Abstract
The direct photoactivation of the red-ox process between tryptophan (Trp) and Au3+ through indole moiety of amino acid is the perspective and effective way to obtain tailored gold nanoparticles (Au NPs) in colloids. We systematically varied experimental parameters (concentration and ratio of reagents, pH of the solution) and adjusted UV illumination parameters to influence the kinetics of the process and optical properties of obtained Au NPs. Products of reaction in Au/Trp system were studied using absorption and fluorescence spectroscopies, and mass spectrometry. The complementary analysis evidenced the transformation of tryptophan via the kynurenine pathway, which can improve the biocompatibility of Au NPs. We found that the photoactivated formation of Au NPs proceeded through two stages: reduction of Au3+ ions by an amino acid that leads to the formation of metal “primary clusters” and their further accumulation and growth of Au NPs. The use of UV light for the photochemical activation of reaction promotes up to three times faster formation of more homogeneous and uniform in size Au NPs compare to only chemical reaction pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The immense potential of multifunctional nanosized gold for medical use, namely for hyperthermia and immunotherapy, is proved by the recent start of its clinical trials (Shi et al. 2017). Emerging technology in cancer treatment suggests that use of gold nanoparticles (Au NPs), conjugated with molecules such as RNA, monoclonal antibodies and other, have many advantages such as reduced dose delivered to the target organ, improved detection of tumors and increased effectiveness against cancer cells (Jurj et al. 2017). However, we still face the major drawbacks associated with possible toxic effects of nanoparticle-based nanomedicine. Currently, the primary attention is focused on improving the biocompatibility of NPs for their safe use in medicine.
The essential aromatic amino acid tryptophan (Trp) is advantageous for the design of hybrid biocompatible Au NPs and their further implementation in cancer diagnostics and therapy. First, Trp can be applied as bifunctional agent for reducing of metal ions and stabilizing of NPs instead of commonly used toxic ion reducers and surfactants. Second, optical properties of gold colloids can be easily tracked by fluorescence and absorption of Trp and its oxidation products. Finally, tryptophan oxidation by Au3+ goes through the same kynurenine pathway alike in the human body (Mukha et al. 2016), thus forming biocompatible species.
We showed earlier that it is possible to obtain stable colloids of mono- and bimetallic silver/gold NPs in aqueous solutions in the presence of Trp. Furthermore, they possessed the anticancer effect (Mukha et al. 2017; Shmarakov et al. 2017) and could be used as a component of “chemotherapeutic agent”.
Physical and chemical properties of noble metal NPs, as well as their biological activity, depend on the size that directly results from the procedure of NPs preparation (Daniel and Astruc 2004). Among known synthetic methods, controllable photochemical synthesis is an alternative simple and effective way to obtain NPs. In opposite to chemical ones, photochemical methods are non-toxic and environmentally friendly, remaining exceptionally effective (Dong and Zhou 2007). The promising results were obtained in past several years on amino acids assisted photochemical synthesis (Courrol and de Matos 2016).
The new deep ultraviolet (UVC) light emission diodes (LED), which are currently available on the market, are an advantageous source for the photoinduced synthesis of gold colloids due to the precise influence on Trp at 278 nm, which corresponds to the absorbance maximum of the amino acid. Thus, the direct activation of the red-ox process through indole moiety of Trp can be induced. In its turn, the resulting characteristics of nanoobjects (e.g., size, morphology) can be controlled by varying the intensity and time of irradiation of the reaction mixture.
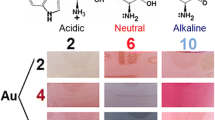
The present investigation is focused on photoinduced processes in Au/Trp system. Due to the fact that properties of Au NPs and their stability strongly depend on the acidity of the reaction medium (Mukha et al. 2016), here we studied the formation of nanoparticles and products of tryptophan conversion in the photochemical reaction conducted in acidic (pH = 2) and neutral (pH = 6) aqueous solution under the UVC LED irradiation at λ = 278 nm. The results evidenced the advantages of the photochemical synthesis of Au NPs in the presence of amino acid tryptophan with the use of UVC LED as the practical and precise light source.
Methods
Photochemical synthesis, as well as the chemical synthesis of Au/Trp colloids, occurred in the same experimental conditions. The reduction of gold ions in the presence of tryptophan in aqueous medium was conducted in a 1 cm2 quartz cuvette (V = 3 ml) at T = 13 °C. The reaction proceeded with a constant metal amount of CHAuCl4 = 1 × 10−4 M. The concentration of amino acid was CTrp = 1 × 10−4 M and 2 × 10−4 M. Thus, the components interacted in a molar ratio of νTrp:νAu = 1:1 and 2:1. In the case of the acidic medium, the initial solution of Trp was adjusted to pH = 2 with HNO3. Control solutions of tryptophan were studied in the same way without adding metallic component.
For the photochemical activation, we applied UVC LED source (LGInnotek) with the wavelength of λ = 278 nm at radiation power P = 1 ± 0.2 mW/cm2.
Absorption spectra of colloids obtained by both chemical and photochemical methods were recorded in the UV–visible region on a spectrophotometer Lambda 35 (Perkin-Elmer, United States) in 1 cm quartz cells, in the same time intervals of reaction starting from t0—the moment when reagents were mixed.
Fluorescence spectra were recorded on a spectrofluorometer LS 55 (Perkin-Elmer, Great Britain) in 1 cm quartz cells with excitation by light in the range of 240–370 nm.
Mass spectrometry analysis was performed by the method of laser desorption/ionization (LDI MS) on an Autoflex II (Bruker Daltonics, Germany) mass spectrometer with a nitrogen laser (λ = 337 nm). Experiments were carried out in reflection mode for positive and negative ions in the mass range from 100 to 950 Da. Resulting in mass spectra were obtained by assuming the data of 200 laser shots and processing by the software FlexAnalysis (Bruker Daltonics, Germany).
Results and discussion
Gold nanoparticles formation
A specific bright red color is a distinguishing feature of gold nanoparticles. It is caused by the phenomenon of localized surface plasmon resonance (LSPR) that appears as a characteristic absorption band in a visible range of the spectrum (Louis and Pluchery 2012).
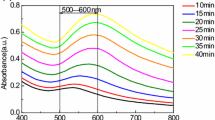
In studied systems we observed LSPR band of gold with a maximum at 550 nm. Its growth is shown in Fig. 1 as an appearance of the green-colored spectral region in time. The changes in absorption spectra of colloids confirm that we can accelerate the formation of gold nanoparticles in the acidic and neutral medium by both chemical and photochemical ways.
The contour plots of absorption spectra of gold colloids (Au/Trp). a Photochemical and b chemical induced changes in absorption spectra were tracked in time and wavelength ranges. The UVC LED source with peak wavelength λ = 278 nm and radiation power P = 1 ± 0.2 mW/cm2 was used to induce a photochemical reaction pathway. The components interacted in a molar ratio of νTrp:νAu = 1:1 and 2:1. In the case of the acidic medium, the initial solution of Trp was adjusted to pH = 2 with HNO3. Optical Density changes are displayed on color bars (right side)
The concentration of reducing agent (or its ratio towards the metal) has an impact on processes of nucleation and growth of nanoparticles. We found that when amino acid:metal molar ratio was increased from 1:1 to 2:1 the intensity of LSPR band in its maximum increased in 1.4 times faster (Fig. 2, right panel). At the same time, the plasmon band in final colloid was more narrow and symmetrical (Fig. 1, left panels). Thus, in the Au/Trp system, we can influence the formation of Au NPs and their further growth chemically, by changing the amount of reducing agent.
The similar trend was observed, when UV light irradiation at the wavelength in the maximum of Trp at 278 nm was used to activate the photochemical reaction between tryptophan and Au3+ (Fig. 2, left panel). Moreover, in this case, LSPR band grows faster, and its intensity is about 1.5 times higher, as well as it is narrower and more symmetrical compared to those for chemically obtained NPs. This result indicates that in photochemical process NPs are formed faster giving less variation in their size.
The shapes of curves, that reflect changes of LSPR absorbance in time (Fig. 2), shows that the reaction in the Au/Trp system proceeds in two stages: “slow” and “rapid”. We assume that the slow stage refers to the reduction of Au3+ ions by an amino acid that leads to the formation of metal “primary clusters”. It represents mostly exponential dynamics. The second stage is characterized by further accumulation and growth of Au NPs from previously formed clusters that was observed by the exponential growth of LSPR band of gold.
These curves were analyzed using the non-linear sigmoid function for the whole time range in the case when two stages are not clearly resolved to fit two stages by two separate exponential functions, or by a single one (Table 1). According to calculated time constants, by applying UV light at 278 nm, we can speed up the formation of NPs, and thus the growth of LSPR band up to three times (Table 1).
As a result, there are two ways: chemical (e.g., varying concentration and pH) and photochemical (e.g., varying wavelength, power density and time of UV light illumination) to control the morphology of obtained Au NPs. Photoactivation of indole moiety by light promoted the formation of nanoparticles with more uniform in size due to the acceleration of reaction rate.
Tryptophan transformation, comparative analysis of absorbance, fluorescence and mass spectra
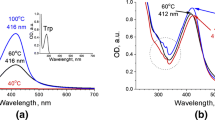
Analyzing the UV–vis spectra of solutions after centrifugation, that is the solutions without nanoparticles, in the region of amino acid absorption, we fixed the following observations.
First, chemical and photochemical processes resulted in the same products of reaction independently on reagent ratio, pH value and presence or absence of UV irradiation, since the spectra were superimposed for both ratios of reagents (1:1 and 2:1) and both pH values (2 and 6), as shown in Fig. 3. Thus, chemical and photochemical reduction of metal proceeds in the same way of Trp destruction.
Second, when the excess of tryptophan was used in the case of 2:1 ratio, we observed higher residual absorption of Trp at 270–290 nm. Also in mass spectra for 2:1 ratio systems with both initial acidic and neutral medium there were peaks with a pitch of 130 Da (positive ions), that corresponds to the ionized indole fragment of tryptophan (Fig. 4). Taking into account a three-electron reduction process of Au3+→ Au0 and the excess of Trp left in solution, we can suggest stages of deep tryptophan oxidation pathways that take place under the influence of Au3+ cation, as it occurs in the case of peroxide or singlet oxygen influence (Simat and Steinhart 1998; Ronsein et al. 2008).
The shape of absorption bands and the position of their maxima allows to assumed two possible type of products formed with: (a) the oxidation of pyrrole moiety to Pic, Oia (2HT) and DiOia (DHT) products, and (b) its following cleavage, resulting in N′-formylkynurenine (NFK) and kynurenine (Kyn) formation (Salminen and Heinonen 2008; Petronio and Valdecir 2013). All of them have low affinity to the formed surface of Au NPs, and thus go out from the surface to bulk solution.
For all studied Au/Trp systems there were three maxima in excitation spectra at λmax(ex) = 245 (most intensive), 300 and 370 nm (Fig. 5). The maxima of fluorescence excited at the mentioned wavelengths had the same position for all Au/Trp samples at 450 nm. In all cases the fluorescence emission and excitation spectra of the solution are close to those obtained for N′-formylkynurenine (Fig. 5 in Fukunaga et al. (1982), λmax(ex) = 320 nm, λmax(fl) = 430 nm). The increased band halfwidth in both emission and excitation spectra points on the presence of deformylated kynurenine (λmax(ex) = 360 nm, λmax(fl) = 475 nm). This feature suggests that oxidation process is going on in the direction to deeper oxidation of tryptophan species and NFK hydrolysis: PIC → 2HT → DHT → NFK → KYN.
At the same time, exciting Au/Trp systems at λex = 280 nm one more fluorescence maximum appeared at 370 nm. As no emission of both NFK and Kyn fluorescence took place on excitation at 280 nm (Fukunaga et al. 1982), this maximum corresponded to residual Trp emission.
Deprotonated molecular ion of Trp was identified in the mass-spectrum of control solution of amino acid. For the obtained samples there are a series of repeated peaks with m/z = 122, 147, 156, 164 and 184 Da (negative ions). For the samples obtained in acidic medium, the clear peak with m/z = 136 is present that can be attributed to NFK (Ronsein et al. 2008), as well as to KYN (Vazquez et al. 2001) species. Considering the mass of nitrate anion, we can assume a series of peaks in the range of 198–246 (Fig. 4) as adducts of ionized fragments with m/z = 136, 147, 164 and 184 with NO3−, since there is a repeatable difference in masses of 62 Da.
Further study on Au/Trp system aims to improve the efficiency of the current photochemical reaction through adjusting experimental parameters such as power density and time of UV light illumination and verify the biocompatibility of obtained gold nanoparticles.
Conclusions
Direct photochemical activation of the red-ox reaction between amino acid tryptophan and Au3+ at 278 nm promotes up to 3 times faster formation of Au NPs compare to chemical process conducted at 13 °C. Photoactivated process resulted in Au NPs more homogeneous and uniform in size, that was reflected as changes in position, shape and intensity of localized surface plasmon resonance band of gold. The analysis of the absorption intensity changes of Au NPs revealed steps of “primary clusters” formation, their accumulation and further particles growth. Due to signals in mass spectra attributed to N-formylkynurenine and its fluorescence emission and excitation spectra, we suggested deep oxidation of tryptophan via kynurenine pathway in both acidic and neutral mediums in both chemical and photochemical reactions.
References
Courrol LC, de Matos RA (2016) Synthesis of gold nanoparticles using amino acids by light irradiation. In: Mishra NK (ed) Catalytic application of nano-gold catalysts, IntechOpen, London, pp 83–99. https://doi.org/10.5772/63729
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104(1):293–346. https://doi.org/10.1021/cr030698
Dong S, Zhou S (2007) Photochemical synthesis of colloidal gold nanoparticles. Mater Sci Eng B 140(3):153–159. https://doi.org/10.1016/j.mseb.2007.03.02014
Fukunaga Y, Katsuragi Y, Izumi T, Sakiyama F (1982) Fluorescence characteristics of kynurenine and N’-formylkynurenine. Their use as reporters of the environment of tryptophan 62 in hen egg-white lysozyme. J Biochem 92(1):129–141
Jurj A, Braicu C, Pop L, Tomuleasa C, Gherman CD, Berindan-Neagoe I (2017) The new era of nanotechnology, an alternative to change cancer treatment. Drug Des Devel Ther 11:2871–2890. https://doi.org/10.2147/DDDT.S142337
Louis C, Pluchery O (2012) Gold nanoparticles for physics, chemistry, and biology. Imperial College Press, London
Mukha Iu, Vityuk N, Severynovska O, Eremenko A, Smirnova N (2016) The pH-dependent structure and properties of Au and Ag nanoparticles produced by tryptophan reduction. Nanoscale Res Lett 11:101–107. https://doi.org/10.1186/s11671-016-1318-8
Mukha Iu, Vityuk N, Grodzyuk G, Shcherbakov S, Lyberopoulou A, Efstathopoulos EP, Gazouli M (2017) Anticancer effect of Ag, Au, and Ag/Au bimetallic nanoparticles prepared in the presence of tryptophan. J Nanosci Nanotechnol 12:8987–8994. https://doi.org/10.1166/jnn.2017.14106
Petronio M, Valdecir F (2013) Light emission from tryptophan oxidation by hypobromous acid. Luminescence 28:853–859. https://doi.org/10.1002/bio.2445
Ronsein GE, Oliveira MCB, Miyamoto S, Medeiros M, Mascio P (2008) Tryptophan oxidation by singlet molecular oxygen [O2 (1Δg)]: mechanistic studies using 18O-labeled hydroperoxides, mass spectrometry, and light emission measurements. Chem Res Toxicol 21(6):1271–1283. https://doi.org/10.1021/tx800026g
Salminen H, Heinonen M (2008) Plant phenolics affect oxidation of tryptophan. J Agric Food Chem 56:7472–7481. https://doi.org/10.1021/jf800708t
Shi J, Kantoff PW, Wooster R, Farokhzad OC (2017) Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer 17:20–37. https://doi.org/10.1038/nrc.2016.108
Shmarakov I, Mukha Iu, Vityuk N, Borschovetska V, Zhyshchynska N, Grodzyuk G, Eremenko A (2017) Antitumor activity of alloy and core-shell type bimetallic AgAu nanoparticles. Nanoscale Res Lett 12:333. https://doi.org/10.1186/s11671-017-2112-y
Simat TJ, Steinhart H (1998) Oxidation of free tryptophan and tryptophan residues in peptides and proteins. J Agric Food Chem 46:490–498. https://doi.org/10.1021/jf970818c
Vazquez S, Truscott RJW, O’Hair R, Weimann A, Sheil MM (2001) A study of kynurenine fragmentation using electrospray tandem mass spectrometry. J Am Soc Mass Spectrom 12:786–794. https://doi.org/10.1016/S1044-0305(01)00255-0
Acknowledgements
National Academy of Sciences of Ukraine (NASU) supported this work in framework of grants of NASU for the research laboratory/group of young scientists of NASU for conducting investigations in priority directions of science and technology development in 2018 (No. 29/2018-2019).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukha, I., Khodko, A., Vityuk, N. et al. Light-driven formation of gold/tryptophan nanoparticles. Appl Nanosci 10, 2827–2833 (2020). https://doi.org/10.1007/s13204-019-01171-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-019-01171-6