Abstract
The current investigation was aimed to biologically synthesize silver nanoparticles utilizing Pyrostegia venusta leaf aqueous extract. The synthesized nanoparticles were characterized using UV–Vis spectroscopy, Fourier transform infrared spectroscopy (FTIR), atomic force microscopy (AFM), X-ray diffraction (XRD), high-resolution-transmission electron microscopy (HR-TEM), zeta potential, and scanning electron microscopy (SEM) with energy-dispersive X-ray (EDX) analysis. The antimicrobial activity was done against bacterial and fungal pathogens like Enterococcus faecalis, Escherichia coli, Candida albicans and Candida glabrata and finally subjected to anticancer activity and cell apoptotic pathway assessment against African green monkey kidney (COS-7) cell line. The UV–Vis observation showed a maximum absorbance at 381 nm; the AFM data confirmed spherical shape and poly-dispersed nature of particles. Later, FTIR analysis revealed the patterning of IR spectral bands and TEM images determined the smallest size of biological AgNPs as 12.56 nm. Zeta potential showed a sharp peak at − 31.7 mv, whereas SEM/EDX depicted spherical shape and poly-dispersed nature of AgNPs with 42.95% presence of silver in sample. The antagonistic potential of synthesized AgNPs was well indicated by the zone of inhibition formed around the agar well against pathogens at varying concentrations. The AgNPs showed highest and lowest activity against E. coli and C. albicans, respectively. The AgNPs displayed significantly increased cytotoxic activity against COS-7 cells in vitro, with IC50 value of 50.48 µg/ml. The anticancer activity was also assessed using cell apoptosis; the results depicted the potential of AgNPs to kill COS-7 cells by differential staining to visualize both dead and live cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous studies have been conducted on the biological synthesis of silver nanoparticles (AgNPs) using plants and have shown their highly dynamic applications in different fields, such as biomedical, agricultural, industrial, electric properties, biotechnological and many others. The recent advancement in green nanotechnology is evolving into a vital branch in material science. To best exploit green synthesis of AgNPs, we need to know what is there and how it can be used in better way (Rauwel et al. 2015; Ndikau et al. 2017). AgNPs are generally having size range from 1 to 100 nm and have high surface to volume ratio. The dimension of nanoparticles gives certain unique potential biological properties and is useful in different fields in science. There are various methods, which are being used for characterization of AgNPs to determine particle size, shape, crystallinity, surface area, surface volume and fraction of dimensions (Zhang et al. 2016).
The development of eco-friendly synthesis of AgNPs is a crucial aspect in the area of nanoparticle research (Singhal et al. 2011). However, AgNPs have multidisciplinary applications in the fields of science including pharmaceutics, industries and medicine because of their well-known antibacterial and antifungal activity (Govindaraju et al. 2010; Devi and Bhimba 2014; Krithiga et al. 2015). Among the different synthesis methods for AgNPs, like physical, chemical and biological processes; the biological synthesis of AgNPs is the best mainly because of eco-friendly, non-toxic and cost effectiveness (Lee and Jun 2019). Synthesis of AgNPs is possible by means of using biological entities like bacteria (Ameen et al. 2020), algae (Gopu et al. 2021), plants (Sengottaiyan et al. 2016; Sampath et al. 2021), and vegetable waste (Mythili et al. 2018) etc. The biological synthesis of AgNPs is growing attention of researchers, mainly because of its rapid economical, eco-friendly, non- toxic, large scale production and the biotechnologically potential products (Aziz et al. 2014).
The plant Pyrostegia venusta (Ker-Gawl.) Miers (P. venusta) belongs to the family Bignoniaceae and represents medicinally important and most diverse group of flowering plants. P. venusta is commonly used as traditional medicine to treat induced sickness behaviours like flu and cold and also for the treatment of diarrhoea, vitiligo, and cough as oral tonic (Khalil et al. 2014). The phytochemical profiling of P. venusta revealed the existence of various metabolites, such as steroids, carotenoids, anthraquinones, terpenoids tannins and flavonoids, etc. (Iravani et al. 2014). Cancer causes various changes in metabolic and cellular pathways in the organisms which develop through wide signaling pathway mechanisms including cell proliferation, apoptosis, metastasis and angiogenesis (Seigneuric et al. 2010; Abdel-Fattah and Ali 2018). The metabolic and cellular activities like alteration in genomic expression, aerobic glycolysis, damaged mitochondrial DNA and respiratory chain is caused by the abnormality of cancer cells (Burns and Manda 2017). The nanoparticles have high significance in the field of biomedicine for global health and treatment of different diseases and disorders caused by the free radicals and prevention of biological attacking to give a secure life to mankind. The medicinal importance of the P. venusta provides biotechnological potency and importance of the species in carrying out the green synthesis of nanoparticles.
The current investigation was aimed to synthesize and characterize eco-friendly AgNPs from P. venusta leaf extract for the first time; and to examine the antimicrobial and apoptosis mediated anticancer activity against pathogenic microbes and COS-7 cell line, respectively.
Materials and methods
Collection of plant sample, chemicals and microbial cultures
P. venusta leaves (Fig. 1) were collected from the botanical garden of Karnatak University campus (15° 26′ 09.5" N 74° 58′ 54.5" E) and the authenticity was confirmed by comparing with the herbarium collection of plants at Plants and Animals Museum, Karnatak University, Dharwad. The reagents, such as silver nitrate (AgNO3) and nutrient agar (NA) media were purchased from Sigma-Aldrich and Hi-Media laboratories, respectively. The pathogens, such as Enterococcus faecalis (MTCC6845), Escherichia coli (MTCC40), Candida glabrata (MTCC3019) and Candida albicans (MTCC227) were obtained from IMTECH Chandigarh, India.
Preparation of leaf extract and synthesis of AgNPs
The fresh leaves of P. venusta were thoroughly washed with running tap water for 4 to 5 times followed by double distilled water wash. About 20 g of dried leaves were cut into small pieces into a 250 ml conical flask containing 100 ml distilled water and heated at 60 °C for 2 h to facilitate the formation of aqueous extract. The extract was obtained by filtering the mixture through Whatman No. 1 filter paper and the extract was retained at 4 °C for further analysis (Gnanadesigan et al. 2012).
Aqueous solution of 0.1 mM AgNO3 was prepared and 10 ml of aqueous leaf extract was mixed with 90 ml AgNO3 solution. The solution was adjusted to pH 10.0 and left for incubation in dark chamber for 4 h. The colour change in the reaction mixture indicated the synthesis of AgNPs. After incubation, the mixture was taken out for centrifugation and the residue was collected in Eppendorf tubes for further analyses (Christopher et al. 2015).
Characterizations of AgNPs
The UV–Vis inspection was carried out by scanning the sample from 200 to 700 nm in UV–Vis. spectrophotometer (UV-9600A, Shanghai METASH Instruments Co. Ltd. Series No: AU1701001; whereas, the AFM analysis was performed to understand the morphology, distribution and sizes of AgNPs. On a glass slide, a fine film of the synthesized AgNPs was prepared and allowed to dry for 1 h prior to the analysis by AFM instrument (NanoSurf Flex AFM System). FTIR study was done to determine the involvement of possible biological functional groups involved in stabilization and capping of synthesized AgNPs. The pellet of AgNPs was made with KBr and the FTIR spectrum was obtained by scanning from 400 to 4000 cm−1 (Nicolet 6700, Thermo Fisher Scientific, Massachusetts, USA). The diameter and size of the synthesized AgNPs were determined using HR-TEM (FEI, TECNAI G2, F30) analysis. A drop of sample was dispersed in double-distilled water and spotted on a copper grid followed by carbon coating.
Zeta potential measurement was used as essential parameter for the determination of stability of AgNPs in aqueous suspensions and the analysis was done with the help of Nano-analyser instrument (Horiba, scientific nanoparticle analyser SZ-100, Japan). Imaging, surface morphology, size distributions and shapes of AgNPs were studied by SEM analysis. The freeze-dried AgNPs powder was suspended in distilled water to prepare a thin film on the stab and the sample was analysed after gold coating using SEM (JEOL, JSM-IT500, LA, Japan). The chemical status of AgNPs was ascertained by EDX instrument equipped with SEM. The synthesized AgNPs were subjected to XRD analysis to determine the crystal structure and particle size. The dried powder of AgNPs were placed in sample holder and gently pressed to prepare thin pellets, which was later scanned with XRD instrument (Rigaku SmartLab SE, Texas, US) from 30° to 90° at 2ϴ angle.
Antimicrobial activity
The potential of synthesized AgNPs to inhibit the microbial growth was examined on gram-negative and gram-positive bacteria like Escherichia coli (MTCC40) and Enterococcus faecalis (MTCC6845) along with fungi like Candida glabrata (MTCC3019) and Candida albicans (MTCC227) using agar well diffusion (CLSI 2012) method. The stock solution was prepared by mixing 1 mg of AgNPs in 1 ml of sterile distilled water. About 6 mm diameter wells were bored on Muller-Hinton agar (pH 7.4) plates and 25 µl, 50 µl, 75 µl and 100 µl of synthesized AgNPs was poured into wells. The P. venusta leaf extract was used as control along with ampicillin and fluconazole (50 µg/ml) as standards. Incubation was done by keeping the plates at 37 °C for 18 h and the diameter of clear zone formed around each well was measured after completion of incubation period (Bhat et al. 2021).
Anticancer activity
The African green monkey kidney fibroblast (COS-7) cancer cell line was obtained from NCCS, Pune, India. The Dulbecco’s modified Eagle medium (DMEM) was used as a cell culture media with high glucose for cell proliferation assay at 37 °C at a 5 mg/ml of final concentration and incubated in a 5% CO2 atmosphere. At the density of 20,000 cell/well in 200 μl of medium were loaded in a 96 well plate and the total number of cells used in the assay was determined by cell counter. The cancer cells were treated against individual AgNPs concentrations (12.5, 25, 50, 100, 200 µg/ml) were prepared in DMSO and followed by incubation at 37 °C for 24 h along with 10 µM camptothecin (34.83 µg/ml) as a standard and cells without AgNPs and experimental drug as a untreated. The experimented cells were kept to grow for 3 h at 37 °C with 0.5 mg/ml concentration of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT). After incubation; 100 μl di-methyl sulfoxide were added to solubilize the formazan crystals and the viable cells were determined at 570 nm as an absorbance recorder with a reference wavelength at 630 nm in spectrophotometer and finally, the IC50 value was determined (Mosmann 1983; Sreenivasa et al. 2020).
Assessment of cell apoptosis
COS-7 cell lines were estimated by the fluorescence method. The intracellular cytotoxic generation by AgNPs treated COS-7 cells were detected by staining cells with acridine orange (AO) (20 µg/ml, Thermo Fisher, USA) and ethidium bromide (EB) (50 µg/ml, Thermo Fisher, USA) fluorescence. Cells were cultured in 12 well plates with 2 × 105 cells/2 ml density and kept for incubation at 37 °C overnight inside a CO2 incubator. Later, the cells were treated with IC50 concentration of AgNPs and incubated for 48 h. Further, the cells were stained with 200 µl staining solution for 10 min, mounted and observed under fluorescence microscope (Singh et al. 2021).
Results and discussion
Synthesis of AgNPs and UV–Vis analysis
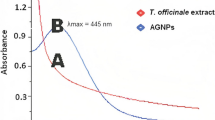
The colourless AgNO3 solution (Fig. 2a) was added to the aqueous leaf extract of P. venusta, which was light orange in colour as shown in Fig. 2b. Later, the mixture was turned dark brown in colour (Fig. 2c) during incubation in dark chamber for 4 h at pH 10.0. The change of colour hinted the synthesis of AgNPs and the characteristic brown colour provided a convenient spectroscopic representation confirming the synthesis of biological AgNPs by displaying a characteristic absorption peak at 381 nm (Fig. 3).
The green synthesis of AgNPs is a challenging research field, with lot of scope and concept. It is growing attention of researchers mainly because of its rapid economical, eco-friendly, non-toxic, large-scale production and biotechnological applications. During the current investigation, the formation of biogenic AgNPs from P. venusta leaf extract was monitored by UV–Vis spectroscopic analysis. The colour change of the reaction mixture from light orange to brown indicated the excitation of surface plasmon vibrations with the biological AgNPs (Sahu et al. 2016; Lakshmanan et al. 2017). Similar results have been reported in recent literatures, where a plant leaf and flower extracts significantly reduced silver ions into elemental silver with the help of secondary metabolites, which also involved in capping of AgNPs (Taranath et al. 2015; Ameen et al. 2019). The UV spectrum showed the maximum absorption peak at 381 nm, which indicated the stability of bio-synthesized nanoparticles. The similar work was reported (Jyoti and Singh 2017), where the absorption spectrum of AgNPs was found at 381 nm.
AFM analysis
The surface morphology and particle distribution of biological AgNPs were obtained from AFM images. AFM micrographs revealed the nature and shape of AgNPs as poly-dispersed and spherical. The size of biological AgNPs ranged from 12 to 20 nm (Fig. 4a) with the height of 14.4 nm in 2D and 3D images (Fig. 4b and c). The distance between AgNPs was approximately 44 nm with a little amount of agglomeration. Similarly, AFM analysis was performed to check the morphological details and particle size of the AgNPs. This result was well supported by the earlier results of AFM analysis on biosynthesized AgNPs (Balashanmugam and Kalaichelvan 2015; Nayak et al. 2020).
FTIR analysis
The FTIR spectrum of AgNPs revealed the presence of possible biological functional groups that acts as bio-reducing molecules and capping agents as shown in Fig. 5. The ionizing radiation bands were exhibited at 3355, 3279, 2930, 2426, 2095, 1771, 1601, 1383, 1056, 873, 838, 781, 576, and 514 cm−1 corresponding to various biological functional groups. The short peak at 3355 cm−1 corresponds to N–H stretching primary and secondary amines/amides; while the short peak at 3279 cm−1 correlates for C–H stretching terminal alkynes. The medium peak at 2930 cm−1 corresponds to C–H stretching alkanes, and the medium, sharp peak at 2426 cm−1 was correlated to O–H stretching carboxylic acids. The weak peak at 2095 cm−1 correspondent to C≡C stretching, mono-substituted alkynes; the weak peak at 1771 cm−1 is corresponded to C–H bending aromatic compounds. The strong intensity peak at 1601 cm−1 correlated to C=C stretching, α, β-unsaturated ketone; strong peak at 1383 cm−1 for S=O stretching sulfate/sulfonyl chloride, and the strong peak at 1056 cm−1 correspondent to C–O stretching primary alcohol. Further, the peaks at 873, 838, 781, 576, and 514 cm−1 were correlated to =C–H bending alkenes, N–H wagging primary and secondary amines, C–Cl stretching alkyl halides, and C–Br stretching alkyl halides, respectively. Similarly, the FTIR analysis revealed the presence of different biomolecules and functional groups in Moringa oleifera and Tectona grandis seeds extract. The previously described reports suggested the role of biomolecules associated with the reduction and capping of AgNPs (Ghosh et al. 2014; Rautela et al. 2019).
HR-TEM analysis
HR-TEM investigation confirmed the size and morphology along with poly-dispersed nature of biologically synthesized AgNPs in Fig. 6a. The spherical shape, smaller size and higher surface area of the AgNPs were found out during the analysis. TEM investigation revealed the lowest size of biological AgNPs as being 12.56 nm as shown in Fig. 6b. Similar results were observed, where the average size of biologically synthesized AgNPs was 18.06 nm and the AgNPs were poly-dispersed with fewer particles of varied sizes (Vijaykumar et al. 2014; Devamma et al. 2015).
Zeta potential analysis
Zeta potential analysis suggested about the surface potential and electrostatic stability of biologically synthesized AgNPs. The zeta potential value of the AgNPs was indicated by a sharp peak at − 31.7 mV (Fig. 7) and the negative potential value was associated with the stability of the synthesized AgNPs. The zeta potential of synthesized AgNPs was determined in water as dispersant, LASER diffraction shows that particles were polydispersed in mixture. The positive or negative charge on the surface of nanoparticles provides stability and prevents aggregation of nanoparticles by pushing the same charges.
The zeta potential is an indicator of surface charge potential which is an important parameter for understanding the stability of nanoparticles in aqueous suspensions and it has been stated that, AgNPs with a zeta potential higher than + 30 mV or lower than − 30 mV are considered to be very stable in the dispersion medium. The negative zeta potential value of AgNPs suggests that the particles were and the higher negative value indicated the high stability and repulsion among the particles (Ali et al. 2015; Skiba et al. 2020).
SEM with EDX analysis
The nanometer level resolution of biological AgNPs was supervised by SEM analysis. The SEM study showed the particles were poly-dispersed and spherical in shape with no agglomeration (Fig. 8a). The EDX study showed 42.95% of silver in the AgNPs along with 19.85% of carbon, 28.04% of oxygen, 3.44% of chlorine, 2.17% of sodium, 1.12% of calcium, 0.90% of aluminium, 0.79% of magnesium, and 0.74% of sulphur (Fig. 8b). The SEM study of AgNPs shows the particles as spherical, poly-dispersed and there was a little or no agglomeration between the particles. The similar earlier result was reported (Moodley et al. 2018; Han et al. 2020). The EDX analysis indicated the presence of carbon, oxygen, sodium, magnesium, aluminium, sulphur, chlorine, calcium and silver in AgNPs. An earlier report was also recorded the elemental composition of plant based AgNPs (Srirangam and Rao 2017).
XRD analysis
The AgNPs were examined by XRD analysis to understand composition and phase purity of particles (Fig. 9). The XRD spectrum depicted Bragg’s lattice plane values for five characteristic diffraction peaks at 37.52°, 44.38°, 64.55° and 77.49°, 81.59° indexed at (111), (200), (220), (311) and (222) planes of face-centred cubic structure. Later, the result was correlated with JCPDS standard diffraction card were compared to our results. The approximate estimation of the average particle size was 15.56 nm. Similar reports were published, where the particles of AgNPs were spherical with some amount of agglomeration (Peiris et al. 2017; Shaik et al. 2018). It clearly suggests the crystallization of the surface bio-organic phase of the AgNPs (Srirangam and Rao 2017).
Antimicrobial activity
The antimicrobial efficiency of biological synthesized AgNPs were studied on different microbial pathogens (Fig. 10a–d). The inhibition zones formed by AgNPs at various concentrations against pathogens illustrated the potential of AgNPs to hinder the growth of pathogens (Fig. 10e). Among all the pathogens, E. coli was observed to be the most sensitive whereas; C. albicans was the least sensitive. The antimicrobial efficiency of biologically synthesized AgNPs was studied against different microbial pathogens at various concentrations.
Similarly, the antimicrobial efficiency of nanoparticles was reported in an earlier study, where the antagonistic potential of bio-synthesized AgNPs from Moringa oleifera leaf extract was studied against E. faecalis (Padalia et al. 2015; Kgatshe et al. 2019). A similar result was also observed in anti-fungal study of AgNPs from marigold flower exhibited maximum zone of inhibition against pathogens, like E. coli, C. albicans and C. glabrata (Zafar and Zafar 2019). The earlier reports on plant based synthesized AgNPs using Phoenix dactylifera fruits and Tinospora cordifolia extracts showed highest inhibition zone for C. albicans and Staphylococcus sp. followed by E. coli and Bacillus sp., respectively (Gurunathan et al. 2013; Selvam et al. 2019). One of the modes of antimicrobial action of AgNPs was expected through a slow break of silver ions via oxidation outside or within the cells and the disruption of membrane permeability of microbial and other cells.
Anticancer activity
The anti-cancer activity of COS-7 cell line was carried using MTT cytotoxic assay. The results clearly indicated a steady increase against the growth of cancer cell line with 12.5, 25, 50, 75, 100, and 200 µg/ml concentrations of AgNPs. Among all these the concentration of AgNPs, the 200 µg/ml was highly effective in destroying more cancer cells (Fig. 11a–g). Finally, a graph was implemented taking the percentage of cell viability versus concentration of AgNPs (Fig. 11h).
The result depicted considerable reduction in the proliferation of COS-7 cells by half maximal inhibitory concentration (IC50) value of 50.48 μg/ml of AgNPs, which have the capacity to kill 50% of viable cells. The in-vitro MTT assay is most often being carried out for measuring cytotoxic effects of AgNPs against cancer cell lines (Han et al. 2014;). The AgNPs are more toxic at higher level of dose and the MTT assay depicted a gradual shrinkage of cells with increasing concentration of AgNPs resulting to death of COS-7 cancer cells. The IC50 concentration against the COS-7 cells was 50.48 µg/ml, after the treatment of 24 h incubation at 37 °C temperature in in-vitro. Nanoparticles are known to generate free radicals that are capable of damaging the target cells and tissues by inducing oxidative stress. The exact mechanism of action is not elucidated; however, several studies suggested that, AgNPs can hinder cell growth by interacting with cell membrane proteins and activating respective signaling pathways. AgNPs enter into a cell via endocytosis or diffusion and disturb the mitochondrial electron transfer chain. Similarly, reactive oxygen species (ROS) can also affect various processes such as DNA damage, interference with cellular signaling pathways, changes in the gene expression, and finally leading to cell death (Azandeh et al. 2017; Al-Sheddi et al. 2018; Mousavi et al. 2018).
Assessment of cell apoptosis
AO and EB dyes intercalate with nucleic acid (DNA or RNA) present in living cells, and emit respective fluorescence colours that help to differentiate cellular organelles. The dual staining study against the COS-7 cell line revealed the AgNPs cytotoxic potential at IC50 concentration of 50.48 µg/ml. The images with AO (green colour cells) represented the viable cells and EB (red colour cells) represented the dead cells and the image with both fluorescent dyes merged gave the clear idea about the activity of AgNPs on tumour cells by displaying both viable and dead cells (Fig. 12). The possible mechanism of action of AgNPs to induce apoptosis in cancer cells is proposed in Fig. 13.
Apoptosis study by AO/EB double staining; a Untreated cells, b AO stained untreated cells, c EB stained untreated cells, d AO/EB double stained untreated cells, e AgNPs treated cells, f AgNPs treated cells stained with AO, g AgNPs treated cells stained with EB, and h AgNPs treated cells double stained with AO/EB
Cell apoptotic pathway is well known as programmed cell death, which includes major changes in morphology, condensation of chromatin material and fragmentation of DNA (Aceituno et al. 2017; Sorayya et al. 2019). To study cell apoptosis on COS-7 cell line, nuclear morphology of COS-7 cells was investigated with dual staining of EB and AO at IC50 concentration. Similar results about AgNPs induced apoptosis were also reported on various cancer cell lines suggesting that, the oxidative pressure generated by the free radicals produced due to the external stimuli is the major cause of apoptosis in cancer cells. Previous investigations indicated that, AgNPs cause oxidative stress and also suppress the normal functioning of tumor suppressor genes, reduce mitochondrial potential, and induce lipid peroxidation which collectively results in cell apoptosis (Ullah et al. 2020). Similarly, mitochondria are known to play a crucial role in the induction and regulation of apoptosis, as the restricted permeability of mitochondrial inner membrane is one critical criteria that regulates apoptosis. The permeability of the inner mitochondrial membrane gets altered under the influence of various intrinsic and extrinsic stimuli, which in turn leads to the disruption of oxidative phosphorylation. The mitochondrial membrane rupture results in the release of cytochrome coenzyme and initiates the apoptosis process and induction of apoptosis is mainly characterized by cell shrinkage, nuclear condensation, and DNA fragmentation (Acharya et al. 2020; Singh et al. 2021).
Conclusion
Recently, biological AgNPs were successfully synthesized by plant extracts and the present study was aimed at synthesis of AgNPs from P. venusta leaf extract and their biological activity. The maximum absorption peak in UV–Vis spectrum was obtained at 381 nm, spherical shape and poly-dispersed nature of AgNPs were confirmed by AFM and SEM, the bio-fabrication of AgNPs was illustrated by FTIR, while the lowest particle size of 12.56 nm was confirmed by HR-TEM. Zeta potential analysis revealed the peak at − 31.7 mV and 42.95% of silver in the AgNPs was exhibited by EDX. The biologically synthesized AgNPs displayed broad-spectrum antimicrobial activity against microbial pathogens with the formation of notable inhibition zones against pathogenic microorganisms at varying concentrations. The IC50 concentration of 50.48 µg/ml against COS-7 cells indicated the cytotoxic potential of AgNPs on tumour cells. These in vitro results hinted at the possible use of AgNPs as an alternative to antibiotics and to control cancer cell growth after successive clinical trials.
Availability of data and material
Not applicable.
References
Abdel-Fattah WI, Ali GW (2018) On the anti-cancer activities of silver nanoparticles. J App Biotech Bioeng 5:1–4. https://doi.org/10.15406/jabb.2018.05.00116
Aceituno VC, Abbai R, Moon SS (2017) Pleuropterus multiflorus (Hasuo) mediated straight forward eco-friendly synthesis of silver, gold nanoparticles and evaluation of their anti-cancer activity on A549 lung cancer cell line. Biomed Pharma 93:995–1003. https://doi.org/10.1016/j.biopha.2017.07.040
Acharya D, Satapathy S, Somu P, Parida UK, Mishra G (2020) Apoptotic effect and anticancer activity of bio-synthesized silver nanoparticles from marine algae Chaetomorpha linum extract against human colon cancer cell HCT-116. Biol Trace Elem Res 199:1812–1822. https://doi.org/10.1007/s12011-020-02304-7
Ali ZA, Rosiyah YS, Devi S, Puteh R (2015) Green synthesis of silver nanoparticles using apple extract and its antibacterial properties. Adv Mater Sci Eng 2016:4102196. https://doi.org/10.1155/2016/4102196
Al-Sheddi ES, Farshori NN, Al-Oqail MM, Al-Massarani SM, Saquib Q, Rizwan W, Musarrat J, Al-Khedhairy AA, Siddiqui MA (2018) Anticancer potential of green synthesized silver nanoparticles using extract of Nepeta deflersiana against human cervical cancer cells (HeLA). Bioinorg Chem Appl 2018:9390784. https://doi.org/10.1155/2018/9390784
Ameen F, Srinivasan P, Selvakumar T, Kamala-Kannan S, Al-Nadhari S, Almansob A, Dawoud T, Govarthanan M (2019) Phytosynthesis of silver nanoparticles using Mangifera indica flower extract as bioreductant and their broad-spectrum antibacterial activity. Bioorg Chem 88:102970. https://doi.org/10.1016/j.bioorg.2019.102970
Ameen F, Alyahya S, Govarthanan M, Aljahdali N, Al-Enazi N, Alsamhary K, Alshehri WA, Alwakeel SS, Alharbi SA (2020) Soil bacteria Cupriavidus sp. mediates the extracellular synthesis of antibacterial silver nanoparticles. J Mol Struct 1202:127233. https://doi.org/10.1016/j.molstruc.2019.127233
Azandeh SS, Abbaspour M, Khodadadi A (2017) Anticancer activity of curcumin-loaded PLGA nanoparticles on PC3 prostate cancer cells. Iranian J Pharm Res 16:868–879 (PMCID: PMC5610743)
Aziz N, Tasneem F, Ajit V, Ram P (2014) Biogenic synthesis of silver nanoparticles using Scenedesmus abundans and evaluation of their antibacterial activity. J Nanopart 16:868–879. https://doi.org/10.1155/2014/689419
Balashanmugam P, Kalaichelvan PT (2015) Biosynthesis characterization of silver nanoparticles using Cassia roxburghii (DC). aqueous extract, and coated on cotton cloth for effective antibacterial activity. Int J Nanomed 10:87–97. https://doi.org/10.2147/IJN.S79984
Bhat M, Chakraborty B, Kumar RS, Almansour AI, Arumugam N, Kotresha D, Pallavi SS, Dhanyakumara SB, Shashiraj KN, Nayaka S (2021) Biogenic synthesis, characterization and antimicrobial activity of Ixora brachypoda (DC) leaf extract mediated silver nanoparticles. J King Saud Univ Sci 33:101296. https://doi.org/10.1016/j.jksus.2020.101296
Burns JS, Manda G (2017) Metabolic pathways of the Warburg effect in health and disease: perspectives of choice, chain or chance. Int J Mol Sci 18:1–28. https://doi.org/10.3390/ijms18122755
Christopher JSG, Saswati B, Ezilrani PS (2015) Optimization of parameters for biosynthesis of silver nanoparticles using leaf extract of Aegle marmelos. Brazilian Arch Bio Tech 58:702–710. https://doi.org/10.1590/S1516-89132015050106
CLSI (2012) Performance standards for antimicrobial disk susceptibility tests; approved standard-eleventh edition. CLSI document M02–A11. Clinical and Laboratory Standards Institute, Wayne
Devamma MN, Durga GP, Devi PSL, Prasad TNV (2015) Synthesis, characterization and evaluation of the antimicrobial efficacy of medicinal plants extract mediated silver nanoparticles. Int J Sci Eng Res 6:86–94
Devi JS, Bhimba BV (2014) Antibacterial and antifungal activity of silver nanoparticles synthesized using Hypnea muciformis. Biosci Biotech Res Asia 11:235–238. https://doi.org/10.13005/bbra/1260
Ghosh N, Paul S, Basak P (2014) Silver nanoparticles of Moringa oleifera: Green synthesis, characterization and its antimicrobial efficacy. J Drug Delivery Ther 4:42–46. https://doi.org/10.22270/jddt.v0i0.906
Gnanadesigan M, Anand MR, Ravikumar S, Maruthupandy M, Ali S, Vijayakumar M, Kumarageru VAK (2012) Antibacterial potential of biosynthesized silver nanoparticles using Avicennia marina mangrove plant. Appl Nanosci 2:143–147. https://doi.org/10.1007/s13204-011-0048-6
Gopu M, Kumar P, Selvankumar T, Senthilkumar B, Sudhakar C, Govarthanan M, Kumar RS, Selvam K (2021) Green biomimetic silver nanoparticles utilizing the red algae Amphiroa rigida and its potent antibacterial, cytotoxicity and larvicidal efficiency. Bioprocess Biosyst Eng 44:217–223. https://doi.org/10.1007/s00449-020-02426-1
Govindaraju K, Tamilselvan S, Kiruthiga V, Singaravelu G (2010) Biogenic silver nanoparticles by Solanum torvum and their promising antimicrobial activity. J Biopest 3:394–399
Gurunathan S, Jae WH, Vasuki E, Muniyandi J, Jin-Hoi K (2013) Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. BioMed Res Int 2013:535796. https://doi.org/10.1155/2013/535796
Han JW, Gurunathan S, Jeong JK, Choi YJ, Kwon DN, Park JK, Kim JH (2014) Oxidative stress mediated cytotoxicity of biologically synthesized silver nanoparticles in human lung epithelial adenocarcinoma cell line. Nanoscale Res Lett 9(459):1–14. https://doi.org/10.1186/1556-276X-9-459
Han JK, Madhusudhan A, Bandi R, Park CW, Kim JC, Lee YK, Lee SH, Won JM (2020) Green synthesis of AgNPs using lignocellulase nanofibrils as a reducing and supporting agent. Bioresour 15:2119–2132. https://doi.org/10.15376/biores.15.2.2119-2132
Iravani S, Korbekandi H, Mirmohammadi S, Zolfaghari VB (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9:385–406 (PMCID: PMC4326978)
Jyoti K, Singh A (2017) Evaluation of antibacterial activity from phytosynthesized silver nanoparticles against medical devices infected with Staphylococcus spp. J Taibah Uni Med Sci 12:47–54. https://doi.org/10.1016/j.jtumed.2016.08.006
Kgatshe M, Aremu OS, Seru LK, Gopane R (2019) Characterization and antibacterial activity of the bio-synthesized silver nanoparticles using the ethanolic extract of Pelargonium sidoides (DC.). J Nanomater 2019:3501234. https://doi.org/10.1155/2019/3501234
Khalil MMH, Ismail EH, El-Baghdady KZ, Mohamed D (2014) Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arabian J Chem 7:1131–1139. https://doi.org/10.1016/j.arabjc.2013.04.007
Krithiga N, Rajalakshmi A, Jayachitra A (2015) Green synthesis of silver nanoparticles using leaf extracts of Clitoria ternatea and Solanum nigrum and study of its antibacterial effect against common nosocomial pathogens. J Nanosci 2015:928204. https://doi.org/10.1155/2015/928204
Lakshmanan G, Sathiyaseelan A, Kalaichelvan PT, Murugesan K (2017) Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: assessment of their antibacterial and anticancer activity. Karbala Int J Modern Sci 4:61–68. https://doi.org/10.1016/j.kijoms.2017.10.007
Lee SH, Jun BH (2019) Silver nanoparticles: synthesis and applications for nano-medicine. Int J Mol Sci 20:1–24. https://doi.org/10.3390/ijms20040865
Moodley JS, Krishna SBN, Pillay K, Sershen GP (2018) Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv Nat Sci Nanosci Nanotech 9:015011. https://doi.org/10.1088/2043-6254/aaabb2
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Mousavi B, Tafvizi F, Bostanabad SZ (2018) Green synthesis of silver nanoparticles using Artemisia turcomanica leaf extract and the study of anti-cancer effect and apoptosis induction on gastric cancer cell line (AGS). Artif Cell Nanomed Biotechnol 46:499–510. https://doi.org/10.1080/21691401.2018.1430697
Mythili R, Selvankumar T, Kamala-Kannan S, Sudhakar C, Ameen F, Al-Sabri A, Selvam K, Govarthanan M, Kim H (2018) Utilization of market vegetable waste for silver nanoparticle synthesis and its antibacterial activity. Mater Lett 225:101–104. https://doi.org/10.1016/j.matlet.2018.04.111
Nayak S, Bhat MP, Udayshankar AC, Lakshmeesha TR, Geetha N, Jogaiah S (2020) Biosynthesis and characterization of Dillenia indica-mediated silver nanoparticles and their biological activity. Appl Organomet Chem 34:e5567. https://doi.org/10.1002/aoc.5567
Ndikau M, Noah NM, Andala DM, Masika E (2017) Green synthesis and characterization of silver nanoparticles using Citrullus lanatus fruit rind extract. Int J Anal Chem 2017:8108504. https://doi.org/10.1155/2017/8108504
Padalia H, Moteriya P, Chanda S (2015) Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arabian J Chem 8:732–741. https://doi.org/10.1016/j.arabjc.2014.11.015
Peiris MK, Gunasekara CP, Jayaweera PM, Arachchi NDH, Fernando N (2017) Biosynthesized silver nanoparticles: are they effective antimicrobials? Mem Inst Oswaldo 112:537–543. https://doi.org/10.1590/0074-02760170023
Rautela A, Jyoti R, Mira D (2019) Green synthesis of silver nanoparticles from Tectona grandis seeds extract: characterization and mechanism of antimicrobial action on different microorganisms. J Anal Sci Technol 10:5. https://doi.org/10.1186/s40543-018-0163-z
Rauwel P, Siim K, Stanislav F, Erwan R (2015) A Review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Adv Mater Sci Eng 2015:682749. https://doi.org/10.1155/2015/682749
Sahu C, Kalyani G, Baghel DBD, Singh R (2016) Bioanalytical determination of irbesartan in bulk powder and pharmaceutical preparation using spectrophotometric method. Int J Chem Pharm Anal 3:1029
Sampath G, Govarthanan M, Rameshkumar N, Dai-Viet NV, Krishnan M, Sivasankar P, Kayalvizhi N (2021) Eco-friendly biosynthesis metallic silver nanoparticles using Aegle marmelos (Indian bael) and its clinical and environmental applications. Appl Nanosci. https://doi.org/10.1007/s13204-021-01883-8
Seigneuric R, Markey L, Nuyten DSA, Dubernet C, Evelo CTA, Finot E, Garrido C (2010) From nanotechnology to nanomedicine: applications to cancer research. Curr Mol Med 10:640–652. https://doi.org/10.2174/156652410792630634
Selvam K, Sudhakar C, Govarthanan M, Thiyagarajan P, Sengottaiyan A, Senthilkumar B (2019) Eco-friendly biosynthesis and characterization of silver nanoparticles using Tinospora cordifolia (Thunb.) Miers and evaluate its antibacterial, antioxidant potential. J Radiat Res Appl Sci 10:6–12. https://doi.org/10.1016/j.jrras.2016.02.005
Sengottaiyan A, Sudhakar C, Selvam K, Selvankumar T, Govarthanan M, Senthikumar B, Manoharan K (2016) Biogenic synthesis of Adhatoda vasica L. Nees mediated silver nanoparticles and their antibacterial, anticancer activity on Hep-G2 cell lines. Int J Tradition Complement Med 1:0001–0009
Shaik MR, Mujeeb K, Mufsir K, Abdulrahman AW, Hamad ZA, Mohammed RHS, Jilani PS, Anis A, Adeem M, Merajuddin K, Syed FA (2018) Plant extract assisted green synthesis of silver nanoparticles using Origanum vulgare L. extract and microbicidal activities. Sustainability 10:913. https://doi.org/10.3390/su10040913
Singh SP, Mishra A, Shyanti RK, Singh RP, Acharya A (2021) Silver nanoparticles synthesized using Carica papaya leaf extract (AgNPs-PLE) causes cell cycle arrest and apoptosis in human prostate (DU145) cancer cells. Biol Trace Elem Res 199:1316–1331. https://doi.org/10.1007/s12011-020-02255-z
Singhal G, Riju B, Kunal K, Ashish RS, Rajendra PS (2011) Biosynthesis of silver nanoparticles using Ocimum sanctum leaf extract and screening its antimicrobial activity. J Nanopart Res 13:2981–2988. https://doi.org/10.1007/s11051-010-0193-y
Skiba MI, Victoria IV, Pivovarov A, Makarshenko NP (2020) Green synthesis of silver nanoparticles in the presence of polysaccharide: optimization and characterization. J Nanomater 2020:3051308. https://doi.org/10.1155/2020/3051308
Sorayya B, Fahimeh BA, Sahar A, Seyed A, Sadat S (2019) Green engineered biomolecule-capped AgNPs fabricated from Cichorium intybus extract: in vitro assessment on apoptosis properties toward human breast cancer (MCF-7) cells. Bio Trace Elem Res 187:392–402. https://doi.org/10.1007/s12011-018-1392-0
Sreenivasa N, Meghashyama BP, Pallavi SS, Bidhayak C, Dattatraya A, Muthuraj R, Shashiraj KN, Halaswamy H, Dhanyakumara SB, Vaishnavi MD (2020) Biogenic synthesis of silver nanoparticles using Paenibacillus sp. in-vitro and their antibacterial, anticancer activity assessment against human colon tumour cell line. J Environ Biol 42:118–127. https://doi.org/10.22438/jeb/42/1/MRN-1401
Srirangam GM, Rao PK (2017) Synthesis and characterization of silver nanoparticles from the leaf extract of Malacha capitata (L.). Rasayan J Chem 10:46–53. https://doi.org/10.7324/RJC.2017.1011548
Taranath TC, Bheemanagouda NP, Santosh TU, Sharath BS (2015) Cytotoxicity of zinc nanoparticles fabricated by Justicia adhatoda L. on root tips of Allium cepa L. a model approach. Environ Sci Pollut Res 22:8611–8617. https://doi.org/10.1007/s11356-014-4043-9
Ullah I, Khalil AT, Ali M, Iqbal J, Ali W, Alarifi S, Shinwari ZK (2020) Green-synthesized silver nanoparticles induced apoptotic cell death in MCF-7 breast cancer cells by generating reactive oxygen species and activating caspase 3 and 9 enzyme activities. Oxid Med Cell Longevity 2020:1215395. https://doi.org/10.1155/2020/1215395
Vijaykumar PPN, Pammi SVN, Pratap K, Satyanarayana KVV, Shameem U (2014) Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their antibacterial activity. Ind Crops Prod 52:562–566. https://doi.org/10.1016/j.indcrop.2013.10.050
Zafar S, Zafar A (2019) Biosynthesis and characterization of silver nanoparticles using Phoenix dactylifera fruits extract and their in vitro antimicrobial and cytotoxic effects. Open Biotechnol J 13:37–46. https://doi.org/10.2174/187407070
Zhang XF, Liu ZG, Shen W, Gurunathan S (2016) Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17:1534. https://doi.org/10.3390/ijms17091534
Acknowledgements
The authors are thankful to the P.G. Department of Studies in Botany for extending laboratory facility and SAIF (USIC), Karnatak University, Dharwad for providing instrumentation facilities. This project was supported by seed grant for research program (2021-22/62), Karnatak University, Dharwad, Karnataka, India. The project was supported by Researchers Supporting Project number (RSP-2021/142), King Saud University, Riyadh, Saudi Arabia.
Funding
The project was supported by Researchers Supporting Project number (RSP-2021/142), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
SN designed the concept and supervised the experiments. MPB wrote the manuscript and carried out experimental analysis along with MR. RSK, AIA, NA, KD and KP interpreted the anticancer analysis results with data validation. DSB and PSS carried out characterizations. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhat, M.P., Kumar, R.S., Almansour, A.I. et al. Characterization, antimicrobial activity and anticancer activity of Pyrostegia venusta leaf extract-synthesized silver nanoparticles against COS-7 cell line. Appl Nanosci 13, 2303–2314 (2023). https://doi.org/10.1007/s13204-021-02120-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-021-02120-y