Abstract
Over a past few decades, the major contemplation in the petroleum industry has been to develop a drilling fluid that minimizes the wellbore instability problems and shows high performance in drilling shale formations. This paper focuses on the formulation of a novel water-based drilling fluid using nano-particle graphene oxide (GO) and micron particle Pure-bore in varying concentration ranging from 0.1 to 0.5 wt%. To prove their efficacy, mud containing these particles was used to mitigate the swelling characteristics of the Murree shale formation obtained from the Northern region of Pakistan. Results reveal that the addition of GO and Pure-bore in different concentrations in water-based drilling fluid, significantly improves the rheological properties, filtration characteristics and swelling inhibition of the base mud. Almost 50% swelling of the Murree formation was reduced while using these two particles. Additionally, when the comparison was done between the two particles, it was concluded that GO mud provides a better filtrate control mud and swelling inhibition in the shale formation than Pure-bore. The reason of its better performance is because it develops a better membrane like mud cake having a better surface quality, toughness and stiffness. Moreover, the active functional group especially hydroxyl and carboxyl present in GO interacts actively with Smectite clay mineral with the help of hydrogen and chemical bonds resulting in a better filtration control. These properties of GO ensure that the hydrogen ions do not penetrate into the nano-spacing of the formation thus providing a better wellbore stability in Murree shale formation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drilling fluids used in oil and gas drilling sector provide numerous functions, which includes transportation and removal of drill cuttings, lubrication and cooling of the drill bit and the most important of all is to provide stability to the borehole. Selection of an appropriate drilling fluid is the fundamental task for the successful drilling operations, especially in shale formations (Bayat et al. 2018), where the wellbore stability issues are quite prominent. Nearly, 75% of the shale formations are drilled with water-based drilling mud (WBDM) (Aftab et al. 2020b), as this mud is relatively inexpensive and ecofriendly in comparison with oil-based mud (OBM) (Huang et al. 2017; Wajheeuddin and Hossain 2018; Aftab et al. 2020a). Nevertheless, there are some problems of using WBDM in shale which results in dispersion and swelling problems, thus reducing the stability of shale formation (Saleh and Ibrahim 2019; Jiang et al. 2020).
Almost 70% of the wellbore stability problems are associated with shale (Aftab et al. 2017). The structure of this formation comprises of crystal platelets that are tetrahedral and octahedral in shape and are link with oxygen atoms and having nano-scale pore spacing between them. The minerals that are linked with this formation are silts, clays and mudstone (Wang et al. 2020). The presence of clay minerals in particularly Smectite and Kaolinite which have great affinity to water is the main reason behind the shale instability problems (Aftab et al. 2017). The migration of hydrogen ions from the water-based drilling fluids into the nano-platelets of the clay minerals, thereby yield an increase in swelling percentage, which causes a dispersion effect (Khan et al. 2020). The swelling and dispersion in shale creates destructive impacts on the stability of the wellbore, which ultimately further causes loss of circulation; mechanical as well as differential pipe sticking, and wellbore caving problems (Bayat et al. 2018; Ewy and Morton 2009). The migration of these smaller radii hydrogen ions is a complex mechanism as it encompasses hydraulic, physical and chemical phenomena (Al-Bazali et al. 2009). As these H+ ion moves into the shale formation, it gives rise to pore pressure, which subsequently surge the wellbore stresses and arises wellbore instability issues (Khan et al. 2020). Several conventional additives are used for the plugging of these nano-spacing layers in shale formation; however, their effectiveness fails up to a certain extent because of the nano-spacing in shale formation. To begin with, Potassium chloride (KCl) mud was commonly adopted for the better swelling resistance against these H+ ions (Aftab et al. 2017) but there were two major limitations. First, because of large ionic radii of K+, it was unable to stop the migration of small H+ ions. Second, at higher concentration of KCl, the mud starts to dispersed into two distinct phases (Aftab et al. 2017). Owing to these limitations, application of the use for polymer-based mud came into existence (Chesser et al. 1987). On the other hand, at elevated temperature, the performance of these polymer-based fluid was dubious (Aftab et al. 2017, 2016; Growcock et al. 2011). More recently, importance is given to the designing of smart drilling fluid that involves the use of nano-particles to improve drilling fluid efficiency (Al-Yasiri and Al-Sallami 2015; Al-Yasiri et al. 2019), control the amount of filtration loss, minimize the swelling and dispersion effect and to improve the rheological properties of the fluid (Aftab et al. 2020a). This study aims to present a comparison work between micron particle Pure-bore and nano-particle graphene oxide in inhibition the Pakistan’s shale formation.
Pure-bore (PB) is a high shear thinning natural polysaccharides micro-particle (Yang et al. 2017) with the particle size ranging from 5 to 200 µm (Yang et al. (2017)). The bulk density for this particle ranges from 0.3 to 0.8 g/cm3.. It is considered as an environmental friendly particle as no metallic element is associated with it. The major constituents present in Pure-bore are carbon, oxygen and chlorine (Yang et al. 2017). This chemical compound is normally classified as a polymer, so when its molecules interrelates with each other they form a network of structure which in due course improves the filtration performance of the drilling fluid by encapsulating the shale hydration (Yang et al. 2017). Nevertheless, as the size of Pure-bore is in microns, therefore, the plugging performance debilitated after a certain extent. As a result, it is essential to use a more suitable nano-size particle in shale inhibition process.
Graphene is basically derived from graphite with unique chemical and physical properties (Moraes et al. 2020). It is a light-weighted compound with a relatively high surface area of 2600 m2/g. This material exhibits pronounced tribological properties (Moraes et al. 2020; Dasari et al. 2017), high tensile strength, thermal conductivity and is a good conductor of heat and electricity (James and Tour 2012). Nevertheless, because of its hydrophobic nature, this particles demonstrates difficulty in the formulation of nano-sheets solutions in different solvents especially in water (Moraes et al. 2020). Subsequently, an alternate compound named graphene oxide (GO), which is normally used as a precursor in the production of graphene is widely used in different drilling applications (Wang et al. 2020). GO has a two-dimensional (2D) structure comprising of sheets of 1 nm in thickness (Wang et al. 2020). Moreover, these sheets are soft and flexible in nature and when used as an additive in drilling fluid shows a vast variation in rheological properties (Soares et al. 2020). Different studies also shows that GO develops a fine thin filter cake around any formation that drastically reduces the filtration loss (Kosynkin et al. 2012). It also offers a better thermal stability along with shear thinning effect at higher temperature in contrast with conventional clay additives such as bentonite (Ghafuri and Talebi 2016). These interesting properties of GO encourage its use in the drilling fluid, particularly when drilling shale formation.
Several researchers have worked on the preparation of GO and at the same time demonstrates its application in improving the rheological properties, filtration loss and providing lubricity to WBDFs (Kusrini et al. 2020). Dmitry et.al in a research also showed that GO are well-exfoliated and their substantially lower concentration develops a more desirable performance in drilling fluid in comparison with the conventional clay additives (Kosynkin et al. 2012). Furthermore, particles of GO combines with each other to form a large surface film with interconnected layered structure, which is effective in controlling the fluid loss and produces a fine thin integrated film of filter cake on the surface of wellbore, which helps in preventing the flow of water in the formation (Xuan et al. 2014). An et al. studied the interaction of GO and Smectite, with the intention to elucidate the interaction mechanism of these two particles (Yuxiu et al. 2016). In contrast to GO, study related to Pure-bore is relatively limited in drilling industry. Xianyu et al. studied the shale inhibition mechanism for Pure-bore. It was reported that using Pure-bore with NaCl salt, inhibition in shale increases up to 61.93%, which further upgraded to 62.4%, if a larger ionic radius salt such as KCl is used with it (Yang et al. 2017). The study also shows that the combination of different brines and Pure-bore exhibits a some strong volatility characteristics (Yang et al. 2017).
The main reason behind this study is to examine the performance of GO and Pure-bore containing drilling fluids in Murree shale formation that is situated in the Northern region of Pakistan. The particles are characterized by Fourier-transformed infrared spectroscopy (FTIR), QXRD analysis and SEM imaging. Filtration test was also carried out to demonstrate the plugging performance of these two particles in shale formation. Additionally, Linear Dynamic swell meter test was performed on Murree formation with three different mud samples that comprises of GO mud, Pure-bore mud, and fresh water. The research work in this paper is carried out at ambient condition. The scope of the work is to check the compatibility of WBDF in the Murree shale, with the intention of reducing the non-productive time (NPT) and to improve the drilling efficiency especially in the Northern region of Pakistan.
Materials and methods
Materials for mud preparation
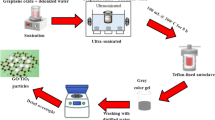
The materials used in the preparation of drilling muds were acquired from different sources. Graphene nano-particles were purchased from Xi’an Lyphar Biotech Co. Ltd. (Shaanxi Province, China). Pure-bore (PB) was acquired from a drilling company located in Pakistan. Polyanionic cellulose (L) technical grade, sodium carbonate (Na2 CO3, also known as soda ash), amine and Barite were all obtained from the labs of Petroleum Engineering Department, NED University of Engineering & Technology. Xanthan gum used as viscosifiers, was attained from a service company operating in Pakistan. Samples of Murree shale formation were collected from the Northern part of Pakistan with the help of a drilling company working in Pakistan. The experimental design/methodology is given in Fig. 1
Characteristics of both particles
Graphene oxide (GO) comprises of 99.7% carbon content that was determined with the help of Burming method. Conductivity test was carried out on the GO sample using sheet square resistance which showed a conductivity of 925 S/cm (Wang et al. 2018; Eda et al. 2008; Kobayashi et al. 2010). The GO sample also demonstrated 0.08 g/cm3 and 0.12 g/cm3 of apparent and tap density from vibrating density meter whereas the thickness of this nano-particle ranges from 2 to 7 nm. Fourier-transform infrared spectroscopy (FTIR) of graphene oxide and Pure-bore was recorded using Bruker ALPHA II spectrometer (IAC, Karachi), with the spectral range from 500 to 6000 cm−1, and a resolution of better than 2 cm−1. High-resolution transmission electron microscopy (HRTEM) was conducted on thin layer of graphene nano-plates for the purpose of attaining the number of plies. 3–7 numbers of plies were gathered from the test data. Furthermore, characteristics of both particles were conducted using QXRD analysis. This analysis was performed using XPERT-PRO Diffractometer system using Ni-filtered radiation (λ = 1.54060 Å). The scan range (°2Th.) starts at 10.0125°–79.9875°. Both the samples were placed in powder form in the cells, respectively. Additionally, morphology of both the particles was studied using scanning electron microscope (SEM), situated in IAC Karachi.
Preparation of nano-based mud and micron-based mud
One Barrel of water-based drilling mud (350 cc), considered equivalent to 1 barrel of field mud, was prepared by mixing soda ash, PAC-L, Xanthan gum, Barite and amine. The preparation was designed with the purpose of achieving a mud weight of 10 ppg. To make the base mud, 0.25 g of soda ash was added in 303 cc of distilled water and the mixture was stirred for 2 min. After 2 min, 5 g of PAC-L was added in the solution and the mixture was again mixed from 5 min. Then, 1 g of Xanthan gum was added and the solution was blended again for 5 min. 7% of amine was added in the mixture and was stirred for 2 min. Lastly, 60 g of Barite as a weighing agent was added and the solution was again mixed in Hamilton Beach Mixer for 30 min to achieve uniform mixture. According to API standard (API-1608), the mud was kept at room temperature (27 °C) for 16 h before any testing. All the testing of the water-based drilling fluids was conducted according to API Recommended Practice 13B-1. Both of the particles were then added in dry powder form to the base mud in different concentrations ranging from 0.1 to 0.5 wt%. The mud was again agitated for 30 min in the Hamilton Beach Mixer and 40 min in heavy duty mixture at 10,000 rpm to obtain a proper consistency. It can be perceived from Fig. 2 that all the prepared mud samples proved to be stable and there was no sign of phase segregation observed after 24 h. For rheological property experimentation, API 13D was scrupulously followed.
Rheological properties
Rheological properties of water-based drilling fluid, which includes plastic viscosity (PV) in centipoise (cP), apparent viscosity (AV) in cP, yield point (YP) in lb./100 ft2, gel strength (GS) measured at 10 s and 10 min, were measured using Fann Rheometer Model 286 at standard condition. Filtration loss was determined using the Series 300 API Low Pressure Low Temperature (LPLT) Filter Press at 100 psig. All the tests including filtration test and tests for mud rheological properties were carried out using API Recommended Practice 13B-1 and 13B-2. Figure 3 shows the API filter press schematic that was used during the testing. Readings at 600, 300, 200, 100, 6 and 3 revolutions per minute (RPM), were gathered.
These values were then used to determine the rheological parameters as indicated in Eq. 1 till Eq. 3. For GS at 10 s and 10 min, the fluid was first rotated at 600 RPM for 10 s, and then was left undisturbed for 10 s and 10 min, respectively, before shifting the dial to gel and recording the deflection.
Figure 4 shows the graphically representation of shear stress (lb. /100ft2) against the shear rate (RPM) for the based fluid without nano-particles at standard condition. It was observed that the behavior of the drilling fluid exhibits the Herschel Buckley non-Newtonian model (William et al. 2014). The model also confirms that the fluid requires certain minimum shear stress to initiate the flow. The mathematical expression for the model that relates the shear stress and shear rate is given in Eq. 4, where K and n are defined as the consistency index and power law index.
Swelling characteristics
It is extremely important to design a fluid that provides the wellbore stability. Wellbore instability can occur as a result of (1) mechanical failure of the formation, (2) tensile failure of the formation, (3) compressive failure of the formation and (4) swelling. Formations that are rich in clay minerals are the potential source of swelling (Wang et al. 2020). The swelling characteristic of Murree formation was determined using the OFITE Dynamic Linear Swell Meter with compactor (Model 150-80-230 V). According to standard OFITE testing procedure, fifteen grams of Murree formation was placed in compactor at 8000 psi for 1.5 h (Aftab et al. 2016) to get four pellets ready for testing. Once the samples were ready, all three cells for Swell meter were calibrated using calibration procedure.
Once the calibration process was completed, the pellets were then placed in the swell meter cell. In each cup, 20 mL of drilling fluid was introduced. Three shale pellets were tested simultaneously with three different drilling fluids. The linear dynamic swell meter has capacity of testing maximum of four samples at the same time. The swelling test was conducted for 18 h before the sample was removed. In Cell 1, the shale sample was tested with Pure Bore mud, in cell 2, graphene oxide (GO) mud was used, whereas cell 3comprises of fresh water sample.
The main principle behind the apparatus is to record any change in height of the sample once it comes in contact with the fluid. The change in height is recorded by a transducer (Aftab 2020) that is the part of the equipment. The greater the migration of ions inside the shale platelets, the greater will be the swelling recorded against the time. Equation 5 (Aftab et al. 2020a) shows the formula for the % swelling that is been calculated from the linear swell meter. The % swelling was then plotted against the time and it was observed that greater the swelling % poor will be the inhibition quality of the fluid (Aftab et al.2020a).
Results and discussion
Murree formation characterization
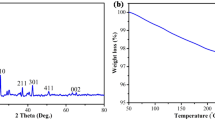
Murree formation mainly comprises of fine red shale along with thick layer of mudstone. The QXRD of this formation was conducted by the Diffractometer (XPERT-PRO), using Cu filter and wavelength of 1.54060 Å. The 0.5 g of sample was air dried in moisture extraction oven (UE 200) before being ready for QXRD. Figure 5 shows the QXRD result of Murree formation. The first strong peak at 2° Theta = 20.90° corresponds to an interlayered spacing of 4.25 nm that is referred to as Kaolinite (Butt 2012). The most powerful peak was observed at 2° Theta = 26.65° that provide the interlayered spacing of 3.35 nm, which according to Bragg’s equation is referred to as Quartz (Butt 2012). The peak at 2° Theta = 27.78° is stated as Diopside. Another strong peak was observed at 2° Theta = 44.71°, corresponding to an interlayer spacing of 2.02 nm, which relates to an interlayered spacing range of Montmorillonite (Fukushima 1984). This clay mineral is more susceptible to swelling as it comprises of higher content of Smectite (Aftab et al. 2020a, 2016; Asef and Farrokhrouz 2013). Table 1 indicates the detail of the Murree formation mineralogy that was obtained from QXRD analysis.
Based on the result from QXRD, the minerals were congregated into four major sections, which includes silicates, clay, iron ores and carbonates. It was perceived that this formation comprises of 63% of silicates, 26% clay contents that comprises of 13.6% Smectite, 6.7% Kaolinite and 5.7% is Illite. The remaining two sections are carbonates and iron ores, which are 6% and 5%, respectively.
FTIR and QXRD analysis of graphene oxide and pure-bore
To determine the functional groups associated with any sample, FTIR spectroscopy is a useful tool. The FTIR spectrum for both the GO and Pure-bore is shown in Fig. 6. From the figure, it is evident that there are more than five absorption bands in both the particles, hence both of these particles can be referred to as a complex molecule (Nandiyanto et al. 2019). The peak in Fig. 6a at 3185 cm−1 confirms the presence of O–H bond. The band at 1718 cm−1 indicates the presence of C=O bond. The characteristics band at 1612 cm−1 indicates the presence of C=C. The absorption band at 1041 cm−1 reflects the fingerprint of C–O bond. The result gathered from FTIR analysis confirms the presence of oxygen functionalized group. The FTIR analysis of Pure-bore is illustrated in Fig. 6b. The absorption peaks at 3278 cm−1, 1717 cm−1, 1595 cm−1, 1017 cm−1 and 698 cm−1 were assigned to O–H bond, C=O bond, carboxylate, fingerprint of C–O bond and stretching vibration of Aliphatic chloro-carbon (Nandiyanto et al. 2019).
The QXRD analysis for both Pure-bore and graphene oxide are shown in Fig. 7a and b, respectively. The strong peak in Fig. 7a at 2Ө = 31.67° shows the presence of Na, the peaks at 2Ө = 44.64° and 45.44° in the curve shows carbon and oxygen, which according to Bragg’s equation corresponds to the interlayer spacing of 2.02 Å and 1.99 Å. Similarly, Fig. 7b shows the peak at 2Ө = 11.63° (d = 0.76 nm) that corresponds to GO (Vu et al. 2015).
Figure 8a and b illustrates the SEM images of GO and Pure-bore, respectively. Figure 8a exemplifies that how flaskes of GO are closely related with each other. Figure 8b comprehend that Pure-bore has a needle like morphology. This elongated structure of Pure-bore results in an inimitable colloidal properties exclusively in filtration loss.
Effect of GO and Pure-bore on the rheological properties of the based mud
Effect on plastic viscosity (PV cP) and apparent viscosity (AV cP)
Plastic viscosity (PV) is defined as viscosity that is measured at an infinite shear rate using Bingham plastic model (Güneyisi et al. 2016). In general, drilling fluid with a higher value of PV exhibits a higher pumping rate, which is not always preferred for drilling activities (Bayat et al. 2018). Lower the value of PV more efficient will be the drilling rate (Ismail et al. 2016) as this will demonstrates a low viscosity at the drilling bit. However, drilling fluids that displays a lower magnitude of viscosity will yield a lower value of hydrostatic pressure that is not always considered as an effective outcome (Bayat et al. 2018). The PV and the mud weight for the base mud used in this research were measured to be 17 cP and 10 ppg, respectively. In contrast, the PV and AV for both particles showed an increasing trend at various concentration levels (0.1–0.5 wt%) as depicted in Fig. 9.
Figure 9a illustrates the graphically representation of PV for the two particles. During the experimentation, it was observed that PV was raised from 19 to 21 cp with the addition of graphene oxide at ambient condition. For the case of Pure-bore, PV value shows a continuous increase in trend. It was perceived that when the PV increased to 25 cP, it was almost 47% of an increase in PV in comparison with the base mud. PV is mainly a function of solids concentration and their configuration (Aftab et al. 2016; Paritosh Dhiman et al. 2018). Recently, Ismail and Rashid (2018) used graphene nano-platelets (GNP) in WBDF and found that the targeted PV was achieved at 0.02 ppb concentration, similarly, Husin et al. (2018) also reported the PV for the GNP to be in range of 20–24 cp for similar concentration that is been used in this study. The results of this study for PV are compatible with the Husin et al. (2018) as it shows similar range of PV values for the same concentration. The improvement in PV values in the presence of GO nano-particles is observed largely because of the well dispersion of this particle in base mud. Furthermore, this nano-particle has a larger surface area per unit volume, hence, the interaction of this particle with the drilling fluid matrix further improves the PV (Salam et al. 2018).
Figure 9b depicts the apparent viscosity (AV) variation when the two particles concentrations varies from 0.1 to 0.5 wt%. For the base mud, the AV was measured to be 28 cp. For GO, apparent viscosity almost remained constant at 17 cp. However, for the case of Pure-bore, the AV increased from 17 cp at 0.1 wt% to 26 cp at 0.5 wt%. According to Yang et al. (2017), the AV achieved after the use of Pure-bore was 46 cp at 25 °C. However, in the current study, the maximum AV value that was achieved is 27 cp at the same temperature condition. In all the cases, the mud weight remained relatively constant. For GO, it was maintaining 9.65 ppg whereas for Pure-bore, it was 9.68 ppg.
Effect on yield point (YP lb./100 ft2)
Yield point (YP) is basically defined as the minimum shear stress required by a fluid to flow (Noah et al. 2016). It is the measure of force of attraction that develops in drilling mud as a result of positive and negative charges (Aftab et al. 2016; Abduo et al. 2015). An increase in yield point will result in an increase in efficiency of transportation of the drilling cutting up to the surface (Ismail et al. 2016). However, an increase in YP beyond a certain limit will automatically develop an excessive pump pressure, effect the circulation transition point and productivity of cutting transportation mechanism (Ismail et al. 2016; Noah et al. 2016). The YP of the base mud was measured to be 20 lb./100 ft2. The effect of addition of these two particles on YP is demonstrated in Fig. 10.
The YP for Pure-bore shows an increasing trend with an increase in concentration. The value of YP is almost double to what it was at the initial concentration of 0.1 wt%. The maximum value of YP in the case of Pure-bore is 55 lb./100 ft2 at the concentration of 0.5 wt%. However, the YP of GO approximately remains constant throughout the variation in concentration and reaches to a maximum value of 31 lb./100 ft2 at 0.3 wt%.According to a study by Husin et al. (2018) and Ismail and Rashid (2018) they found the YP for GNP to be in range of 24–22 lb./100 ft2 at 0.1–0.5 wt% and 0.01–0.04 ppb, respectively. The result in this study shows the compatibility with Husin et al. (2018) for the GO. On the other hand, Pure-bore as reported by Yang et al. (2017) found the YP value of 25 at 25 °C which is half of what it is attained in the present study. Both the particles showed an increase in YP in comparison to the base mud. In regard to surface interaction, it could be explained that both GO and Pure-bore exhibits attractive surface force when dispersed in the base mud, which results in a higher YP values (Salam et al. 2018).
Effect on gel strength (GS lb./100 ft2)
Gel strength (GS) is basically defined as the ability of the drilling fluid to make the drill cutting suspended when in stationary condition (Bayat et al. 2018). In general, it is the attraction within the drilling fluid that helps in suspension of the cutting (Bayat et al. 2018). Figure 11a and b exhibits the effect on gel strength after 10 s and 10 min when both particles are added in different concentrations. For the base mud, the gel strength at 10 s and 10 min was 6 and 8 lb./100 ft2, respectively. The gel strength for the base mud showed an increasing trend when there is an increase in concentration of Pure-bore. At 0.5 wt% of Pure-bore, the gel strength at 10 s and 10 min are almost 2.5-fold of what it was for the base mud. In presence of GO, the gel strength at 10 s showed a relatively smaller increase in value, as it showed a change of 2 lb./100 ft2 when concentration increased from 0.1 to 0.5 wt%. On the other hand, for 10 min gel strength, it remained fairly constant at 8 lb./100 ft2 except at 0.2 wt% where it declined slightly to 6 lb./100 ft2.
According to Mohideen et al. (2019) for GO gel strength was 25 and 28 lb./100 ft2 at 10 s and 10 min, respectively. On the other hand, Aftab (2016) and Ji et al. (2012) reported the operating range of gel strength is between 5.5 and 10 lb./100 ft2 that is similar to the one obtained in this study. While for the Pure-bore, Yang et al. (2017) stated gel strength to be 8.5/24 for 10 s/10 min, respectively, at 25 °C which is comparable to the one attained in this study. It is apparent that because of gelation, the gel strength at 10 min is slightly than 10 s gel strength. Nevertheless, it is extremely important for a fluid to maintain higher gel strength so that drilling problem associated with accumulation of drilling cutting at the bottom can be eluded.
Effect on filtrate loss (mL)
Overbalance drilling result in greater hydrostatic pressure than the formation pore pressure. This phenomena result in invasion of the drilling fluid in the pores of the formation, which ultimately develops loss of circulation. The amount of filtrate that is being lost into the formation during drilling operations is not desirable. A number of different wellbore problems such as shale swelling, loss of mud and wellbore instability issues arise as a result of this loss (Deng et al. 2015). In this study, two particles GO and Pure-bore is added as filtrate loss material that prevents the loss of drilling fluid in the pore throat. The API filtrate loss in the base mud was measured to be 25 mL/30 min. According to Aftab et al. (2016), fluid loss of even 5.5 mL is highly vulnerable in shale formation (Aftab et al. 2016). Figure 12 describes the API filer press test result conducted on two different mud samples at varying concentrations. The filter paper used during the study is having 115 µm thickness. Results are plotted after API recommended 30 min of testing in the low-pressure and low-temperature filter press equipment. It was observed that the addition of NPs in the base mud significantly reduces the filtrate volume. For GO, at 0.5 wt%, it was almost one-fifth of what it was observed for the base mud. On the contrary, for Pure-bore, the filtrate volume reduces to almost 15 mL at 0.5 wt%, but still it is considered to be below par for the shale formation. According to Husin et al. (2018) and Ismail and Rashid (2018), the filtrate volume the was obtained for GNP was in range of 3.5–5 mL, which is also similar to the one that is been obtained during this study. On the other hand, Yang et al. (2017) observed filtrate volume of 4 mL at 25 °C in the case of Pure-bore that is different of what is perceived during this study. Based on the results, it was concluded that GO exhibits a better plugging capacity especially in the shale formation where the pore sizes are in nano-meter (nm) because of the strong force of attraction and layered structure.
Linear dynamic swell meter analysis for pure-bore and GO
The swelling test was performed using OFITE Linear Dynamic Swell meter. Three samples of 20 g were placed in the three different cells that were calibrated before testing. The samples were placed in the cells initially for 18 h. However, to notice any further increase in swelling trend, the time period for the testing was increased to 23 h. It was discerned that after recommended time period of 18 h. of testing, the samples showed almost a linear trend in their swelling % and change in height.
Figure 13a shows the swelling % of Murree formation in three different systems. It was observed that the GO mud sample shows the minimum swelling characteristics of 9.81% after the test. 9% of the swelling was achieved within 7 h of testing; however, remaining 0.81% was attained after 16 h. Similarly for Pure-bore, the swelling % after 23 h of testing was 10.77%, though similarly after 7 h of testing the sample shows almost 10.06% of swelling behavior. On the other hand, the interaction between the fresh water and Murree formation proved to be most lethal, as within in 3 h of testing the sample showed 17.4% of swelling which almost 50% greater swelling compared with GO and Pure-bore samples. Figure 13b) illustrates the corresponding change in height of the samples. Initially, the height of samples in Cell 1, 2, and 3 were 13.31 mm, 14.08 mm, and 14.92 mm, respectively. The final height of the samples after 23 h of testing was 14.74, 15.48 mm, and 17.79 mm. Using Eq. 5, these heights were then transformed to swelling % as indicated in Fig. 13a. It is clearly evident that addition of NPs in the mud system drastically reduces the swelling % of Murree formation. This indicates that the NPs adsorbed on the surface of the formation and developed a membrane-like structure thereby preventing the migration of smaller H+ ions from the water in the shale sample, resulting in decrease in swelling percentage.
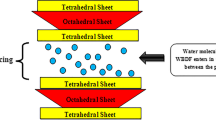
Figure 14 shows the schematic of the GO material adheres on the surface of the shale formation thereby improving its stability when it is contact with the water. Montmorillonite has 2:1 structure as demonstrated in Fig. 14. Since the outer layers are tetrahedral in nature, so there is no likelihood of hydrogen bonding (Chen and Peng 2018). This arrangement of Montmorillonite exhibits weak van der Waals forces and thereby an H + ion from the water molecules easily penetrates into the loosely held structure and cause swelling. To mitigate this effect, the active functional group especially hydroxyl and carboxyl in GO interacts with the active group present in the Montmorillonite clay mineral with the help of hydrogen and chemical bonds(An et al. 2018), thus reducing the swelling of shale and improving its stability.
The quality of mud cake
The quality of the mud cake should be analyzed based on the stiffness, smoothness and durability. It is considered as an important factor during drilling operation (Bayat et al. 2018). The quality of the mud cake is the characteristic factor, which ensures the wellbore stability by preventing the amount of filtrate loss inside the formation (Bayat et al. 2018). Different fluid loss additives are added in the drilling fluid so as to minimize the amount of filtrate loss. Figure 15 demonstrates the SEM analysis of the mud cake that was developed during the API filter press of mud system comprises of GO and Pure-bore. From the SEM results in Fig. 15a, it was observed that GO forms a smooth mud cake that proves to be an effective seal for the filtrate loss. This was also verified by the API filter press results in Fig. 13. In contrast, Pure-bore filter cake exhibits a low-quality cake that can be observed in Fig. 15b. It was perceived that still some void space left as indicated by the circles in the figure. These part proves to be the zone from where filtration occur, as a result filter volume for this particle is greater in comparison to GO.
Conclusion
During this study, the experimental work was performed to find out the effect of NP graphene oxide and micron particle Pure-bore on Murree shale formation obtained from the Northern region of Pakistan. According to QXRD, this formation comprises of 26% of the clay contents in which 13.6% is Smectite, 6.7% Kaolinite and 5.7% is Illite. This 13.6% because of weak Van der Waals forces is utterly responsible for the swelling characteristics of the formation. It was perceived that the addition of these particles does not significantly compromise the mud weight of the base system, and at the same time also improves the rheological properties of the WBDF. Both the particles increase the plastic viscosity of the mud. However, Pure-bore increases 47% of the PV in comparison with the base mud. Similarly, both the particles increased the YP of the base mud. For Pure-bore, it reaches a maximum value of 55 lb./100 ft2 at 0.5 wt%, while GO it reaches a maximum value of 31 lb./100 ft2 at 0.3 wt%. Gel strength of the mud was also increased by adding these two particles, which exhibits a better carrying capacity of the mud. It was further observed that both the particles displays a better filtrate control, however, for GO filtrate volume was under acceptable limit of what it should be in shale formations. Likewise, the swelling % describes that both the particles are effective in this shale formation as both of them significantly reduces the swelling of the formation. However, GO illustrates a better swelling control in contrast to Pure-bore, since this particle interrelates with the functional group present in the Montmorillonite with the help of hydrogen and chemical bonding and provides a sound stability to the Murree formation. These two materials can prove to be a vital addition to the drilling fluid industry, as they can help in mitigating the sloughing of shale formation, which minimizing the NPT events.
Abbreviations
- AV:
-
Apparent viscosity
- EDS:
-
Energy-dispersive X-ray analysis
- FTIR:
-
Fourier-transformed infrared spectroscopy
- GS:
-
Gel strength
- GO:
-
Graphene oxide
- LPLT:
-
Low pressure low temperature
- OBM:
-
Oil-based mud
- PV:
-
Plastic viscosity
- PAC-L:
-
Polyanionic cellulose (L)
- PB:
-
Pure-bore
- RPM:
-
Revolutions per minute
- SEM:
-
Scanning electron microscope
- WBDM:
-
Water-based drilling mud
- YP:
-
Yield point
References
Abduo MI, Dahab AS, Abuseda H, AbdulAziz AM, Elhossieny MS (2015) Comparative study of using water-based mud containing multiwall carbonnano-tubes versus oil-based mud in HPHT fields. Egypt J Pet 25:459–464
Aftab A et al (2020a) Influence of tailor-made TiO2/API bentonite nanocomposite on drilling mud performance: towards enhanced drilling operations. Appl Clay Sci 199:105862
Aftab A et al (2020b) Environmental friendliness and high performance of multifunctional Tween 80/ZnO-nanoparticles-added water-based drilling fluid: an experimental approach. ACS Sustain Chem Eng 8:11224–11243
Aftab A et al (2016) Novel zinc oxide nanoparticles deposited acrylamide composite used for enhancing the performance of water-based drilling fluids at elevated temperature conditions. J Petrol Sci Eng 146:1142–1157
Aftab A et al (2017) Nanoparticles based drilling muds a solution to drill elevated temperature wells: a review. Renew Sustain Energy Rev 76:1301–1313
Aftab A, Ismail AR, Ibupoto ZH (2017) Enhancing the rheological properties and shale inhibition behavior of water-based mud using nanosilica, multi-walled carbon nanotube, and graphene nanoplatelet. Egypt J Pet 26(2):291–299
Al-Bazali T et al. (2009) An experimental investigation on the impact of capillary pressure, diffusion osmosis, and chemical osmosis on the stability and reservoir hydrocarbon capacity of shales. In: Society of petroleum engineers—offshore Europe oil and gas conference and exhibition 2009, OE 2009, vol 1
Al-Yasiri MS, Al-Sallami WT (2015) How the drilling fluids can be made more efficient by using nanomaterials. American J Nano Res Appl 3(3):41–45
Al-Yasiri M, Awad A, Pervaiz S, Wen D (2019) Influence of silica nanoparticles on the functionality of water-based drilling fluids. J Petrol Sci Eng 179:504–512
An YX, Qu WJ, Yu PZ, Lü JG (2018) The assembly of a composite based on nano-sheet graphene oxide and montmorillonite. Pet Sci 15(2):366–374
Asef M, Farrokhrouz M (2013) Shale engineering: mechanics and mechanisms, 1st edn. CRC Press, Boca Raton
Bayat AE, Moghanloo PJ, Piroozian A, Rafati R (2018) Experimental investigation of rheological and filtration properties of waterbased drilling fluids in presence of various nanoparticles. Colloids Surf A 555:256–263
Butt AS (2012) Shale characterization using X-ray diffraction. In: Petroleum engineering program. 2012, Dalhousie University: Halifax
Chen X, Peng Y (2018) Managing clay minerals in froth flotation—a critical review. Miner Process Extr Metall Rev 39(5):289–307
Chesser B et al (1987) Designconsiderationsforaninhibitive, stablewater-based mud system. SPE Drill Eng 2(04):331–336
Dasari BL, Nouri JM, Brabazon D, Naher S (2017) Graphene and derivatives-synthesis techniques, properties and their energy applications. Energy 140:766–778
Deng Z, Livanec PW, Deville JP (2015) Novel water based fluids for oil sands drilling. In: SPE Canada heavy oil technical conference. 2015. SPE, Calgary
Eda G, Fanchini G, Chhowalla M (2008) Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat Nanotechnol 3:270–274
Ewy RT, Morton EK (2009) Wellbore-stability performance of water-based mud additives. SPE Drill Complet 24:390–397
Fukushima Y (1984) X-ray diffraction study of aqueous montmorillonite emulsions. Clays Clay Miner 32(4):320–326
Ghafuri H, Talebi M (2016) Water-soluble phosphated graphene: preparation, characterization, catalytic reactivity, and adsorption property. Ind Eng Chem Res 55(11):2970–2982
Growcock FB, Patel AD, Swaco MI (2011) The revolutioninnon-aqueousdrilling fluids. in In:Proceedings of the at the 2011 AADE National Technica lConference and Exhibition. Hilton Houston North Hotel, Houston
Güneyisi E, Gesoglu M, Algın Z, Yazıcı H (2016) Rheological and fresh properties of self-compacting concretes containing coarse and frine recycled concrete aggregates. Constr Build Mater 113:622–630
Huang W, Li X, Qiu Z, Jia J, Wang Y, Li X (2017) Inhibiting the surface hydration of shale formation using preferred surfactant compound of polyamine and twelve alkyl two hydroxyethyl amine oxide for drilling. J Pet Sci Eng 159:791–798
Husin H, Elraies KA, Choi HJ, Aman Z (2018) Influence of Graphene nanoplatelet and silver nanoparticle on the rheological properties of water based mud. Appl Science 8:1386
Ismail AR, Rashid MS (2018) Application of nanomaterials to enhanced the lubricity and rheological properties of water based drilling fluid. In: IOP conf. series: materials science and engineering. IOP Publishing
Ismail AR, Ibupoto ZH, Zolkifile N (2016) The novel approach fo rthe enhancement of rheological properties of water-based drilling fluids by using multi-walled carbon nanotube, nanosilica and glass beads. J Pet Sci Eng 139:264–275
James DK, Tour JM (2012) Graphene: powder, flakes, ribbons, and sheets. Acc Chem Res 46(10):2307–2318
Ji L, Guo Q, Friedheim JE, Zhang R, Chenevert ME (2012) Laboratory evaluation and analysis of physical shale inhibition of aninnovative water-based drilling fluid with nanoparticles for drilling unconventional shales. In: SPE Asia Pacific oil and gas conference, SPE, Editor. 2012. SPE, Perth
Jiang G, Wang K, He Y, Yang L, Li X, Deng Y (2020) Synthesis of an amphoteric polymer as a high temperature resistant shale stabilizer in water based drilling fluids. J Appl Polym Sci 137:49016
Khan MA et al (2020) Experimental study and modeling of water-based fluid imbibition process in middle and lower indus basin formations of Pakistan. J Pet Explor Product Technol 11(1):425–438
Kobayashi T, Kimura N, Chi J, Hirata S, Hobara D (2010) Channel-length-dependent field-effect mobility and carrier concentration of reduced graphene oxide thin-film transistors. Small 6(11):1210–1215
Kosynkin DV, Ceriotti G, Wilson KC, Lomeda JR, Scorsone JT, Patel AD, Friedheim JE, Tour JM (2012) Graphene oxide as a high-performance fluid-loss-control additive in water-based drilling fluids. ACS Appl Mater Interfaces 4(1):222–227
Kusrini E, Oktavianto F, Usman A, Mawarni DP, Alhamid MI (2020) Synthesis, characterization, and performance of graphene oxide and phosphorylated graphene oxide as additive in water-based drilling fluids. Appl Surf Sci 506:145005
Mohideen AA, Saheed MS, Mohamed NM (2019) Multiwalled carbon nanotubes and graphene oxide as nano-additives in water-based drilling fluid for enhanced fluid-loss-control & gel strength. In: AIP conference proceedings
Moraes LR, Ribeiro H, Cargnin E, Andrade RJ, Naccache MF (2020) Rheology of grahene oxide suspended in yield stress fluid. J Non Newton Fluid Mech 286:104426
Nandiyanto AB, Oktiani R, Ragadhita R (2019) How to read and interpret FTIR spectroscope of organic material. Indones J Sci Technol 4(1):97–118
Noah AZ, El Semary MA, Youssef AM, El-Safty MA (2016) Enhancement of yield point at high pressure high temperature wells by using polymer nanocomposites based on ZnO & CaCO3 nanoparticles. Egypt J Pet 26(1):33–42
Paritosh Dhiman YC, Zhang Y, Patil S (2018) Experimental investigation of the infuence of nanoparticles on water-based mud. Appl Nanosci 8:511–526
Salam M, Al-Zubaidi NS, Al-Wasiti AA (2018) An Enhancement in lubricating, rheological, and filtration properties of unweighted water-based mud using XC polymer NPs. J Eng 25:96–115
Saleh TA, Ibrahim MA (2019) Advances in functionalized Nanopartices based drilling inhibitors for oil production. Energy Rep 5:1293–1304
Soares YCF, Cargnin E, Naccache MF, Andrade RJE (2020) Influence of oxidation degree of graphene oxide on the shear rheology of poly (ethylene glycol) suspensions. Fluids 5(2):41
Vu TH, Tran TT, Le HN, Nguyen PH, Bui NQ, Essayem N (2015) A new green approach for the reduction of graphene oxide nanosheets using caffeine. Bull Mater Sci 38(3):667–671
Wajheeuddin M, Hossain ME (2018) Development of an environmentally-friendly water-based mud system using natural materials. Arab J Sci Eng 43(5):2501–2513
Wang Y, Chen Y, Lacey SD, Xu L, Xie H, Li T, Danner VA (2018) Reduced graphene oxide film with record high conductivity and mobility. Mater Today 21(2):186–192
Wang K, Jiang G, Li X, Luckham PF (2020) Study of graphene oxide to stabilize shale in water-based drilling fluids. Colloids Surf A 606:12457
William JKM, Ponmani S, Samuel R, Nagarajan R, Sangwai JS (2014) Effect of CuO and ZnO nanofluids in xanthan gum on thermal, electrical and high pressure rheology of water-based drilling fluids. J Pet Sci Eng 117:15–27
Xuan Y, Jiang G, Li Y (2014) Nanographite oxide as ultrastrong fluid-loss-control additive in water-based drilling fluids. J Dispers Sci Technol 35(10):1386–1392
Yang X, Shang Z, Liu H, Cai J, Jiang G (2017) Environmental-friendly salt water mud with nano-SiO2 in horizontal drilling for shale gas. J Petrol Sci Eng 156:408–418
Yuxiu A, Guancheng J, Yourong Q, Xianbin H, He S (2016) High-performance shale plugging agent based on chemically modified graphene. J Nat Gas Sci Eng 32:347–355
Acknowledgements
The authors in this article are extremely grateful to the Petroleum Engineering Department at NED University of Engineering & Technology for providing the drilling lab facilities to perform this study. The authors are also extremely grateful to Mr. Hasnain Athar, Mr. Muhammad Yousuf Siddiqui, Mr. Syed Ibtehaj Hashmi, Mr. Muhammad Armaan Shariq and Mr. Syed Furqan Ahmed who provided assistance during the experimental work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Rights and permissions
About this article
Cite this article
Lalji, S.M., Khan, M.A., Haneef, J. et al. Nano-particles adapted drilling fluids for the swelling inhibition for the Northern region clay formation of Pakistan. Appl Nanosci 13, 503–517 (2023). https://doi.org/10.1007/s13204-021-01825-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-021-01825-4