Abstract
Curcumin possesses remarkable chemotherapeutic activity owing to its anti-inflammatory and anti-oxidant properties. Purpose of the research work was to prepare curcumin-loaded nanoparticles of Eudragit E 100 for topical skin applications through a simple, cost-effective method of nanoprecipitation. Resultant formulation will enhance the water solubility, permeability and bioactivity of curcumin. The particle size and morphology were investigated by dynamic light scattering and electron microscopy. The encapsulation efficiency, drug loading, in vitro drug release were determined by UV spectroscopy. In vitro drug release was conducted at acidic and neutral pH. The interaction of drug with polymers was investigated by Fourier-transform infrared spectroscopy. Ex vivo skin penetration study was conducted using vertical Franz diffusion cell to assess the potential of curcumin nanoparticles to cross the stratum corneum. In vivo evaluation was done in mice model. Results illustrated that particles were in nanometers (˂ 120 nm), with narrow size distribution, spherical in shape and with encapsulation efficiency of more than 75%. Drug release demonstrated the acid-responsive behavior of curcumin nanoparticles while ex vivo permeation and in vivo studies show prominent results of the formulation. It can be concluded from the results that curcumin nanoparticles have a potential to be designed successfully in future as a topical dosage form for the treatment of skin cancer. The present research work concludes that Eudragit E100 can fulfill the requirements related to delivery of curcumin for topical use for the local treatment of skin cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer is considered as one of the leading cause of death worldwide commonly affecting Caucasian population (Kurzrock et al. 2019). Skin melanoma was the most frequent kind of malignancy in Canada and the United States from 1970 to 2007 (Shakeri et al. 2019; Mirzaei et al. 2018). Skin cancer is defined as an elevated rate of cell proliferation and a sluggish rate of apoptosis in the epidermis of the skin (Hamzehzadeh et al. 2018; Ramirez et al. 2018). Exposure to ultraviolet (UV) radiation, as well as other potential causes, such as genetics, viruses, traumas, X-rays, and mutagens, in chemicals and food, might be the primary causes of skin cancer (Simonetti et al. 2009). Those with pale complexions are more prone to skin cancer because melanin serves to prevent UV radiation damage up to a certain limit, which is why people with dark complexions have a lower risk of acquiring skin cancer (Fang et al. 2009).
The various cancer therapy techniques each have their own set of benefits and drawbacks (Kurzrock et al. 2019; Shakeri et al. 2019). Major disadvantages associated with these chemotherapies include severe adverse effects, such as multiple drug resistance, drug efflux system, numerous drug targets, and changed pharmacokinetic behavior (Kumari et al. 2021; Kachuri et al. 2013). A variety of techniques, including new molecular carriers like as the nano-carrier system, have been used to overcome these difficulties (Wahlang et al. 2011; Chinembiri et al. 2014).
Curcumin, a natural polyphenolic molecule with a yellowish orange hue, is a nutraceutical that may be used topically (Kachuri et al. 2013). It has a powerful chemotherapeutic impact and has the potential to cure skin disorders, such as psoriasis, dermatitis, and skin cancer (Kurzrock et al. 2019; Chinembiri et al. 2014; Markman et al. 2016). Curcumin suppresses cancer proliferation, invasion, angiogenesis, and metastasis through interacting with a variety of cell signaling proteins (Maeda et al. 2009).
One method of non-invasive medicine administration is the topical drug delivery system. It entailed a collection of technologies used to transfer therapeutically active moieties to superficial regions of skin, eyes, nose, and vagina for the treatment of local illnesses (Kunnumakkara et al. 2008; Chen and Fang 2000). Skin is one of the biggest organs in the body, and its tasks include body temperature control, defense against ultraviolet radiations (UV), and protection against the entry of hazardous materials and microorganisms from the external environment (Lee et al. 2006; Taveira and Lopez 2011). The stratum corneum (SC), or outer layer of skin, serves a barrier function that not only prevents microorganisms from entering but also prevents the penetration of a wide range of medicines. Topical drug delivery required well-designed formulation to enhance its penetration into thick layers of SC and also the deep layers of skin where ailment exists (Tomren et al. 2007; Maeda et al. 2009). In a recent study, their results reinforce Curcumin’s established anti-inflammatory effects in the skin and highlight its potential as a photo-protective adjuvant when delivered through nanoparticles (Adusumilli et al. 2021).
In this study, we used a precipitation method to load curcumin into an amino methacrylate copolymer (Eudragit E 100, (EuE)). Curcumin is a hydrophobic molecule that is nontoxic, non-sensitizing, and has a broad safety profile (Simonetti et al. 2009; Xie et al. 2018). It has limited solubility and permeability, making it a BCS class IV medication, and it is very unstable when exposed to high temperatures, light, and oxidative conditions (Priyadarsini 2014). It has a fast first-pass impact and is eliminated quickly after oral treatment, resulting in poor absorption and low bioavailability due to the breakdown of the, unsaturated di-ketone moiety found in the curcumin structure. (Jantarat 2013). Curcumin's difficulties might be overcome utilizing innovative pharmaceutical technology, using chemical penetration enhancers, and creating an alternate drug delivery mechanism other than the oral route (Ahmadi Nasab et al. 2018) or the first time, a combination of the polymer Eudragit E 100 and the surfactant polyvinyl alcohol (PVA) was utilized in the nano-precipitation technique to create curcumin nanoparticles. Because of their greater surface area and high penetration over the skin, nanoparticles may preserve drugs from degradation and improve their entry into tumor sites. HPMC gel is superior to other because hydroxypropyl methylcellulose (HPMC) has good stability even after exposure to heat and in humid conditions will not experience significant changes observed in homogeneity, pH, clarity, texture profile analysis and rheological properties of HPMC gels. HPMC gel bases are often used in the production of cosmetics and drugs because they can produce gels that are clear, are easily soluble in water, and have low oxidation properties. In addition, HPMC produces a neutral gel, clear, colorless, stable at pH 3–11, has good resistance to microbial attack, and provides good film strength when it dries on the skin. The result of previous studies mentions that the base HPMC has a good rate of drug release, and widespread power (Oktay et al. 2020).
Materials and methods
Materials
Curcumin powder was obtained from Sami Lab Limited (India). Eudragit E 100 (EE100) was kindly provided by Evonik (Pakistan). Poly-vinyl alcohol (PVA), monobasic potassium dihydrogen phosphate and sodium acetate were purchased from Sigma-Aldrich (Germany). Ethanol and methanol were obtained from BDH laboratory supplies (England). Hydroxyl propyl methyl cellulose (HPMC) was purchased from Anhui Sunhere Pharmaceutical excipients Co. Ltd. (China).
Preparation of EE–PVA blank and curcumin-loaded nanoparticles
The blank and curcumin nanoparticles were prepared by nanoprecipitation method described in the literature with minor modification (Lee et al. 2006). First, 0.5% PVA solutions were prepared in water and 40 ml of this solution was taken in a beaker and placed it for continuous stirring on a hot plate at room temperature and at 650 rpm. Then the internal organic-phase solution containing 50 mg of curcumin and 200 mg of Eudragit E 100 dissolved in 10 ml of ethanol were taken in a syringe and injected dropwise into PVA solution resulting in the formation of nanoparticles. The organic phase was further evaporated in open air. Then the preparation was centrifuged at 5525 g to obtain the nanoparticles pellet. For ease of application of formulation on skin, nanoparticles were dispersed into HPMC gel. A gel containing 2% HPMC was prepared in water separately and curcumin nanoparticles were dispersed into it. To prepare blank nanoparticles, the rest of process was same as described above except that the organic phase does not contain drug (curcumin).
Characterization of nanoparticles
The particle size of blank and curcumin-loaded nanoparticles was determined by dynamic light scattering (DLS). The formulation was diluted tenfold with milli-Q water and placed in a cuvette for particle analysis. Polydispersity index, an indicator of size distribution, and zeta potential of formulation were also measured performing the experiment in triplicate. The morphological characteristics of nanoparticles were observed by scanning electron microscopy (SEM) performed on liquid samples. The dilution of samples was made similarly as done for the determination of particle size by DLS.
Fourier-transform infrared spectroscopy
The samples of the pure drug (curcumin), the blank and drug-loaded formulation were analyzed using FTIR and the spectra obtained over the scanning range of 4000–500 cm−1were recorded and interpreted for possible drug–polymer interaction.
Process parameters
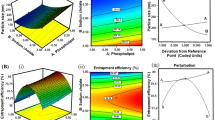
The curcumin nanoparticles were optimized on the basis of different process parameters. The concentration of PVA (%), injection time (min), stirring rate (rpm), and ratio of organic to aqueous phase (keeping organic phase constant and increasing the aqueous phase only) were selected to be studied as process parameters (Tang et al. 2011; Araki et al. 2020). These parameters were analyzed on the basis of particle size and physical stability. In each set of experiment, only one variable of the formulation was changed at a time while the other parameters were kept constant (Tang et al. 2011). For this purpose, more than 20 formulations were prepared rendering at least five for each parameter. The following protocol modifications as shown in Table 1 were used to study the effect of various process and preparative variables for respective formulations. Sample of 5 ml was prepared for each formulation and particle size was determined by DLS. The effect of each process parameter on the physical stability was assessed visually. Here, the physical stability represents the condition of formulation for 1 week after preparation. If there is formation of precipitates, cakes or separation of phases occurs, then that formulation is considered as “unstable” while formulation which remains stable and uniform for more than a week is considered as “stable”.
Loading capacity
Loading capacity of the formulation was calculated using an established procedure (Tomren et al. 2007). Accordingly, nanoparticles were dissolved in 20 ml of methanol and stirred continuously for drug extraction. From this solution, 1 ml of solution was transferred to volumetric flask and final volume was made up to 50 ml with methanol. Absorbance was measured using UV–visible spectrophotometer at 426 nm. The concentration of curcumin was determined from a standard calibration curve of curcumin in methanol using formula (Priyadarsini 2014; Xie et al. 2018).
Encapsulation efficiency
To determine the percent encapsulation efficiency, 10 ml of nano-formulation was centrifuged at 13,500 rpm for 15 min. The supernatant was collected, diluted accordingly and absorbance was measured by UV–visible spectrophotometer at ƛmax of 426 nm. The concentration of curcumin was determined from the standard calibration curve of curcumin in methanol. The percentage encapsulation efficiency is calculated using the formula below (Priyadarsini 2014).
In vitro drug release
The release of curcumin from the nanoparticles was determined at pH 4.5 and pH 7.4 according to the method described previously with minor modifications (Tang et al. 2011). Briefly, 7 mg of curcumin nanoparticles equivalent to 1.75 mg of curcumin was dispersed into 50 ml of 0.2 M sodium acetate buffer (pH 4.5), because in cancerous lesions, pH of the skin became more acidic (Sahu et al. 2019) and 0.2 M monobasic potassium dihydrogen phosphate buffer (pH 7.4) separately in a beaker. Both buffers contained 0.1% Tween 80 to prevent the settling down of particles at the bottom. Beakers were provided with a magnetic bar and placed on a magnetic stirrer at low rpm of 100 and temperature of 37 ± 2 °C. The whole experiment was carried out under dark condition. At pre-determined time intervals of 0.5, 1, 2, 4, 6, 12, 24 and 48 h, 2 ml samples were withdrawn from each beaker. Equal volume of the fresh dissolution medium was added to the beaker after each sample withdrawal to keep the volume constant. Samples were centrifuged and assayed spectrophotometrically at a wavelength of 426 nm (Sahu et al. 2019). The concentration of drug released was calculated from the linear regression equation obtained for the calibration data.
Determination of best-fit kinetic release model
To determine the best-fit kinetic model, release data of curcumin nanoparticles at pH 7.4 were fitted to zero-order Kinetics, first-order kinetics, Hixson–Crowell’s cube root equation, Higuchi’s square root of time equation (diffusion model) and Korsmeyer–Peppas model, and equation of each model is given below.
Zero-order Kinetics (Xu and Sunada 1995)
First-order Kinetics (Xu and Sunada 1995)
Hixson–Crowell’s cube root equation (erosion model) (Xu and Sunada 1995)
Higuchi’s Square Root of Time Equation (diffusion model) (Higuchi 1963)
Korsmeyer–Peppas Kinetic (Ritger and Peppas 1987)
In the above equations, W represents percent drug release at time t, k1 − k4 are release rate constants, depending on the kinetic model used. Fractional drug released into the dissolution medium is presented by Mt/M∞ and k5 is a constant incorporating structural and geometric characteristic of the formulation. The parameter, n, represents the diffusion exponent that characterizes the drug release transport mechanism. When the value of n = 0.5, it means the drug diffuses through and is released from the polymeric matrix with a quasi-Fickian diffusion mechanism. When n > 0.5, anomalous, non-Fickian drug diffusion occurs while when n = 1, a non-Fickian, Case II or zero-order release kinetics occurs. All these analyses were performed by applying multiple linear regression analysis using online excel formula sheet.
Determination of pH, viscosity spreadability and physical appearance
The pH of curcumin nanoparticles-loaded HPMC gel was measured using digital pH meter at room temperature. The formulation was inspected visually for physical appearance (Papadimitriou and Bikiaris 2009). Viscosity was measured by Brookfield viscometer. Spreadability was found by procedure of Ahad and his followers using following equation (Ahad et al. 2016).
where A1 = 2 cm, and A2 = area after spreading.
Drug content of gel
Drug content of curcumin nanoparticles-based gel was calculated using an established procedure (Liu et al. 2012). Accurately weighed amount of gel (0.5 ml) was dissolved in about 10 ml methanol and stirred continuously for drug extraction. The solution of 0.5 ml was taken and appropriate dilution was made with methanol. The solution was filtered before subjecting to the analysis by spectrophotometer (Patel et al. 2009a; b). Drug content was calculated from the linear regression equation obtained for the calibration data.
Stability study of nano-formulation
The optimized formulation F2 was selected to investigate the stability of nanoparticles against environmental changes. To check the effect of temperature on the particle size, encapsulation efficiency and physical stability of the curcumin, the formulation was exposed to two types of temperature conditions, 5 ± 2 °C and room temperature 25 ± 2 °C. The stability study was carried out for duration of 3 months. The formulation was taken into two separate glass vials, one stored at 5 ± 2 °C and the other at 25 ± 2 °C. Samples for particle size were analyzed by DLS. Encapsulation efficiency was determined by UV spectrophotometer. Physical stability was examined by visual inspection.
Ex vivo skin permeation study
The ex vivo permeation study was carried out in Vertical Franz diffusion (FD) cell mounted with the abdominal skin of rat (Krishnakumar et al. 2011). First, the rat was killed and hairs on the dorsal surface of skin were removed using the razor. The skin was thoroughly washed with phosphate buffer (pH 7.4), wiped with a cotton swab and dried. Then skin was separated from the underlying cartilage and subcutaneous fats, cut into appropriate sizes and stored at − 22 °C after wrapping into aluminum foil (Xu and Sunada 1995).
The skin was sandwiched between donor and acceptor compartment of the FD cells with the cross-sectional area of 0.77 cm2. Stratum corneum was faced toward donor compartment. Two cells were used with one contained the sodium acetate buffer of pH 4.5 and the other contained the phosphate buffer of pH 7.4. The receptor compartment has the volume capacity of 5.2 ml and the temperature of receptor phase was maintained at 32 °C by circulating water jacket (Higuchi 1963). The magnetic stirrer gently stirred the acceptor fluid. The optimized formulation of curcumin nanoparticles loaded into HPMC gel of 0.5 ml was spread uniformly on the stratum corneum side of the skin (Ritger and Peppas 1987). After predetermined time interval, aliquots of 0.5 ml were withdrawn periodically and replaced with an equal volume of buffers to maintain the sink conditions. The whole experiment was run for 24 h and samples were taken at 1, 2, 4, 6, 10 and 24 h time interval. Samples were analyzed spectrophotometrically at the wavelength of 426 nm. The concentration of drug permeated from each buffer was calculated from the linear regression equation obtained for the calibration data.
Stability of curcumin nanoparticles-loaded HPMC gel
The stability study of the gel of optimized F2 formulation was performed according to the method reported previously (Higuchi 1963). Preliminarily, the curcumin nanoparticles-loaded gel was subjected to centrifugation at 3000 rpm for 30 min. Then the gel was subjected to thermal stress cycles alternating 24 h in the oven at 40 ± 2 °C and for 24 h in a refrigerator at 5 ± 2 °C. The gel was characterized for pH and phase separation at the end of each cycle.
Development of chemically induced skin papilloma in Balb/c mice
For induction of skin tumors, a two-step chemical induction method is adopted. Eight-week-old Balb/c mice were shaved. Mice with resting hair cycle were applied with 7,12-Dimethylbenz[a] anthracene (DMBA, 100 µl/50 µl of acetone) on clear area. Two weeks later, croton oil (1% in 100 µl of acetone) was applied at the area, thrice a week for 3 weeks. Skin papilloma (2–3 per mice) appeared after 3 weeks of application of croton oil. Mice were divided into group of three. Untreated group is not provided with any treatment. Control negative group and treated group were applied with 10 µl blank and curcumin-loaded nanoparticles twice a day, respectively, for 6 weeks, where all mice in the non-treated group have been dead. Tumor volumes were measured with digital Vernier caliper. Survival is illustrated as Kaplan–Meier graph with GraphPad Software 6.0, presenting cumulative survival percentage per week in different experimental groups (Yallapu et al. 2015).
Statistical analysis
In vitro drug release profiles and ex vivo skin permeability obtained for curcumin nanoparticles at both the pH values were compared statistically by applying T test using MS Excel. The result of the test is indicative of whether a significant difference exists between two pH values or not. Value of p < 0.05 was considered as significant.
Results
Process parameters
The nanoprecipitation method employed for the nanoparticles fabrication is very sensitive to changes in the composition (Prajakta et al. 2009). The effect of different process parameters was assessed on the physical stability and particle size for optimization purpose and an optimized formulation (F2) was obtained as clear from data in Table 1. Stabilizers play an important role in maintaining the stability of formulations (Patel et al. 2009a, b; Huang et al. 2016). The nature and the concentration of stabilizer are principal factors in asserting the stability to a formulation. PVA is a high molecular weight polymer and is commonly used as a stabilizer in the formulation of oil-in-water nano-emulsion (Agarwal et al. 2001; Tzeng et al. 2011). Five samples with different concentration of surfactant were prepared i.e., 0.25, 0.5, 0.75, 1, and 1.5%. It can be seen from the results in Fig. 1 that very low and higher concentration of PVA has a prominent effect on the physical stability and particle size. The size of particles is small with low concentration of surfactant but settles down with the passage of time. Higher concentration of PVA leads to the formulation of micro-particles (Hyvönen et al. 2005; Lamprecht et al. 1999).
The effect of aqueous phase on the stability and particle size was analyzed. Various ratios of aqueous phase (3, 4, 5, 6, 7 ml) were selected with a constant volume of organic phase (1 ml) for the preparation of nanoparticles. It is cleared from the Fig. 2 that with increasing volume of the aqueous phase, the particle size is also increased. On the basis of above data, the volume of 4 ml was selected as an optimum aqueous phase volume (Lee et al. 1999).
The rate at which the organic phase is incorporated into aqueous phase is a critical parameter for determining the size of nanoparticles. It was evaluated in terms of time used to inject the whole organic phase. The injection times of 2.30, 3, 3.30, 4, 4.30 min were chosen and 1 ml of the organic phase was injected into aqueous phase at the prescribed time. It is evident from the Fig. 3 that the slow injection rate (higher time) gives smaller nanoparticles as compared to fast injection rate (less time). Small sized particles were formed as the injection was completed in 4.30 min for injecting 1 ml while a large size particle was fabricated with consuming less time. Injection speed of 1 ml in 4.30 min was selected as an optimum injection time in formulating nanoparticles present in formulation F2.
Stirring rate is the also one of the important parameters to form a homogenous mixture and to provide sufficient amount of energy to a system during formation of nanoparticles (Yang et al. 2001). The samples were prepared at different rpm. Five speeds were selected 600, 650, 700, 750, and 800 rpm. As shown in Fig. 3, stirring speed has no prominent effect on the size up to certain limits, but at 800 rpm, the particle size is increased. The speed of 650 rpm was selected as an optimum stirring speed.
Morphology and size of nanoparticles
The particle size of blank and curcumin-loaded nanoparticles (formulation F2) determined by DLS was found to be well in nano range (Maeda et al. 2009) with 92 and 98 nm respectively as shown in Fig. 4. The increase in the size of curcumin nanoparticles can be attributed to the entrapment or loading of drug in the polymeric nanoparticles as compared to blank. SEM analysis was done to elucidate the morphological characteristics of nanoparticles (Patel et al. 2009a; b). It is clear from Figs. 4 and 5 that the particles are in the range of 86–110 and 93–153 nm for blank and curcumin-loaded formulations, respectively. The particles are also in spherical shape with uniform distribution.
Polydispersity index and zeta potential
PDI was performed in triplicate of F2 formulation and results are presented in mean and ± SD i-e, 0.180333 ± 0.0015. The values indicated that particles are homogenous and uniform in size. Zeta potential of formulation was found to be + 18.23 mV. The charge on nanoparticles is positive owing to cationic nature of EuE 100 (Agarwal et al. 2001).
FTIR analysis
The FTIR spectrum of pure curcumin showed a characteristic peak at 3451.76 cm−1 corresponding to phenolic OH groups stretching vibration, 2925.18 cm−1 for C–H stretching vibration and for carbonyl group (C=O) and an aldehyde group (C–OH) stretching vibration at 1745.63 cm−1 and 1160.61 cm−1, respectively, in Fig. 6. The FTIR spectrum of curcumin-loaded EuE100 nanoparticles almost maintained their C=O, COH and CH peak at 1746.01 cm−1, 1162.60 cm−1 and 2929.16 cm−1 respectively. There exists a characteristic shift in the peak of phenolic OH group from 3451.76 to 3403.04 (Cristina et al. 2015).
Drug loading and encapsulation efficiency
Drug loading and encapsulation efficiency are calculated by indirect methods for the calculation of drug content in the polymer. Encapsulation efficiency refers to the percentage of drug entrapped with respect to total amount of drug added during the preparation of nanoparticles. These are the major factors affecting the drug release and absorption (Mora-Huertas et al. 2011). Formulation F2 has optimized having loading capacity and encapsulation efficiency of curcumin-loaded nanoparticles was found to be 68 ± 5.58% and 81.3 ± 0.08%, respectively.
In vitro drug release profile
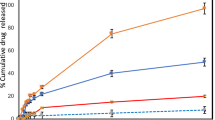
In vitro drug release of curcumin nanoparticles (F2) was performed at acidic and neutral pH 7.4 to assess the impact of pH on the release of curcumin from EuE100 loaded nanoparticles. Tween 80 (0.1%) was added to each solution to prevent the settling of particles at the bottom. The drug is also released in a sustained manner at neutral pH. The release profile depicted in Fig. 7 shows more prominent release at acidic pH as compared to neutral pH value. The release studies were monitored for 48 h.
Curcumin nanoparticles at pH 4.5 have high drug release that is 70% within the first half hour as compared to release at pH 7.4 which is only 18%. It is seen from the data, more than 87% curcumin is released within 24 h when nanoparticles were exposed to 4.5 pH, allowing a better release in an acidic condition of cancer. Release follows the trend of time dependent pattern. However, at the same time interval, only 55% drug is released at neutral pH. The enhanced release is due to the ionization of amino group of EuE100 polymer which quickly solubilized in lower pH and exhibited a fast release (Mangalathillam et al. 2012). Release profile of HPMC gel was also shown in the Fig. 7B that demonstrated almost 35% drug released from gel at 7.4 pH and 60% at pH 4.5 in first 12 h, also follows the same pattern as displayed in nanoparticles release. Statistical analysis by applying T test on release data of curcumin nanoparticles at both the pH values revealed p value of 0.00077 which is < 0.05. It means that there is a significant difference between the drug release patterns of curcumin at two pH values.
Determination of best-fit kinetic release model
To determine the release mechanism of curcumin from nanoparticles, the release data of curcumin were fitted into different mathematical release kinetic models (zero order, first order, Higuchi, Korsmeyer–Peppas and Hixson–Crowell) to illustrate the kinetics of drug release. Drug release at neutral pH was best fitted into Higuchi kinetics with a maximum correlation (R2) value of 0.947 which shows that the nanoparticles follow diffusion mechanism. Hence, it can be concluded that the release mechanism of curcumin from nanoparticles is diffusion.
Determination of best-fit kinetic release model
To determine the release mechanism of curcumin from nanoparticles, the release data of curcumin were fitted into different mathematical release kinetic models (zero order, first order, Higuchi, Korsmeyer–Peppas and Hixson–Crowell) to illustrate the kinetics of drug release. Drug release at neutral pH was best fitted into Higuchi kinetics with a maximum correlation (R2) value of 0.947 which shows that the nanoparticles follow diffusion mechanism. Hence, it can be concluded that the release mechanism of curcumin from nanoparticles is diffusion.
Determination of pH and physical appearance of gel
The pH value of curcumin nanoparticles-loaded gel found to be 7.01 ± 0.0057 which is suitable for application to skin. The physical appearance and color of blank and curcumin-loaded formulation of nanoparticles were inspected visually. It was found that the blank formulation is opalescent in nature, whereas the curcumin nano-formulation was dark red in color, while that of gel is orange. For application on the skin, curcumin nanoparticles were loaded into HPMC gel to enhance the viscosity of nanoparticles. Viscosity studies were carried out at room temperature by Brookfield viscometer and results are showing shear thinning effect of gel. Spreadability of curcumin nanoparticles-loaded gel was 80 times less than the plain gel of curcumin due to compact packing of nanoparticles.
The curcumin nanoparticles were loaded into HPMC gel in 1:1 ratio. HPMC has been reported as a thickening agent previously.
Drug content of gel
Drug content of optimized formulation of curcumin nanoparticles-based gel was found approximately to be 90% (4.5 mg/5 mg in HPMC gel). Hence, it can be concluded that the HPMC gel has a high ability to entrap the loaded drug.
Stability study of nanoformulation
The stability study of nanoformulation F2 was performed at 5 ± 2 °C and 25 ± 2 °C for 3 months to investigate the effect of temperature on the particle size, encapsulation efficiency and physical stability. The results presented in Table 2 showed that at temperature of 5 ± 2 °C, the size of particles was 115 nm. The size of particles is also increased at room temperature from 98 to 120 nm. Encapsulation efficiency is also decreased at both temperature, but formulation was stable physically.
It is evident from the results that temperature has pronounced effect on particle size and encapsulation efficiency. Particle size is increased and encapsulation efficiency is reduced after 3 months. It might be due to aggregation of particles or degradation of polymers as reported in earlier studies (Alves et al. 2017).
Ex vivo skin permeation study
Permeability studies for the topical treatment of skin cancer reported in the literature previously are mostly performed at pH 4.5 (Rachmawati et al. 2015) and 6.5 (Zafar et al. 2019) where DMSO is as control solution of curcumin. But in the present permeability study, potassium dihydrogen phosphate buffer of pH 7.4 was considered as a control just to assess the permeation at normal condition of body.
Permeability study was performed at two pH conditions, i.e., 7.4 and 4.5. The samples were collected at different time intervals and the experiment was carried out for 24 h. The results are presented in µg/cm2. It is clear from Fig. 8 that curcumin nanoparticles did not cross the skin during the first 2 h. After 3 h, the drug started to release, showed high penetration at pH 4.5 that is 16 µg/cm2 and 7.729 µg/cm2 at 7.4 pH. At 24th h, the drug exhibited 145.707 and 45.53 µg/cm2 permeation at 4.5 and 7.4 pH values, respectively. The penetration of curcumin at acidic pH (4.5) is 3.2-fold higher that the penetration at neutral pH value.
The possible reason is the cationic charge and the hydrophobic nature of the polymer, the two important factors responsible for increasing the skin permeation (Govender et al. 2000). The cationic polymer interacts with negatively charged skin lipid and enhances the penetration. Hence, it can be suggested from the study that curcumin, nanoparticles-based gel could be a possible option for the topical application of skin cancer (Mora-Huertas et al. 2011). Statistical analysis using T test on permeability data revealed p value of 0.002898 which is less than p value of 0.05. It shows significant difference between the permeability patterns of drug at both pH values.
Stability of curcumin nanoparticles-loaded HPMC gel
The data of stability study of prepared gel show that the curcumin nanoparticles-loaded HPMC gel did not undergo phase separation when subjected to mechanical stress at 3000 rpm for 30 min (Table 2). Formulation remains light orange color. The pH had undergone minor changes at the end of each thermal cycle of 24 h at 40 and 5 °C.
In vivo therapeutic efficacy of curcumin-loaded nanoparticles
In vivo therapeutic efficacy of curcumin-loaded nanoparticles is realized against chemically induced skin papilloma in Balb/c mice. Post application, tumor growth is presented in Fig. 9A. Results indicated the tumor growth suppression on topical application of curcumin nanoparticles. The untreated and blank nanoparticles-treated tumors continue to grow for the next 2 weeks and the tumor volume then remained static. Whereas application of curcumin-loaded nanoparticles led to the rapid regression in the tumor volume owing to the effectiveness of direct drug application and efficient skin permeation. Application of blank nanoparticles or curcumin-loaded nanoparticles on non-tumor bearing skin did not show any irritation or allergic response.
All mice survived for about 2 weeks of the papilloma appearance. At 2 weeks and onwards, the papilloma mice without any treatment showed an average 20% death rate each week (Fig. 9B). The overall survival rate was similar in the mice treated with blank nanoparticles. Mice with curcumin-loaded nanoparticles however survived till sixth week of the post-application. However, these changes were not significantly different than the pH of gel initially made. The overall picture for development of skin cancer is shown in Fig. 10.
Discussion
The research is centered on the creation, formulation, and assessment of curcumin nanoparticles. The nanoparticles-based topical medication delivery system is a potential strategy for the effective treatment of skin cancer because it combines the benefits of a nano-sized carrier system with topical administration. The study's premise claimed that curcumin nanoparticles at the acidic pH of malignant cells would increase release and enable targeted delivery at the designated location. A positively charged polymer such as EuE100 was used for this purpose. EuE100 was chosen as a polymer because of its acidic-dissolving characteristics, i.e., it is soluble at pH 5 and swellable above pH 5. When it reaches the acidic pH of the skin cancer, it dissolves quickly and provides targeted medication release at that location. PVA, which was employed as a surfactant, was used to keep the nanoparticles stable.
Because of its presence at the interface between the organic and aqueous phases, surfactant incorporation in the formulation decreases interfacial tension, thereby stabilizing the formulation and reducing particle size. (Krishnakumar et al. 2011). Furthermore, the polymer-and-stabilizer combination provides a twofold impact to the composition. This combination not only synthesizes and stabilizes nanoparticles, but it also has the inherent ability to improve medication penetration through the skin (Xu and Sunada 1995).
Curcumin is a chemotherapeutic agent due to its anti-inflammatory and anti-oxidant properties. It suppresses the proliferation, invasion, angiogenesis, and metastasis of many malignancies by interacting with numerous cell signaling proteins (Govender et al. 2000). It was chosen because of its poor solubility and permeability, classification as a BCS class IV medication (Simonetti et al. 2009) and high instability when exposed to high temperatures, light, and oxidative conditions (Maeda et al. 2009). It has a fast first-pass impact and is quickly eliminated after oral administration, resulting in poor absorption and low bioavailability. Topical drug delivery is an alternate drug delivery method, chemical penetration enhancers solve permeability problems, and nanostructures such as polymeric nano-emulsions result in the production of curcumin nanoparticles with high permeability (Lee et al. 2006). The polymer coating on the surface of curcumin assists in its resistance to environmental conditions. Topical curcumin administration might also improve patient compliance and acceptance of treatment.
Furthermore, curcumin nanoparticles were produced mechanically using a hotplate at a low speed (650) and a polymer mixture of EuE100-PVA. This is also a simple approach with fewer stages that is used to create nanoparticles. The measurement of particle size is a crucial stage in characterization because it determines particle solubility and permeability through the cell membrane. The increased size of curcumin nanoparticles can be ascribed to drug entrapment or loading in polymeric nanoparticles as opposed to plain blank. The morphology of nanoparticles is an essential feature for predicting particle transport across the cell membrane. The results show a spherical shape with a homogeneous distribution.
The particle density index (PDI) is an essential metric for determining particle homogeneity and uniformity. It is a dimensionless measure of particle size range. The wide dispersion of nanoparticles makes it challenging for them to overcome the biological barrier and generate a pharmacological impact (Mangalathillam et al. 2012). The results showed that the particles are homogeneous and uniform in size. Because EuE 100 is cationic, the charge on nanoparticles is positive. The FTIR analysis was used to study the intermolecular interaction of polymers (EuE100-PVA) with the medication to assess their compatibility. The FTIR data suggest that the phenolic OH group of curcumin may be involved in hydrogen bonding with the polymer complex. The presence of intermolecular hydrogen bonding most likely leads to the improved water solubility of curcumin nanoparticles. As a result, the EuE100-PVA complex serves as a viable polymer combination for encapsulating curcumin and forming stable nanoparticles throughout the manufacturing of nano-precipitation process (Ibraheem et al. 2015).
For optimization purposes, the impact of various process parameters on physical stability and particle size was evaluated. The role of the stabilizer in the stability of formulations is critical (Yallapu et al. 2015). The nature of the stabilizer and the concentration of the stabilizer are the most important elements in determining the stability of a formulation. PVA is a polymer with a high molecular weight that is widely employed as a stabilizer in the preparation of oil-in-water nano-emulsion. Micro-particles are formed when the concentration of PVA is increased (Yallapu et al. 2015). This might be because the aqueous phase's viscosity rose above a specific limit, causing particles to become bigger and precipitate, resulting in physical instability (Syng-ai et al. 2004).
Particle size is also affected by phase volume. Because it is difficult for the organic phase to evaporate from the formulation when there is a large volume of aqueous phase, particle size rises. This causes particle coalescence and increases particle size. The rate at which the organic phase is integrated into the aqueous phase is a crucial parameter for defining the size of nanoparticles, and it was assessed in terms of the time required to incorporate the organic phase into the aqueous phase. Slow injection (longer time) was used to create small particles, whereas quick injection (shorter time) was used to create larger particles. This may be seen as the organic phase's contact time with the aqueous phase increasing as it is slowly injected, resulting in tiny particles (Tzeng et al. 2011).
Stirring rate is another essential element in forming a homogeneous mixture and providing enough energy to a system during nanoparticle production. Large particles are broken down into little particles due to the rapid churning speed. In the current study, the converse is true, with low rpm producing tiny particles compared to high rpm. It might be because the contact period of the organic phase with the aqueous phase is shorter at high speeds, resulting in the production of big particles. An optimal stirring speed of 650 rpm was chosen. The loading capacity and encapsulation efficiency of curcumin-loaded nanoparticles were reported to be 68.558 and 81.30.08%, respectively (Tzeng et al. 2011).
The release of drugs is greater in an acidic environment than in a neutral one, which is significant for two reasons. For starters, curcumin degrades rapidly at and above neutral pH, preventing it from having any effect on normal cells. Second, due to the acidic environment of the tumor, site-specific medication release provides greater drug release at the desired location. The pH-dependent release of curcumin nanoparticles would be a significant advantage in the clinical application of curcumin nanoparticles for malignant tissues (Oungbho and Müller 1997; Tzeng et al. 2011). Statistical examination suggests that greater of curcumin nanoparticles at both pH levels indicated a p value of 0.00077, which equals 0.05. It indicates that there is a substantial variation in curcumin controlled release patterns at two pH levels.
Curcumin releasing data were fitted into several quantitative release kinetic models (zero order, first order, Higuchi, Korsmeyer–Peppas, and Hixson–Crowell) to show the kinetics of release of drug to determine the mechanism of curcumin release from nanoparticles. The results demonstrate that the (Lamprecht et al. 1999) range may be easily matched to skin, whereas the orange hue of the gel is attributable to curcumin. The drug content of the improved formulation of curcumin nanoparticles-based gel demonstrated that the HPMC gel has a strong capacity to entrap the loaded medication. At different temperatures, the particle size and encapsulation effectiveness of the formulation were evaluated. After 3 months, particle size rose, but encapsulation efficiency decreased. As previously documented in research, it might be related to particle aggregation or polymer breakdown (Lamprecht et al. 1999).
Curcumin penetration is 3.2 times greater at acidic pH values than at neutral pH values. The cationic charge and the hydrophobic property of the polymer, both of which are key variables in enhancing skin penetration (Oungbho and Müller 1997). The cationic polymer interacts with negatively charged skin lipids to improve penetration. As a result of the study, it can be concluded that curcumin nanoparticles-based gel might be a viable alternative for the topical treatment of skin cancer. The findings from the stability analysis of produced gel show that the pH had experienced small variations that were not substantially different from the pH of the gel when it was first created (Yadav and Sawant 2010). Curcumin is a pharmacologically active phytopolyphenol with anti-inflammatory, antioxidant, and anti-metastatic properties (Yallapu et al. 2015). Figure 9A shows antineoplastic capabilities by reducing tumor development and destructing tumor through different methods, such as blocking signaling pathways, growth factors, transcription factors, and pro-inflammatory cytokines, that are crucial for tumor growth and proliferation (Esfandiarpour-Boroujeni et al. 2017).
Curcumin has already been used as an anti-cancer drug in several pilot trials with human participants, demonstrating that it is well tolerated and safe to use even at large dosages. It has been shown to be effective against prostate cancer, breast cancer, high-grade squamous intraepithelial neoplasia, leukoplakia, stomach cancer, and colon cancer. Despite the numerous therapeutic advantages of curcumin, its use is hampered by low solubility, bioavailability, physicochemical instability, and fast degradation in biological systems (Yavarpour-Bali et al. 2019) Nano-mediated curcumin delivery is developing as a unique and successful approach that increases the aqueous solubility and permeability. This allows for its targeted administration at a specific dosage to the site of action (Esfandiarpour-Boroujeni et al. 2017; Yavarpour-Bali et al. 2019).
Curcumin-loaded nanoparticles were used in this work to combat chemically induced cutaneous papilloma in Balb/c mice. These nanoparticles were topically administered to the location of a skin tumor, which provides a significant benefit since it combines the benefits of both a nano-sized carrier system and a topical method of administration. The mice were split into three groups: untreated, negative control and treated, with 10 l of blank and curcumin-loaded nanoparticles administered twice daily for 6 weeks. The first two groups of mice, who received no therapy and were treated with blank nanoparticles, had tumor development for two and a half weeks, after which the tumor volume remained unchanged. Curcumin-loaded nanoparticles, on the other hand, exhibited substantial decrease in tumor development after 6 weeks due to efficient transport and increased permeability to the tumor site (Ombredane et al. 2021).
All mice lived for around 2 weeks after the tumor appeared. After 2 weeks, the untreated mice died at a rate of 20% per week. Similarly, animals treated with blank nanoparticles had a comparable survival rate, and by the end of 6 weeks, all mice in these two groups had died. In comparison, mice treated with curcumin-loaded nanoparticles had a 100% survival rate until the fifth week, and the majority of the mice in this group lived even beyond the sixth week, as seen in the graph.
This study demonstrates the efficacy of curcumin nano-formulation for treating Cutaneous Pappillomas due to its targeted administration and improved bioavailability to the site of action (Ghosh et al. 2021). Furthermore, this study underlines the prospective function of curcumin in the treatment of different human diseases, as well as the future potential of curcumin nano-formulation in the field of medicine and health care.
Conclusion
The study's assumption was that curcumin nanoparticles at the acidic pH of malignant cells would enhance release and allow for targeted administration. Curcumin is a chemotherapeutic drug owing to its anti-inflammatory and anti-oxidant effects. It inhibits the proliferation, invasion, angiogenesis, and metastasis of several cancers. Curcumin nanoparticles were created mechanically using a hotplate at a low speed (650) and a polymer combination of EuE100-PVA. PVA is a polymer with a high molecular weight that is widely employed as a stabilizer in the preparation of oil-in-water nano-emulsion. The pace at which the organic phase is integrated into the aqueous phase is an important element in determining the size of nanoparticles. Slow injection (longer time) was used to generate microscopic particles, whereas rapid injection (shorter time) was used to create bigger particles. The optimum stirring speed was determined to be 650 rpm. Curcumin is a pharmacologically active phytopolyphenol having anti-inflammatory, antioxidant, and anti-metastatic effects. It has been proven to be useful against prostate cancer, breast cancer, high-grade squamous intraepithelial neoplasia, leukoplakia, and other types of cancer. The cationic polymer interacts with negatively charged skin lipids to enhance penetration. Curcumin has numerous medicinal characteristics; however, its use is hindered by poor solubility, bioavailability, physicochemical instability, and rapid degradation in biological systems. Nano-mediated curcumin delivery is emerging as a novel and effective method for delivering curcumin to the site of action. These nanoparticles were applied topically to a skin malignancy.
References
Adusumilli NC, Mordorski B, Nosanchuk J, Friedman JM, Friedman AJ (2021) Curcumin nanoparticles as a photoprotective adjuvant. Exp Dermatol 30(5):705–709
Agarwal R, Katare O, Vyas S (2001) Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int J Pharm 228:43–52
Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M (2016) The ameliorated longevity and pharmacokinetics of valsartan released from a gel system of ultradeformable vesicles. Artif Cells Nanomed Biotechnol 44(6):1457–1463
Ahmadi Nasab N, Hassani Kumleh H, Beygzadeh M et al (2018) Delivery of curcumin by a pH-responsive chitosan mesoporous silica nanoparticles for cancer treatment. Artif Cells Nanomed Biotechnol 46:75–81
Alves MP, Scarrone AL, Santos M et al (2017) Human skin penetration and distribution of nimesulide from hydrophilic gels containing nanocarriers. Int J Pharm 341:215–220
Araki K, Yoshizumi M, Kimura S, Tanaka A, Inoue D, Furubayashi T, Sakane T, Enomura M (2020) Application of a microreactor to pharmaceutical manufacturing: preparation of amorphous curcumin nanoparticles and controlling the crystallinity of curcumin nanoparticles by ultrasonic treatment. AAPS PharmSciTech 21(1):17
Chen HY, Fang J-Y (2000) Therapeutic patents for topical and transdermal drug delivery systems. Expert Opin Ther Pat 10(7):1035–1043
Chinembiri TN, Plessis LH, Gerber M et al (2014) Review of natural compounds for potential skin cancer treatment. Molecules 19:11679–11721
Esfandiarpour-Boroujeni S, Bagheri-Khoulenjani S, Mirzadeh H et al (2017) Fabrication and study of curcumin loaded nanoparticles based on folate-chitosan for breast cancer therapy application. Carbohydrate 168:14–21
Fang YP, Huang YB, Wu PC et al (2009) Topical delivery of 5-aminolevulinic acid-encapsulated ethosomes in a hyper proliferative skin animal model using the CLSM technique to evaluate the penetration behavior. Eur J Pharm Biopharm 73:391–398
Ghosh S, Dutta S, Sarkar A, Kundu M, Sil PC (2021) Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency. Colloids Surf B Biointerfaces 197:111–404
Govender T, Riley T, Ehtezazi T et al (2000) Defining the drug incorporation properties of PLA–PEG nanoparticles. Int J Pharm 199:95–110
Hamzehzadeh L, Atkin SL, Majeed M et al (2018) The versatile role of curcumin in cancer prevention and treatment: a focus on PI3K/AKT pathway. J Cell Physiol 233:6530–6537
Higuchi T (1963) Mechanism of sustained action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. Int J Pharm 52:1145–1149
Huang PH, Hu SCS, Lee CW et al (2016) Design of acid-responsive polymeric nanoparticles for 7,3′,4′-trihydroxyisoflavone topical administration. Int J Nanomed 11:1615
Hyvönen S, Peltonen L, Karjalainen M et al (2005) Effect of nanoprecipitation on the physicochemical properties of low molecular weight poly (l-lactic acid) nanoparticles loaded with salbutsamol sulphate and beclomethasone dipropionate. Int J Pharm 295:269–281
Ibraheem D, Iqbal DM, Agusti G et al (2015) Effects of process parameters on the colloidal properties of polycaprolactone microparticles prepared by double emulsion like process. Colloid Surf A Physicochem Eng Asp 445:79–91
Jantarat C (2013) Bioavailability enhancement techniques of herbal medicine: a case example of curcumin. Int J Pharm Pharm Sci 5:493–500
Kachuri L, De P, Ellison LF, Semenciw R (2013) Cancer incidence, mortality and survival trends in Canada, 1970–2007. Chronic Dis Inj Can 33(2):55–59
Krishnakumar N, Sulfikkarali N, Rajendra Prasad N et al (2011) Enhanced anticancer activity of naringenin-loaded nanoparticles in human cervical (HeLa) cancer cells. Prev Nutr Food Sci 1:223–231
Kumari M, Sharma N, Manchanda R, Gupta N, Syed A, Bahkali AH, Nimesh S (2021) PGMD/curcumin nanoparticles for the treatment of breast cancer. Sci Rep 1:1–7
Kunnumakkara AB, Anand P, Aggarwal BB (2008) Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett 269:199–225
Kurzrock R, Li L, Mehta K et al (2019) University of Texas system, liposomal curcumin for treatment of cancer. US Patent Appl 16:205–816
Lamprecht A, Ubrich N, Hombreiro Pérez M et al (1999) Biodegradable monodispersed nanoparticles prepared by pressure homogenization-emulsification. Int J Pharm 184:97–105
Lee SC, Oh JT, Jang MH et al (1999) Quantitative analysis of polyvinyl alcohol on the surface of poly (d, l-lactide-co-glycolide) microparticles prepared by solvent evaporation method: effect of particle size and PVA concentration. J Controll Release 59:123–132
Lee SH, Jeong SK, Ahn SK (2006) An update of the defensive barrier function of skin. Yonsei Med J 47:293–306
Liu J, Xu L, Liu C et al (2012) Preparation and characterization of cationic curcumin nanoparticles for improvement of cellular uptake. Carbohyd Polym 90:16–22
Maeda H, Bharate G, Daruwalla J (2009) Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm 71:409–419
Mangalathillam SR, Ejinold NS, Nair A et al (2012) Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale 4:239–250
Markman JL, Rekechenetskiy A, Holler E et al (2016) Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv Drug Deliv Rev 65:1866–1879
Mirzaei H, Masoudifar A, Sahebkar A et al (2018) MicroRNA: a novel target of curcumin in cancer therapy. J Cell Physiol 233:3004–3015
Mora-Huertas C, Fessi H, Elaissari A (2011) Influence of process and formulation parameters on the formation of submicron particles by solvent displacement and emulsification–diffusion methods: critical comparison. Adv Colloid Interface Sci 163:90–122
Oktay AN, Ilbasmis-Tamer S, Han S, Uludag O, Celebi N (2020) Preparation and in vitro/in vivo evaluation of flurbiprofen nanosuspension-based gel for dermal application. Eur J Pharm Sci 155:105548
Ombredane AS, Silva VR, Andrade LR, Pinheiro WO, Simonelly M, Oliveira JV, Pinheiro AC, Gonçalves GF, Felice GJ, Garcia MP, Campos PM (2021) In vivo efficacy and toxicity of curcumin nanoparticles in breast cancer treatment: a systematic review. Front Oncol 11:612903
Oungbho K, Müller BW (1997) Chitosan sponges as sustained release drug carriers. Int J Pharm 156:229–237
Papadimitriou S, Bikiaris D (2009) Novel self-assembled core–shell nanoparticles based on crystalline amorphous moieties of aliphatic copolyesters for efficient controlled drug release. J Control Release 138:177–184
Patel NA, Patel NJ, Patel RP (2009a) Formulation and evaluation of curcumin gel for topical application. Pharm Dev Technol 14:83–92
Patel RD, Singh S, Navin S et al (2009b) Development and characterization of curcumin loaded transfersome for transdermal delivery. J Pharm Sci Res 2009(1):71–80
Prajakta D, Ratnesh J, Chandan K et al (2009) Curcumin loaded pH-sensitive nanoparticles for the treatment of colon cancer. J Biomed Nanotechnol 2009(5):445–455
Priyadarsini KI (2014) The chemistry of curcumin: from extraction to therapeutic agent. Molecules 19:20091–20112
Rachmawati H, Budiputra DK, Mauludin R (2015) Curcumin nanoemulsion for transdermal application: formulation and evaluation. Drug Dev Ind Pharm 41:560–566
Ramirez CN, Li W, Zhang C et al (2018) In vitro-in vivo dose response of ursolic acid, sulforaphane, PEITC, and curcumin in cancer prevention. AAPS J 20:19
Ritger PL, Peppas NA (1987) A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release 5:37–42
Sahu P, Kashaw SK, Sau S, Kushwah V, Jain S, Agrawal RK, Iyer AK (2019) pH triggered and charge attracted nanogel for simultaneous evaluation of penetration and toxicity against skin cancer: in-vitro and ex-vivo study. Int J Biol Macromol 128:740–751
Shakeri A, Ward N, Panahi Y et al (2019) Anti-angiogenic activity of curcumin in cancer therapy: a narrative review. Curr Vasc Pharmacol 17:262–269
Simonetti LD, Gelfuso GM, Barbosa JC et al (2009) Assessment of the percutaneous penetration of cisplatin: the effect of monoolein and the drug skin penetration pathway. Eur J Pharm Biopharm 73:90–94
Syng-ai C, Kumari AL, Khar A (2004) Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol Cancer Ther 3:1101–1108
Tang J, Xu N, Ji H, Liu H et al (2011) Eudragit nanoparticles containing genistein: formulation, development, and bioavailability assessment. Int J Nanomed 2011(6):2429
Taveira SF, Lopez RFV (2011) Topical administration of anticancer drugs for skin cancer treatment, in Skin Cancers-Risk Factors. Prevention and Therapy. InTech
Tomren M, Másson M, Loftsson T, Tønnesen HH (2007) Studies on curcumin and curcuminoids: XXXI. Symmetric and asymmetric curcuminoids: stability, activity and complexation with cyclodextrin. Int J Pharm 338:27–34
Tzeng CW, Yen FL, Wu TH et al (2011) Enhancement of dissolution and antioxidant activity of kaempferol using a nanoparticle engineering process. J Agric Food Chem 2011(59):5073–5080
Wahlang B, Pawar YB, Bansal AK (2011) Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur J Pharm Biopharm 77:275–282
Xie J, Fan Z, Li Y et al (2018) Design of pH-sensitive methotrexate prodrug-targeted curcumin nanoparticles for efficient dual-drug delivery and combination cancer therapy. Int J Nanomed 13:1381
Xu G, Sunada S (1995) Influence of formulation change on drug release kinetics from hydroxypropylmethylcellulose matrix tablets. Chem Pharm Bul 43:483–487
Yadav KS, Sawant KK (2010) Modified nanonanoprecipitation method for preparation of cytarabine-loaded PLGA nanoparticles. AAPS PharmSciTech 11:1456–1465
Yallapu MM, Nagesh PKB, Jaggi M, Chauhan S (2015) Therapeutic applications of curcumin nanoformulations. AAPS J 17(6):1341–1356
Yang YY, Chung TS, Ng NP (2001) Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials 22:231–241
Yavarpour-Bali H, Ghasemi-Kasman MP, Pirzadeh M (2019) Curcumin-loaded nanoparticles: a novel therapeutic strategy in treatment of central nervous system disorders. Int J Nanomed 2019(14):4449–4460
Zafar S, Beg S, Panda SK, Rahman M, Alharbi KS, Jain GK, Ahmad FJ (2019) Novel therapeutic interventions in cancer treatment using protein and peptide-based targeted smart systems. Seminar Cancer Biol 69:249–267
Zamarioli CM, Martins RM (2015) Nanoparticles containing curcuminoids (Curcuma longa): development of topical delivery formulation. Rev Bras Farmacogn 25:53–60
Acknowledgements
Authors are thankful to Evonik Pakistan for providing Eudragit, the polymer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bibi, N., ur Rehman, A., Rana, N.F. et al. Formulation and characterization of curcumin nanoparticles for skin cancer treatment. Appl Nanosci 12, 3421–3436 (2022). https://doi.org/10.1007/s13204-022-02346-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-022-02346-4