Abstract
A novel method employing atmospheric pressure discharge plasma has been developed to reduce supported Ag nanoparticles (Ag NPs) and deposited on TiO2 powder without the use of any environmentally and biologically hazardous reducing chemicals. Ag/TiO2 nanocomposite was prepared by the atmospheric direct current plasma in water solution. Trisodium citrate and CMC was used as a capping agent for Ag nanoparticles. The nanocomposites were characterized using UV–Vis spectroscopy, X-ray diffraction, scanning electron microscopy. The results showed that Ag NPs with an average diameter of 20–35.5 nm were uniformly distributed on the powders surface. The presence of nanosilver particles in TiO2 matrix is clearly visible. The bandgap of TiO2/Ag was confirmed (2.65–2.85 eV) through UV–vis DRS, which was lower than TiO2 (3.8 eV). The photocatalytic effect was conducted by methylene blue degradation test. The photocatalytic activity of a series of 2 wt. % Ag/TiO2 composites was evaluated in the degradation of MB (used as a model pollutant) in aqueous solution under solar light. The 2.0 mol % of Ag-doped TiO2 shows 96% degradation of methylene blue (MB) within 50 min under visible light irradiation and exhibit highest photocatalytic performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution is one of the most leading problems that badly affects the human as well as aquatic life (Guerra et al. 2018). The main cause of water contamination is the discharge of the industrial effluents that contain mainly toxic chemicals and pose a major threat to the living systems. It has been well known that these pollutants are mutagenic as well as carcinogenic in nature and their elimination through the traditional techniques is a difficult task. The advanced oxidation processes (AOPs) involving heterogeneous semiconductor photocatalysts have attracted enormous attention owing to their compatibility as a pollution mediator (Nahirniak at. al. 2018; Amin et al. 2014; Saikia et al. 2019). Due to its high efficiency, low toxicity, excellent physico-chemical stability, and low relative costs, titanium dioxide (titania, TiO2) is considered as the most promising photocatalyst for environmental purification. However, TiO2 possesses a wide bandgap which restricts its practical environmental application under visible light irradiation which comprises a wide range of the solar spectrum.
Silver particle at nanoscale size is of particular interest as a novel excellent antimicrobial regent in recent years and have been widely investigated as an important enhanced phase in designing plasmonic metal–semiconductor photocatalysts since they may activate wide bandgap semiconductors (e.g. TiO2) towards visible-light (Berekaa et al. 2016; Dauthal et al. 2016). A number of synthesis procedures have been used for the preparation of Ag/TiO2 nanocomposite such as electrochemical hydrothermal method, electrochemical rout, chemical vapor deposition of precursors and solution synthesis method (Amruta et al. 2016; Chaturvedi et al 2012; Giuseppina et al 2019; Seery et al 2007; Ling et al. 2019; Sahoo et al. 2012). Despite the advantages of the mentioned techniques, they never achieve full industrial potential due to their expensive process and long process time (Ajmal et al 2014; Atarod et al 2016). In the meantime, plasma electrochemical method has recently received much attention in a wide variety of applications from bacterial inactivation to nanoparticle synthesis in liquid phases (Mahmoudabadi et al 2018; Cheng et al 2017). The main advantages of this method are related to its facility for any kind of complex structure, acceptable reaction times and more homogeneous reaction conditions with low temperature. Among plasma-chemical discharges, contact non-equilibrium low-temperature plasma (CNP) is a promising option from the point of view of practical application (Pivovarov et al. 2015). This new class of plasma organization has some fascinating features such as small physical size, atmospheric pressure stability. It usually consists of ultraviolet (UV) radiation, excited species and charged particles which interact with the surface of the solution. The plasma-liquid interface becomes a reaction region where many particular chemical and physical processes occur under the electron /positive ion irradiation from the plasma. Therefore, the plasma discharge is coupled with the electrochemical systems to induct reactions without using a solid electrode in the solution.
In our previous works the Ag NPs was synthesized by using the plasma-assisted electrochemical with different capping agents (Skiba et al. 2017, 2018a; b). The present study deals with the formation of stable silver nanoparticles using one-pot plasma-chemical assisted synthesis, doping of the prepared nanoparticles on TiO2 and application of Ag/TiO2 as a catalyst in degradation of water pollutant.
Preparation of Ag–TiO2 nanocomposites
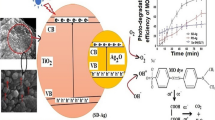
The Ag–TiO2 nanocomposites were synthesized by a one-step deposition method. The commercial titanium oxide was immersed into AgNO3 and capping agent solution with certain concentration. The mixture was treated by plasma discharge in a homemade reactor. A schematic of the experimental configuration used for the preparation of Ag/TiO2 nanocomposite is shown in Fig. 1. The duration of the plasma treatment was limited to 5 min. The various publications about Ag/TiO2 indicated that 2.0 wt. % of Ag exhibited the highest photocatalytic activity (Amruta et al. 2016; Stucchi et al. 2018; Giuseppina Cerrato et al. 2019; Seery et al 2007). Therefore, we decide to fabricate Ag–TiO2 with 2.0 wt.% Ag. First, 0.0425 g AgNO3 was added into a 100 mL DI. Stabilizer agent was added 1:2 (molar ratio Ag+:Stab). As stabilizer agent was used Carboxy Methyl Cellulose (CMC) and sodium citrate (Cit). Then, the solution was magnetically stirred with 1.0 g TiO2 powder. The resulting mixture was placed in a plasma-chemical reactor. Impregnated TiO2 was then filtered, washed by distilled water and dried at room temperature for 12 h. The modified catalysts were labelled Ag-2.0/TiO2/CMC; Ag-2.0/TiO2/Cit.
Photocatalytic activity
The photocatalytic activity of the materials was evaluated by the degradation efficiency of MB solutions under sunlight 60 mL of MB at 10 mg/L and 0.02 g of catalyst were magnetically stirred for 60 min in the dark for the equilibrium of the adsorption/desorption process of MB on the materials to be achieved. At the given time intervals, 5 mL of aliquot was withdrawn, then filtered it to remove the photocatalysts. Then the mixture was subjected to sunlight irradiation. The photocatalytic degradation of MB has been estimated from the reduction in absorption intensity of MB at the characteristic lambda max 663 nm by employing UV–visible spectrophotometer. The absorption spectrum of the MB solution was regularly recorded fixed minutes of irradiation. The experiments were carried out from 10:00 to 13:00 o’clock on a sunny day in Ukraine. All the experiments were repeated three times. The photocatalytic efficiency was estimated using the following expression: η (%) = 100 × (C0 − Ct)/C0, where C0 and Ct were the of MB before and t min after sunlight exposure, respectively.
Experimental methods
Spectra of colloidal solutions were obtained using the spectrophotometer UV-5800PC and quartz cuvettes in the wavelength range of λ = 190–700 nm (FRU, China). Particle size of TiO2 powder was determined by the particle size analyzer Zetasizer Nano-25 (Malvern Instruments Ltd., Malvern, England). Scanning Electron Microscopy REM 106I (Selmi, Ukraine) were used for characterization of particle’s size and morphology of the obtained samples. The X-ray diffraction analyses (XRD) were carried out with Bruker D2 Phaser X-ray diffractometer using Cu-Kα radiation (λ = 1.5406 Aº).
Results and discussion
Figure 2 illustrates the solution including Ag and TiO2 after plasma treatment. Changing the colors during plasma processing in solutions at 120 mA during 4 min confirm the formation of Ag nanoparticles in solutions.
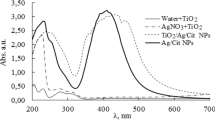
It studied the optical properties and effects of Ag light absorption deposited on TiO2. As the reference material, unmodified TiO2 was used. All metal-modified photocatalysts revealed an enhanced absorption of visible light in comparison to bare TiO2.
It was observed that the metal ions capping agent not strongly influences the shape of absorption properties. For plasma reduced photocatalysts in present sodium citrate and CMC (4 min treated), absorption bands were narrower with SPR at 415–425 nm, as shown in Fig. 3 a–b. The absorption, with a maximum near 415–425 nm, can be attributed to the plasmon absorption of spherical Ag nanoparticles (Skiba et al. 2018a, b; Guerra et al. 2018).
The bandgap of the pure TiO2 and Ag/TiO2 composite were calculated using a well-known Tauc's plot method and the following relation (1):
where α, h, υ, E and A are the absorption coefficient, Planck’s constant, light frequency, bandgap and a constant, respectively. The index n relies on the type of electronic transition of semiconductor, where n = 2 for direct-gap semiconductor and n = 0.5 for indirect-gap semiconductor.
The optical band gap energy of pure and Ag NPs -doped TiO2 has been calculated by extrapolating the linear region of the plot of hν verses (αhν)2 as shown in Fig. 4. The energy gap values of the pure TiO2 and Ag-doped TiO2 were calculated to be approximately 3.8 eV and 2.65–2.85 eV respectively. The variations of energy bandgap with type silver NPs doping Fig. 4 demonstrates that after doping with silver ions the bandgap energy decreases, which shows the red shift. Because of this red shift the recombination rate of photoinduced electrons and holes decreases which improved photocatalytic activity.
Figure 5 shows the DLS analyses made on TiO2 (Ag-2.0/TiO2/CMC) particles of powder before and after the synthesis of silver nanoparticles. These analyses were used to probe whether silver nanoparticles were really attached to the surface of TiO2 particles. From Fig. 5a, the TiO2 particles, before silver synthesis, have a mean diameter of 273 nm; after silver synthesis their mean diameter slightly grows (309 nm) due to the presence of silver nanoparticles (Fig. 5b). This result confirms that silver nanoparticles really are attached to TiO2 particles; if this were not the case we would see two peaks in Fig. 5b, one corresponding to silver nanoparticles and the other one corresponding to TiO2 particles.
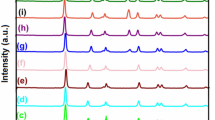
The XRD analysis Ag NPs doped TiO2 (Ag-2.0/TiO2/Cit) was used for the identification of the crystalline structure of the samples and the evaluation of the solids phase purity. The XRD analysis results of 2.0% silver doped TiO2 catalyst is shown in Fig. 6. All obtained photocatalysts revealed a high level of crystallinity and consist of an rutile phase and metallic silver nanoparticles. The main peaks assigned to rutile phase due to the following planes and 2θ angles of (110), 27.4°, (101), 36°, (111), 41.2°, (210), 44.2°, appear in the pattern of sample. The XRD peaks at 32.16, 38.1,º46.1°, 66.74°, and 76.84° correspond to 122, 111, 200, 220, and 311 crystalline planes for cubic crystalline structure of metallic silver (JCPDS Card No. 04-0783). From the figure, it is clear that the silver present in TiO2 catalyst is not as oxide (Mohammadi et al. 2019).
SEM image of nanosilver doped TiO2 catalyst (Ag-2.0/TiO2/Cit) and particle size distribution are shown in Fig. 7. It is observed from this figure that the silver nanoparticles are successfully immobilized on the surface of TiO2 support.
Thus Ag/TiO2 composite was successfully fabricated using a CNP plasma-assisted electrochemical. The use of the plasma in the synthesis of Ag/TiO2 composite in comparison with other methods accelerates the processes involved in the Ag/TiO2 composites synthesis. Compared with the conventional hydrothermal, wet chemical, or laser irradiation technique, approaches based on plasma are found to be simple because of the limited number of processing steps that do not require specific expertise. Furthermore, plasma-based processes do not involve the long consumption time and the use of reducing agents, simplifying further nanoparticle functionalization or surface treatments. The photocatalytic efficiency of as prepared composite has been investigated by measuring the decomposition of MB as a model pollutant under solar light irradiation. The oxidized and reduced forms of MB have different absorption bands in the UV–Vis spectrum. Hence, the progress of decoloration or reduction reaction from MB to leuco MB (LMB) can be monitored by measuring the decrease in absorption of MB on UV–Visible spectrum at a λmax of 664 nm. The time-dependent electronic absorption spectrum of MB during solar light irradiation is presented in Fig. 8. After 10 min of irradiation under solar light in the presence of Ag co-doped TiO2 suspension, more than 98% of dye got degraded and the solution became colorless (Fig. 8b). Also, no new bands appeared in the UV–Vis spectrum, confirming the absence of reaction intermediates during the degradation process.
Conclusions
A series of Ag-doped TiO2 composite were obtained by impregnation of the commercial powder TiO2 by plasma-chemically synthesized silver nanoparticles. The results showed that Ag NPs with an average diameter of 20.0–35.5 nm were uniformly distributed on the powders surface. The presence of nanosilver particles in TiO2 matrix is clearly visible. The photocatalytic activity of pure and Ag-doped TiO2 catalyst was examined by performing the degradation of methylene blue dye. The experimental result exhibited that Ag-doped TiO2photocatalyst can effectively degrade MB under visible light irradiation and 2.0 mol % Ag-doped TiO2 showed the highest photocatalytic activity among all the samples.
References
Ajmal A, Majeed I, Malik RN, Idriss H, Nadeem MA (2014) Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: a comparative overview. RSC Adv 4:37003–37026
Amin MT, Alazba AA, Manzoor U (2014) A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv Mater Sci Eng 2014:1–24
Amruta S, Vidya SK (2016) Photocatalytic degradation of phenol using Ag core-TiO2 shell (Ag@TiO2) nanoparticles under UV light irradiation. ESPR 23(20):20055–20064
Atarod M, Nasrollahzadeh M, Mohammad SS (2016) Euphorbia heterophylla leaf extract mediated green synthesis of Ag/TiO2 nanocomposite and investigation of its excellent catalytic activity for reduction of variety of dyes in water. J Colloid Interface Sci 462:272–279
Berekaa MM (2016) Nanotechnology in wastewater treatment; influence of nanomaterials on microbial systems. Int J Curr Microbiol App Sci 5:713–726
Chaturvedi S, Dave PN, Shah NK (2012) Applications of nano-catalyst in new era. J Saudi Chem Soc 16(3):307–325
Cheng X, Dong P, Huang Z, Zhang Y, Chen Y, Nie X, Zhang X (2017) Green synthesis of plasmonic Ag nanoparticles anchored TiO2 nanorod arrays using cold plasma for visible-light-driven photocatalytic reduction of CO2. J CO2 Util 20:200–207
Dauthal P, Mukhopadhyay M (2016) Noble metal nanoparticles: plant-mediated synthesis, mechanistic aspects of synthesis, and applications. Ind Eng Chem Res 55:9557–9577
Giuseppina C, Federico G, Boffito DC, Operti L, Bianchi CL (2019) Correlation preparation parameters/activity for microTiO2 decorated with SilverNPs for NOx photodegradation under LED light. Appl Catal B 253:218–225
Guerra F, Attia M, Whitehead D, Alexis F (2018) Nanotechnology for environmental remediation: materials and applications. Molecules 23:1760–1768
Ling L, Feng Y, Li H, Chen Y, Wen J, Zhu J, Bian Z (2019) Microwave induced surface enhanced pollutant adsorption and photocatalytic degradation on Ag/TiO2. Appl Surf Sci 483:772–778
Mahmoudabadi ZD, Eslami E, Narimisa M (2018) Synthesis of Ag/TiO2 nanocomposite via plasma liquid interactions: Improved performance as photoanode in dye-sensitized solar cell. J Colloid Interface Sci 529:538–546
Mohammadi SH, Hekmatara RS, Darehkordi MA (2019) Preparation and optimization photocatalytic activity of polymer-grafted Ag@AgO core-shell quantum dots. Environ Sci Pollut Res 26(13):13401–13409
Nahirniak S, Dontsova T, Astrelin I (2018) Nanochemistry, biotechnology, nanomaterials, and their applications. In: Fesenko O, Yatsenko L (eds) NANO 2017. Springer Proceedings in Physics, vol 214. Springer, Cham
Pivovarov A, Kravchenko A, Tishchenko A et al (2015) Contact nonequilibrium plasma as a tool for treatment of water and aqueous solutions: theory and practice. Russ J Gen Chem 85:1339–1350
Sahoo C, Gupta AK, Sasidharan Pillai IM (2012) Photocatalytic degradation of methylene blue dye from aqueous solution using silver ion-doped TiO2 and its application to the degradation of real textile wastewater. J Environ Sci Health Part A 47(10):1428–1438
Saikia J, Gogoi A, Baruah S (2019) Nanotechnology for water remediation. In: Dasgupta N, Ranjan S, Lichtfouse E (eds) Environmental nanotechnology. Environmental Chemistry for a Sustainable World 21. Springer, Cham
Seery MK, George R, Floris P, Pillai SC (2007) Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J Photochem Photobiol A 189:258–263
Skiba M, Pivovarov A, Makarova A, Pasenko O, Khlopytskyi A, Vorobyova V (2017) Plasma-chemical formation of silver nanodisperssion in water solutions. East Eur J Enterp Technol 6(9–90):59–65
Skiba M, Vorobyova V, Pivovarov A, Shakun A, Gnatko E, Trus I (2018a) "Green" synthesis of nanoparticles of precious metals: Antimicrobial and catalytic properties. East Eur J Enterp Technol 5(6–95):51–58
Skiba M, Pivovarov A, Makarova A, Vorobyova V (2018b) Plasma-chemical synthesis of silver nanoparticles in the presence of citrate. Chem J Mold 13(1):7–14
Stucchi M, Bianchi CL, Argirusis C et al (2018) Ultrasound assisted synthesis of Ag-decorated TiO2 active in visible light. Ultrason Sonochem 40(Pt A):282–288
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Skiba, M., Vorobyova, V. Synthesis OF AG/TIO2 nanocomposite via plasma liquid interactions and degradation methylene blue. Appl Nanosci 10, 4717–4723 (2020). https://doi.org/10.1007/s13204-020-01422-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-020-01422-x