human colon tumour cell line. J Environ Biol 42:118–127. https://doi.org/10.22438/jeb/42/1/MRN-1401
Ssekatawa K, Byarugaba DK, Kato CD, Wampande EM, Ejobi F, Nakavuma JL, Maaza M, Sackey J, Nxumalo E, Kirabira JB (2021) Green strategy-based synthesis of silver nanoparticles for antibacterial applications. Front Nanotechnol 3:697303. https://doi.org/10.3389/fnano.2021.697303
Swamy MK, Akhtar Mohd S, Mohanty SK, Sinniah UR (2015) Synthesis and characterization of silver nanoparticles using fruit extract of Momordica cymbalaria and assessment of their in vitro antimicrobial, antioxidant and cytotoxicity activities. Spectrochim Acta Part A Mol Biomol Spectrosc 151:939–944. https://doi.org/10.1016/j.saa.2015.07.009
Thakur S, Krishna MG, Sandhya RM (2018) Plant-mediated synthesis of silver nanoparticles-A critical review. Phyto. https://doi.org/10.25258/phyto.v9i07.11161
Veerasamy R, Xin TZ, Gunasagaran S, Xiang TFW, Yang EFC, Jeyakumar N, Dhanaraj SA (2011) Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc 15:113–120. https://doi.org/10.1016/j.jscs.2010.06.004
Wu S, Rajeshkumar S, Madasamy M, Mahendran V (2020) Green synthesis of copper nanoparticles using Cissus vitiginea and its antioxidant and antibacterial activity against urinary tract infection pathogens. Artif Cells Nanomed Biotechnol 48:1153–1158. https://doi.org/10.1080/21691401.2020.1817053
Acknowledgements
The authors of the research work greatly acknowledge the P.G. Department of Studies in Botany, Karnatak University, Dharwad for extending laboratory resources. The authors are even thankful to SAIF, Karnatak University, Dharwad for essential instrumentation facilities.
Funding
This project was supported by seed grant for research program (2021–22/62), Karnatak University, Dharwad, Karnataka, India. This project was also supported by Researchers Supporting Project number (RSP-2021/231), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
SN designed the concept and supervised the experiments. SKN wrote the manuscript and carried out characterizations and experimental analysis along with RSK, HM, PVG and BC, AIA and KP interpreted the anticancer analysis results with data validation. All the authors have no issues and approved the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Ethics approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nagaraja, S.K., Kumar, R.S., Chakraborty, B. et al. Biomimetic synthesis of silver nanoparticles using Cucumis sativus var. hardwickii fruit extract and their characterizations, anticancer potential and apoptosis studies against Pa-1 (Human ovarian teratocarcinoma) cell line via flow cytometry. Appl Nanosci 13, 3073–3084 (2023). https://doi.org/10.1007/s13204-022-02386-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-022-02386-w