Abstract
The coal-rock reservoir exhibits a dual porous medium characteristic, where fractures are the primary contributor to permeability, while pore structure influences the gas adsorption properties of coal rock. Gas adsorption induces swelling in the coal matrix, leading to a reduction in fracture width and subsequently causing decreased permeability and reduced well production. Investigating the impact of geological characteristics of coal-rock on gas adsorption and desorption properties can enhance our understanding of the patterns governing changes in coal-layer production. This study focused on the 3# coal seam in China's Qinshui Basin as its research subject. It involved an analysis of mineral composition, physical properties, gas content, and pore structure characteristics to explore the adsorption traits of different gases and conduct experimental studies on variations in gas adsorption and desorption capabilities under diverse conditions. The research findings suggest that the coal rock in the study area is primarily characterized by micropores and small pores, with well-developed larger pores and fractures. The pore connectivity is somewhat limited, and the predominant pore size ranges from 100 to 200 nm. The average permeability measures 0.198 × 10–3 µm2, while the mean specific gas content stands at 21.7 m3/t. Analysis of the isothermal adsorption curve reveals a substantial increase in adsorption when pressure falls below 3.5 MPa due to a steep slope; as pressure continues to rise, there is a gradual upward trend in adsorption until reaching 8 MPa, after which point adsorption increases slowly and stabilizes. Results from binary gas adsorption–desorption experiments indicate low desorption levels and rates for CO2 components compared to relatively higher desorption amounts and rates for CH4 components. Furthermore, it was observed that CO2 has a displacement effect on CH4; higher CO2 concentrations are more conducive to CH4 release and CO2 storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The coal-rock reservoir exhibits the characteristics of a double pore medium, with the pore structure significantly impacting coal adsorption to gas. The majority of coalbed methane is present in the pores through adsorption, leading to expansion of the coal matrix and reduction in permeability due to fracture narrowing. Additionally, gas adsorption weakens the mechanical properties of coal, resulting in poor stability of the coal seam. The formation environmental conditions for coalbed methane refer to in-situ stress, geothermal and groundwater pressure conditions (Boyer et al. 1998; Atkins et al. 2003; Zhang et al. 2010), which are manifestations of energy within Earth’s crust. These conditions control both occurrence and output of coalbed methane (Warwick et al. 2005; Wang et al. 2009; Siriwardane et al. 2009). Coalbed methane primarily exists as adsorption within the coal seam, with its occurrence state varying based on different degrees of coalification and changes in environmental conditions. Changes in in-situ stress, groundwater pressure and geothermal conditions significantly affect desorption, diffusion and seepage processes for both adsorbed gas and free gas within the reservoir (Pashin et al. 2018; Li et al. 2020; Chen et al. 2006; He et al. 2021). Therefore, accurate analysis of environmental conditions within the reservoir and their impact on permeability is crucial for understanding changes in permeability patterns within a coal seam, optimizing engineering operations, and effectively developing coalbed methane.
The coalbed methane (CBM) is present in coal seams primarily in adsorbed, free, and dissolved states. The adsorbed gas is predominantly stored in micro-pores and small pores of the coal matrix, while fractures act as channels for the migration of CBM. Therefore, the pore-fracture structure significantly impacts the adsorption and fluidity of CBM. Gas adsorption results in expansion of the coal matrix, which reduces fracture width and permeability while also weakening the mechanical properties of coal rock, leading to poor stability. The complex double pore structure system makes matrix pores and fractures the main reservoirs and migration channels (Jia et al. 2020; Hu et al. 2014; Zhong et al. 2020; Wang et al. 2023). Consequently, the structure, morphology, and other characteristics of pores and fractures in coal rock have a significant impact on gas concentration and migration ability within coal seams. Matrix pores are influenced by various factors with complex morphology that can be affected by internal factors (Cai et al. 2016; Lau et al. 2017). Yalcin et al.’s detailed investigation on Carboniferous coal in the basin revealed that specific surface area and pore volume were influenced by maturity and microstructure variation characteristics (Cai et al. 2013a; Gürdal et al. 2001; Lin et al. 2024). The coalbed methane undergoes the processes of desorption, diffusion, and percolation. The adsorption and desorption characteristics of coal have garnered significant attention from scholars both domestically and internationally. The adsorption capacity and rate of coal to gas are influenced by various factors such as coal petrological composition, physical and chemical characteristics, coal grade, moisture content, as well as external factors including temperature, pressure, electric field, sound field, low-frequency vibration. Additionally, there are differences in the adsorption capacity between different gas (Cai et al. 2013b; Yang et al. 2014). Research on pore structure indicates that for similarly developed coals, the gas adsorption capacity increases with micropore volume; under high pressure conditions it linearly increases with micropore volume. This suggests that the adsorption capacity of high-pressure gas is primarily controlled by micropore volume (Mohamed et al. 2020; Wang et al. 2021).
The adsorption capacity and rate of gases by coal are influenced not only by its petrographic composition, physical and chemical properties, rank, moisture content, but also by external factors such as temperature, pressure, and low-frequency vibration. Additionally, different gases exhibit varying adsorption properties on coal. The adsorption experiment demonstrates that for coal formations with similar levels of maturity, the gas adsorption capacity of the coal increases as the volume of micropores expands. At high pressures, the adsorption capacity exhibits a linear increase in tandem with the expansion of micropore volume, indicating that under high pressure conditions, the gas adsorption capacity of the studied coal formations is primarily governed by their micropore volume (Li et al. 2023; Zhang et al. 2024). The coal sample used in the isothermal adsorption experiment is typically coal powder smaller than the target grain size, which does not match the grain size of coal in situ. For particles within a certain scale range, the adsorption time increases as the particle size increases in the absence of fractures. The saturated adsorption capacity of dry coal samples with different particle sizes is usually larger than that of saturated water coal samples. The particle size has a minor effect on the adsorption capacity of both dry and saturated water coal samples, primarily resulting in an extended adsorption time with increasing particle size. In saturated water coal samples, at low pressures, larger particle sizes lead to smaller saturated adsorption capacities and a tendency for the adsorption rate constant to decrease (Siemons et al. 2003).
The presence of water in coal rock leads to competition for adsorption with gas. As the water content increases, the adsorption capacity of coal rock for gas decreases. Once the water content reaches a certain level, further increases in moisture have minimal or even negligible impact on the coal rock’s adsorption capacity (Ahamed et al. 2019). The adsorption theory suggests that, for the same coal sample, the maximum adsorption capacity of coal rock for gas should remain consistent under any temperature condition (Perera et al. 2012). However, prior to reaching adsorption equilibrium, the ability of coal rock to adsorb gas weakens with increasing temperature and strengthens with increasing pressure. When considering the combined effects of temperature and pressure, the impact on the gas adsorption capacity of coal rock is more pronounced at lower temperatures and pressures (Wang et al. 2022). Whereas it is primarily influenced by temperature at higher temperatures and pressures. The adsorption capacity of coal rocks for gas is consistently higher when the equilibrium pressure is below a certain threshold, regardless of the degree of metamorphism (Yan et al. 2021). Furthermore, as the degree of metamorphism increases, so does the ability of coal rocks to adsorb gas.
Previous studies have extensively investigated the influencing factors of coal adsorption and desorption capacity, with a focus on coal mining. In comparison to the oil and gas industry, coal mining operates at relatively low pressure and temperature. To meet the requirements of coalbed methane extraction, it is essential to thoroughly explore the physical properties of coal rock reservoirs and their adsorption characteristics for coalbed methane under reservoir pressure and temperature conditions, in order to establish a theoretical foundation for efficient development of coalbed methane. Gas isothermal adsorption experiments were conducted to simulate the adsorption and desorption behavior of CH4-CO2 mixed gases, based on the analysis of mineral composition, physical properties, gas content, and pore structure characteristics of coal rocks. This aimed to clarify the adsorption and desorption patterns of CH4 in coal rocks under different geological conditions. These accomplishments hold significant importance for optimizing coalbed gas recovery processes and enhancing the productivity of coalbed gas wells.
Geological characteristics of coal seam
Analysis of coal seam characteristics
(1) Evaluation of coalbed methane reservoir.
The depth of coal seam is 300–800 m, which belongs to shallow and middle-level. Coal seam gas content is 15–27 m3/t, with an average of 21.7 m3/t, which belongs to the medium and high gas content. The reserve abundance of coal seam 3# is 1.41 × 108 m3/km2, and that of coal seam 15# is 0.58 × 108 m3/km2, which belongs to the medium reserve abundance. The daily gas output of a single well is generally 1 000–3 000 m3/d, with an average of 2138 m3/d, which belongs to medium and low productivity. As a whole, the gas field belongs to the shallow middle-level, middle-low yield and middle-abundance coalbed gas field, which is very suitable for the exploration and development of coalbed methane.
The maceral component of coal seam is mainly vitrinite (Fig. 1), the average content of vitrinite in coal seam 3# is 86.8%, and that in coal seam 15# is 81.9%. The measured vitrinite reflectance of coal seam 3# is 2.54–3.86%, with an average of 3.31%, and the measured vitrinite reflectance of coal seam 15# is 2.67–3.72%, with an average of 3.34%.
(2) Gas bearing property.
The gas content of 3# coal seam is generally 15–27 m3/t, with an average of 21.7 m3/t, and the gas saturation is 89–99%. The gas content of 15# coal seam is mostly 15–21 m3/t, with an average of 18.6 m3/t, gas saturation is 72–94%, which belongs to high saturation coalbed methane field.
Physical property characteristics
(1) Scanning electron microscopy analysis.
The SEM analysis provides direct information on the mineral types, occurrence, and pore filling within the pores, serving as a crucial method for studying pore structure. It not only offers insights into the types, sizes, contents, and coexistence relationships of particles in the pore system but also enables determination of clay mineral types, occurrence, and contents while observing the morphology, size, and connectivity of pore throats. The SEM results indicate that the rock is dense with predominant micropores and microfractures; quartz and imogolite are distributed on grain surfaces; framework feldspar appears striated and weathered; severe quartz dissolution leads to an uneven rough surface; imogolite is interspersed with it along with traces of pyrite weathering. Additionally, localized gas pores (biogenic pores) develop on the matrix surface filled with imogolite and other clay minerals alongside microcrystalline quartz, feldspar, and calcite. Overall porosity in coal rock is relatively good (Fig. 2).
(2) Porosity-permeability property.
Injection/pressure drop well test confirmed that the permeability of coal seam in this area is poor, and the effective permeability is (0.013–0.527) × 10–3 µm2, with an average of 0.198 × 10–3 µm2, of which the 3# coal seam is (0.024–0.516) × 10–3 µm2, with an average of 0.173 × 10–3 µm2, and the 15# coal seam is (0.013–0.072) × 10–3 µm2, with an average of 0.047 × 10–3 µm2.
The pore structure characteristics of coal seam determine the existence form and movement form of pore fluid. Coal reservoir is a kind of double-porosity medium, with matrix pores and fissures. Different from common double-porosity gas reservoirs, coal is divided into several matrix blocks by cleavings in coalbed methane reservoir. The matrix contains a large number of tiny pores and is the main space for gas storage with low permeability. The cleat is the secondary pore system in coal, but it is the main channel for fluid (gas and water) seepage in coal seam.
The coal types in Qinshui Basin are relatively complete, from gas coal to anthracite are distributed, but mainly metamorphic coal and anthracite. Each coal seam is composed of saproic coal, and its macro-coal rock composition is mainly bright coal, followed by dark coal, mirrorcoal and silk carbon less. The pore range of coal rock in southern Qinshui area is relatively wide, the pore size of matrix is from less than 1 nm to several hundred nm, and the fractures in the coal seam can be seen by naked eye. By using mercury injection experiment method to measure the micro-pore structure of coal seam, the capillary pressure curve as shown in Fig. 3 is obtained. The capillary pressure curve of coalbed methane field in southern Qinnan is characterized by about 50–80% mercury intake before the pressure is 0.1 MPa. When the pressure is between 0.1 and 20 MPa, the mercury intake is very small. After 20 MPa, the amount of mercury into the increase, about 4–15% of the amount of mercury into. This shows that the coal samples in Qinshui Basin have relatively developed cleavage and large pores, but few middle pores, followed by transition pores and micropores.
At the same time, it can be found that the capillary pressure curve in the above figure also has the following properties: the maximum mercury saturation is very large, close to 100%. The drainage pressure is not easy to determine. The median pressure is low and the median radius is large. High mercury removal efficiency, large apparent pore-throat volume ratio, and relatively uniform pore-throat sorting. According to the mercury injection experiment, the pore structure of coalbed methane reservoir in southern Qinshui is as follows: the large pore is about 76.56%, the medium pore is about 2.05%, the small pore is about 15.21%, and the micro pore is about 6.18%.
Although large pores account for a large proportion in the coal seam, they have basically no impact on the surface area of the coal seam (Table 1). The main factors affecting the surface area in the coal seam are the small holes and microholes, and the relationship with the volume of the middle holes is not obvious.
Through the low-temperature nitrogen adsorption experiment of Qinshui coalbed methane field, it can be concluded that the pore size is mainly distributed in 100–200 nm. It can be seen from the characteristics of low temperature nitrogen experiment that the coal seam pores in Qinshui Basin are mainly micro-pores and small holes, large pores and fractures are relatively developed, and the pore connectivity is slightly poor.
Due to the development of coal micropores and large specific surface area, it is conducive to the adsorption of gas on the surface of coal matrix, forming the reservoir space, and the cleave-fissures form the seepage channel of coalbed methane. The special chemical structure and pore structure of coal in coalbed methane reservoir determine the different storage and migration forms of coalbed methane in coal seam compared with conventional gas reservoirs.
Composition of coalbed methane
For the phase state research of CBM in southern Qinshui of China, the first research is the composition of CBM. The chemical composition of hydrocarbon in reservoir is the internal factor of phase transformation, while the change of pressure and temperature is the external condition of phase transformation.
The main component of coalbed methane is methane, whose content is generally greater than 85%, which is formed in the process of coalification. The hydrocarbon gases of coalbed methane are mainly methane, ethane, propane, butane and isobutane, while the non-hydrocarbon gases are mainly N2, CO2, CO, H2S, H2 and trace inert gases. The composition of coalbed methane varies with different CBM reservoirs and different positions of the same CBM reservoir. The chemical composition of coalbed methane is related to the composition of coal, reservoir pressure, degree of coalification, hydrogeological conditions and desorption of coalbed methane.
Because the coalbed methane in the southern Qinshui Basin is composed of various gases, the physical properties of coalbed methane in this area are the comprehensive physical properties of various gases. Under the condition of 0 °C, methane is colorless, tasteless, bromine free and non-toxic gas, but the coal reservoir often contains a small amount of other aromatic hydrocarbon gas, so it is often accompanied by some apple aroma. Under the standard conditions of standard atmospheric pressure and temperature, the density of coalbed gas is 0.716 kg3/m, and the specific gravity is about 0.554 compared with air. When the air is mixed with 5.3–16.0% methane, it can burn or explode in the event of fire, and the methane concentration reaches 9.5%, and the explosion is the most violent in the event of open fire. When the methane concentration reaches 43%, people feel shortness of breath. At 57.0%, the person is in a coma. The physical properties of each gas component in coalbed methane, such as water solubility, explosive, toxicity, odor, color and relative density of gas, are shown in Table 2.
It can be seen that although methane itself is non-toxic, it will explode within a certain concentration range and produce a huge safety hazard. Coalbed methane released in coal mine production is also known as mine gas, which affects and threatens the normal safety production of coal mines. It poses a great threat to the safety operation of coal mine mainly in the aspects of gas accumulation, coal and gas outburst and gas explosion. At the same time, the release of coal mine gas into the atmosphere will also produce serious greenhouse effect on the atmospheric environment. Compared with CO2, the greenhouse effect of methane calculated by mass is 20–60 times larger than that of CO2. Mining Coalbed methane and reducing the amount of methane released into the atmosphere are of great importance in protecting the global environment.
Adsorption and desorption characteristics of coal seam
Mathematical model of adsorption and desorption
In order to reflect the migration process of coalbed methane such as desorption, diffusion and seepage, many simulation models have been proposed, which can be roughly divided into three types (Liu et al. 2022; Liu et al. 2021; Zhang et al. 2022).
-
(1)
Empirical model. It is only a simple mathematical description of the observed physical phenomenon.
-
(2)
Equilibrium adsorption model. The coal seam is treated as a single porous medium, and the gas adsorption phenomenon is the simplest treatment. This model assumes that once the gas is desorbed, it immediately enters the fracture, so that the gas concentration in the micropores is only related to the pressure in the fracture and has nothing to do with time, so that the gas adsorbed on the pore of the coal seam is in a continuous equilibrium state with the free gas in the pore.
-
(3)
Non-equilibrium adsorption. It is assumed that the coal seam is a double medium of micropores and fractures, considering the adsorption and desorption of gas in micropores and the time of diffusion from micropores to fractures.
Occurrence state of coalbed methane
Coalbed methane is mainly stored in coal seams in three forms, namely, adsorption state adsorbed on the surface of coal pores, free state in coal pores and fractures, and dissolved state in coal seam water. In general, the methane gas generated in the process of coalification will first meet the adsorption state, and then the free state and the dissolved state. Its main occurrence state is the adsorption state, and the adsorption gas volume accounts for the vast majority of the coalbed methane volume.
(1) Adsorption state.
Coal is a porous medium, the van der Waals force of the particles surface molecules attracts the surrounding gas molecules, is a physical adsorption process on the solid surface, in line with the Langmuir isothermal adsorption equation. Through adsorption, a large amount of methane is absorbed and stored on the wall of coal pores. The grain surface of coal also adsorbs a thin layer of coalbed methane to accumulate on the surface of coal rock, and combines with the large surface of coal grains in the matrix unit, making adsorption the main mechanism of gas storage.
(2) The free state.
Free gas refers to natural gas stored in pores or fractures that can move freely. This part of the gas is subject to the general gas equation, and its amount depends on the pore volume, temperature, gas pressure and gas compression coefficient.
(3) Dissolved gas.
Methane has a certain solubility in pure water at room temperature and atmospheric pressure, but the solubility is very small. The solubility of methane in water mainly depends on the temperature, salinity, ambient pressure and gas composition of the water.
Coalbed gas exists in the above three forms in the coal seam, and when the amount of hydrocarbon generation in the coal seam increases or the external environment changes, the three storage forms can be converted into each other. Under normal circumstances, more than 90% of the gas is stored on the inner surface of the coal in the form of adsorbed gas, less than 10% of the free gas, and only a small part of the dissolved gas.
Under the formation condition, the coal reservoir is a three-phase coupling system of solid-liquid-gas. The interaction of solid-gas, gas-liquid and solid-liquid occurs together. The solid coal matrix is a complex porous medium with microporous development and mainly composed of organic macromolecules. The gas phase is mainly methane, containing a small amount of gaseous water, carbon dioxide, nitrogen, heavy hydrocarbon gas, etc. The liquid phase mainly refers to liquid water, and sometimes liquid hydrocarbons can be seen. The adsorption and desorption of gas by coal matrix is the main form of solid gas action, and the large pore volume of coal matrix provides space for gas migration and storage. Gas-liquid action mainly refers to the dissolution and escape of gas in water. The solid-liquid action mainly includes the wetting of the surface of the coal matrix, the filling and migration of pore water.
Adsorption of coalbed gas
Different from conventional natural gas reservoirs, adsorption is the main way to store CBM in CBM reservoirs, and CBM is mainly stored in coal seams in adsorption state. The adsorption state of gas in coal can be divided into physical adsorption and chemical adsorption. Table 3 lists the comparative characteristics of physical adsorption and chemisorption. It can be seen from the essential difference between physical adsorption and chemical adsorption at the solid-gas interface that the gas adsorbed on the solid surface by physical adsorption is relatively easy to be desorbed, as long as the pressure is reduced or the temperature is increased; The chemical adsorption state of gas desorption is more difficult, from the chemical adsorption state into the physical adsorption state need to climb over the energy barrier, the energy barrier is the adsorbent and the adsorbent surface to form a chemical bond required energy.
Experimental determination and theoretical analysis of coal intermolecular structure show that the adsorption of coalbed methane molecules in coal pore structure is physical adsorption. Its essence is because coal is a porous medium, which is distributed with a large number of pores, with a lot of plant tissue holes, intergranular holes, mold holes, intergranular holes, corrosion holes and polycondensation holes. Coalbed methane is mainly adsorbed in these microvoids. There is van der Waals force interaction between solid surface molecules and adsorbed gas molecules. Van der Waals force interaction mainly includes three aspects: electrostatic force, induction force and dispersion force. Part of the attraction of coal molecules points to the saturation state of the coal molecular structure, while the other part points to the space, the unsaturated state, and produces an adsorption force field on the surface of the coal pore to adsorb the surrounding gas molecules, which is physical adsorption. Due to the existence of van der Waals force between particle surface molecules and adsorbed gas, physical adsorption becomes the main way of coal seam adsorption.
Physical adsorption is characterized by the adsorption of the molecules to maintain its personality, and adsorption on the surface of the adsorbent to maintain the van der Waals force. The adsorbent molecules fall on the surface of the adsorbent and maintain a force field on it for a certain time, and then desorption. When the adsorption rate is greater than the desorption rate, the adsorption continues until the adsorption equilibrium is reached when the adsorption rate is equal to the desorption rate, and the adsorbent molecules form an adsorption layer on the surface of the adsorbent. The adsorption heat of physical adsorption is very low, its speed is fast and reversible, and it has selective adsorption in the presence of a variety of gases, and in the physical adsorption process, it complies with the langmuir isothermal adsorption equation, that is, in the isothermal adsorption process, pressure has a significant impact on the adsorption, and the adsorption amount increases significantly with the increase of pressure. Chemisorption is mainly adsorbed by ionic bond, which requires a large amount of energy to open the ionic bond and desorption methane. The proportion of this state is very small.
Due to the development of micropores in Panhe area and Shizhuangnan area in southern Qinshui Basin, the specific surface area of coal seam is large, the physical adsorption capacity is large, and the chemical adsorption capacity is small.
Single molecular layer adsorption theoretical model - Langmuir model
For the phase analysis, reserve calculation and production analysis and calculation of coalbed methane in various blocks in southern Qinnan area, it is necessary to calculate the adsorption amount of coal and the theory of coal adsorption and desorption. All of them are based on Langmuir isothermal adsorption model. The theoretical models used to describe coal seam gas adsorption include Langmuir model, BET model, potential energy theory model, etc. Various models have different application ranges. Langmuir model is the main model widely used in coal adsorbability at home and abroad, which is used to describe the relationship between the adsorption capacity and pressure of coal physical adsorption.
Langmuir proposed the single molecular layer adsorption theory from the kinetic point of view in 1916. The basic assumptions are: (1) the adsorption equilibrium is dynamic equilibrium. The solid surface is uniform; There is no interaction between adsorbed molecules. Only a single molecular layer is formed by adsorption. Its mathematical expression is
It can be seen that the adsorption capacity of Coalbed methane is a function of pressure, and with the increase of pressure, the adsorption capacity of coal increases. Through the experiment of isothermal adsorption–desorption of coal samples in the laboratory, the isothermal adsorption curve of coal seam can be measured, and then the adsorption constant a and pressure constant b in formula (1) can be obtained. The Langmuir volume and Langmuir pressure are more widely used and can better show the gas adsorbability of coal seam. That is, another expression of Langmuir equation
In general, monolayer adsorption often occurs on the surface of non-reactive solids above the critical temperature of the gas. Therefore, the above Langmuir equation can be used to calculate coalbed methane adsorption in the southern Qinshui area of China. It can be seen that the larger the volume of Langmuir, the greater the adsorption capacity of gas. The larger the Langmuir pressure, the smaller the adsorption capacity of the gas. For a specific component of a specific coal seam, the value of Langmuir volume and Langmuir pressure is constant, so the adsorption law of coal seam gas can be expressed by measuring and calculating its value. Through the application of Langmuir isothermal adsorption equation, the law of mutual transformation between adsorbed state and gaseous state can be obtained.
Adsorption isotherm curve of coal
Through the adsorption isothermal experiment on the coal sample collected in the laboratory, the isothermal adsorption and desorption curve of the coal seam can be obtained, reflecting the adsorption and desorption law of the coal seam under the formation conditions.
(1) Isothermal adsorption curve characteristics in southern Qinshui of China.
The 3# coal seams in Panhe area and Shizhuangnan area in the south of Qinshui Basin are anthracite with high adsorption capacity and high coal rank. The adsorption isotherm curve of anthracite coal seam in Jincheng Mining# area can be measured through the adsorption isotherm experiment of the anthracite coal sample in the third coal seam of Jincheng Mining area. From the isothermal adsorption curve of coal seam, it can be seen that the adsorption capacity of coalbed gas is a function of pressure, and the adsorption capacity increases with the increase of pressure. In the case of low pressure, CBM is easy to be absorbed into the surface of coal grains, and the adsorption amount of coal can be increased a lot with a small increase in pressure; When the pressure rises, the gas molecules repel each other in the small space on the surface of the coal grain, and the increasing pressure can only increase the small adsorption amount and slow down the absorption rate; In the case of high pressure, the gas molecules form close packing, and the absorption rate tends to 0.
Figure 4 shows the isothermal adsorption curve of coal seam 3# in Qinshui Basin, obtained by experimental tests. According to the curve, the Langmuir volume constant and pressure constant can be calculated to obtain the adsorption law, and the recovery rate and recovery efficiency can be predicted. It can be seen from the Fig. 4 that, when the pressure is lower than 3.5 MPa, the slope of the adsorption curve is larger, the adsorption capacity increases greatly, and the adsorption power of coal to methane is enhanced. When the pressure continues to increase, the adsorption curve shows a gentle upward trend. When the pressure increased to 8 MPa, the adsorption capacity increased slowly and basically remained unchanged.
(2) The application of adsorption isotherm curve.
The isothermal adsorption curve of coal seam reflects the adsorption (desorption) characteristics and ability of different coal rocks to methane gas. The reversibility of coal's gas adsorption makes it possible to develop the adsorbed gas in coal seam. The application of adsorption isotherm in coalbed methane research mainly has the following five aspects:
① To evaluate the maximum adsorption capacity of coal seam for gas, the measured value is often low.
② Predict the maximum value and release rate of gas released when the reservoir pressure is reduced in the production process.
③ Determine the critical desorption pressure. The pressure at which the CBM begins to desorption under formation conditions is called the critical desorption pressure. The method to determine the desorption pressure of undersaturated coalbed methane is on the adsorption isotherm curve, and the pressure corresponding to the actual gas content is the critical desorption pressure.
④ Determine the gas saturation. Gas saturation refers to the ratio of the actual gas content under certain conditions (reservoir pressure, temperature and coal quality, etc.) to the theoretical adsorption amount under the corresponding conditions, expressed as a percentage. If the ratio is 100%, it is a gas saturated reservoir. If the ratio is less than 100%, it is undersaturated reservoir. If the ratio is greater than 100%, it is supersaturated, indicating that there are free and dissolved gases in the reservoir. The actual content is obtained by desorption experiment, and the theoretical adsorption is obtained by isothermal adsorption experiment. In general, gas saturation is generally less than 100% because coalbed methane has a certain degree of loss in geological time. If it is less than 100%, especially when the gas is in the unsaturated state, that is, the amount of gas contained does not reach the maximum adsorption capacity, the test to determine the gas saturation is very important.
⑤ To obtain the recovery rate of coalbed gas. The recovery rate of coalbed methane is the percentage of the ratio of the adsorbed gas content in the depressurized desorption zone to the gas content under critical desorption pressure (experimental gas content). This is also a theoretical result under artificial experimental conditions, but the actual formation conditions, in any case, can not reach the laboratory conditions. Therefore, this recovery rate is reliable in evaluating the adsorption characteristics of different coal and rock, but there is a considerable error in determining the final recovery rate of coalbed methane in the actual production process.
Adsorption capacity of coal
Coal is a substance with a large surface area, so it is an excellent natural adsorbent and has a strong adsorption capacity for various gases, which is the material basis for the different gas storage mechanism of coalbed methane and conventional reservoirs. Due to the control of various factors, the adsorption capacity of coal is quite different. The adsorption of coal to Coalbed methane occurs on the solid surface of coal (outer surface and inner surface of pores). Its adsorption capacity is affected by external factors in addition to the characteristics of coal itself. The former such as the material composition of coal, metamorphism degree, particle size; The latter such as temperature, pressure, moisture and so on. The experimental results show that the adsorption capacity of coal is significantly affected by the degree of coal metamorphism, temperature and moisture content.
The adsorption characteristics of coal were studied by using high pressure volumetric method. The method of determining the methane adsorption capacity of coal by high pressure volumetric method is: The treated dry coal sample, into the adsorption tank, vacuum degassing, determine the volume of the adsorption tank, fill a certain volume of methane into the adsorption tank, so that the adsorption tank pressure balance, some gas is adsorbed, some gas is still in the free state in the remaining volume, known to fill the methane volume, deduct the free volume in the remaining volume, that is, the adsorption volume. Connected is the adsorption isotherm.
(1) The influence of coal metamorphism on adsorption capacity.
The adsorption of methane by coal is a physical process that occurs on the inner surface of pores, and the adsorption capacity is affected by the characteristics of pores. In the process of coal metamorphism, the pores are changing, which affects the adsorption capacity of coal. As can be seen from Table 4, from lignite to anthracite, the adsorption capacity also increases with the increase of coal metamorphism. The coal grade is high, mainly anthracite, with high gas capacity and adsorption capacity, gas concentration is generally 10–20 m3/t, the highest up to 35 m3/t.
(2) The effect of temperature on the adsorption property of coal.
At present, the temperature of 30 °C is generally used in the isothermal adsorption experiments of coal samples under the conditions of, 25 °C, 35 °C, 45 °C, and 50 °C. It can be seen that the adsorption capacity of coal changes at different temperatures, and the adsorbability of coal to methane decreases regularly with the increase of temperature. The research shows that the ability of coal to adsorb gas decreases by about 8% with the increase 1 °C of temperature. The reason is that gas activity increases with the increase of temperature, and it is difficult to be adsorbed by coal. At the same time, the adsorbed gas molecules are easy to obtain kinetic energy and escape from the surface of the coal body. At the same time, the influence of temperature on the adsorption capacity of high coal grade coal is stronger. If the methane adsorption capacity of coal at present 30 °C is known, the adsorption capacity at other temperatures can be approximated by the following formula
(3) The effect of water on the adsorption property of coal.
In many areas of China, the enrichment degree of coalbed methane is extremely poor, showing a serious problem of undersaturation of coalbed methane adsorption. The gas geology community interprets this phenomenon as groundwater runoff carrying away methane from coal seams, so that the content of Coalbed methane in these zones is reduced, and even the so-called coal seams are free of gas. In the gas geology community, this rule is summarized as "if there is water, there is no gas, there is no water". The occurrence of this phenomenon indicates the existence of coalbed gas displacement and desorption.
Desorption of coalbed methane
In addition to the adsorption amount of coal, the output of Coalbed methane is also closely related to the desorption of coal. As a representative of low coal rank basins, the coalbed methane content of Powder River Basin in the United States is generally 0.78–1.6 m3/t, the highest is not more than 4 m3/t, and the average daily production of a single well is 4530 m3/d. As a representative of high rank basins, the southern Qinshui Basin in Shanxi Province generally has a CBM content of 10–20 m3/t, and the highest is up to 35 m3/t, while the average daily production of a single well is generally lower than 1000 m3. The reason for this phenomenon is closely related to the degree and rate of desorption of coalbed methane.
(1) Factors affecting coalbed methane desorption.
Factors affecting the desorption rate of coalbed methane are:
① The influence of pressure on desorption rate. When the pressure is closer to the critical desorption pressure, the desorption rate of methane is slower; The lower the pressure, the faster the desorption rate of methane. Therefore, the key to increase the desorption rate is to increase the pressure drop rate in the largest area.
② The influence of coal seam water content on desorption rate. The adsorption amount of methane in coal seam is inversely proportional to the coal seam water content. This shows that the faster the drainage rate, the faster the desorption rate.
③ The influence of coal particle size on desorption rate. Coal particle size has a great influence on the desorption rate. The larger the particles, the greater the distance the methane diffuses and travels, and the longer the desorption time. Reducing the particle size will greatly improve the desorption speed and productivity.
④ The influence of temperature on the desorption rate. The higher the temperature, the faster the desorption rate. Increasing the temperature of the coal seam can increase the desorption rate.
⑤ The influence of permeability. Increasing the permeability end point increases the flow rate of methane after desorption. Increasing permeability also increases the rate of pressure drop during drainage, thus increasing the rate of desorption.
Coalbed methane desorption is a very complicated problem, not only depressurization desorption, but also the displacement of adsorbed methane gas by water and other gases in coal seam is a type of coalbed methane desorption that cannot be ignored. The essence of displacement desorption is that the unadsorbed water molecules or other gas molecules replace the methane molecules in the adsorbed state in order to achieve dynamic equilibrium, so that the methane molecules in the adsorbed state become free. By using this principle, gas can be injected to improve the recovery rate of coalbed methane. CO2 gas injection is widely used.
(2) Coal displacement desorption: the influence of component concentration in CH4-CO2 gas mixture on CH4 desorption.
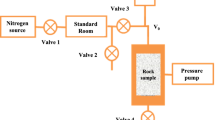
Using the principle of displacement desorption, when CO2 is injected into the coal seam, it will promote the desorption of Coalbed methane adsorbed in the coal seam. By using gas isothermal adsorption desorption instrument, professor of China University of Geosciences conducted isothermal adsorption–desorption experiment on anthracite equilibrium water coal sample of Jincheng coal seam 3#, Qinshui Basin, Shanxi Province. Different CH4—CO2 components were used to study the quantitative influence of different component contents on desorption. In the experiment, the following three different CH4-CO2 mixed components were used for comparative experiments: ① 75% CH4 + 25% CO2 adsorption-desorption test. ② 35% CH4 + 65% CO2 adsorption–desorption test. ③ 15% CH4 + 85% CO2 adsorption-desorption test. For CH4, CO2 pure components of the adsorption–desorption experiment, the data obtained are shown in Table 5.
The results of binary gas adsorption and desorption experiments for different components are shown in Table 6. After comparing the theoretical model established with the experimental test results on the influence of component concentration on CH4 desorption in CH4-CO2 mixed gas, it was found that the predicted results of the theoretical model were in good agreement with the experimental results. Furthermore, the experiment revealed the nonlinear influence of component concentration on the CH4 desorption process and the differences in CH4 desorption rates at different concentrations. Therefore, the experimental test results further proved the reliability and accuracy of the theoretical model and provided strong evidence for predicting the CH4 desorption behavior in CH4-CO2 mixed gas.
Conclusions
This study focuses on the No. 3 coal from the Qinshui Basin in China, analyzing its mineral composition, physical properties, gas content, and pore structure characteristics in depth. Furthermore, it discusses the adsorption characteristics of different gases in coal rock and conducts experimental research to investigate changes in gas adsorption and desorption capacity under varying conditions.
(1) The coal formation in the study area is predominantly characterized by micropores and small pores, with well-developed larger pores and fractures. The pore connectivity is somewhat limited, and the predominant pore size ranges from 100 to 200 nm. The average permeability measures 0.198 × 10–3 µm2, while the mean specific gas content stands at 21.7 m3/t. Vitrinite reflectance has been measured at 3.32%. It provides a comprehensive analysis of the microscopic environmental characteristics of gas adsorption in coal rocks, offering valuable insights into the migration and storage behaviors of gases in underground reservoirs.
(2) The isothermal adsorption curves of coal rock for CH4 indicate that at pressures below 3.5 MPa, the slope of the adsorption curve is steep and there is a significant increase in adsorption; as the pressure further increases, the adsorption curve exhibits a gradual upward trend; once the pressure reaches 8 MPa, the adsorption amount increases slowly and stabilizes. The gas adsorption capacity of coal rock rises with increasing coal rank. These findings offer valuable reference information for further investigating the role played by different types and qualities of coal rock in storing and releasing natural gas in gas reservoirs.
(3) The CO2-CH4 mixed gas demonstrates distinct characteristics during adsorption and desorption: the desorption amount and rate of the CO2 component are lower, while those of the CH4 component are relatively higher. This disparity arises from the diverse interactions between the gas molecules and the solid adsorbent. Due to their stronger dipole moment and greater polarization ability, CO2 molecules tend to form more robust and stable bonds with the solid surface, resulting in a reduced desorption amount and rate compared to CH4 molecules. The non-polar nature of CH4 molecules leads to a weaker bond on the solid surface, resulting in a higher desorption rate and amount. As the relative concentration of CO2 increases, both CH4 desorption rate and CO2 adsorption rate also increases under identical pressure drop conditions. This phenomenon can be attributed to the competitive influence of multi-component mixed gases on the solid surface. With an increase in CO2 concentration, it occupies more available positions and impacts CH4's ability to detach from the solid surface, leading to an escalation in CH4 desorption rate.
Abbreviations
- a :
-
Adsorption constant, the maximum adsorption capacity of the reaction adsorbent, independent of temperature and pressure, but dependent on the nature of the adsorbent and adsorbent, cm3/g.
- b :
-
Pressure constant, depending on the temperature and the nature of the adsorbent, MPa−1.
- n t , n 30 :
-
The temperature coefficient index of temperature t and 30 °C respectively.
- P :
-
Equilibrium gas pressure, MPa.
- \({P}_{L}\) :
-
Langmuir pressure, which represents the equilibrium gas pressure corresponding to the adsorption capacity reaching half of the Langmuir volume, = 1/b, MPa.
- V:
-
Adsorption capacity, cm3/g.
- \({V}_{L}\) :
-
Langmuir volume, its physical meaning is the same as the value of a, \(VLVL\) cm3/g.
- V t, V 30 :
-
The adsorption capacity of dry coal sample methane when the temperature is t and 30 ℃ respectively
- CBM:
-
Coalbed methane
- SEM:
-
Microscopy
- XRD:
-
X-Ray Diffraction
References
Ahamed MAA, Perera MSA, Matthai SK, Ranjith PG, Dong-yin L (2019) Coal composition and structural variation with rank and its influence on the coal-moisture interactions under coal seam temperature conditions–A review article. J Petrol Sci Eng 180:901–917. https://doi.org/10.1016/j.petrol.2019.06.007
Atkins B (2003) Coal Bed Methane—From resource to reserves. Focus. Gaffney, Cline & Associates, pp. 1–2.
Boyer CM II, Qingzhao B (1998) Methodology of coalbed methane resource assessment. Int J Coal Geol 35:349–368. https://doi.org/10.1016/S0166-5162(97)00041-4
Cai Y, Liu D, Pan Z, Yao Y, Li J, Qiu Y (2013a) Pore structure and its impact on CH4 adsorption capacity and flow capability of bituminous and subbituminous coals from Northeast China. Fuel 103:258–268. https://doi.org/10.1016/j.fuel.2012.06.055
Cai JH, Yuan Y, Wang JJ, Li XJ, Cao WJ (2013b) Experimental research on decreasing coalbed methane formation damage using micro-foam mud stabilized by nanoparticles. J China Coal Soc 38(9):1640–1645
Cai J, Gu S, Wang F, Yang X, Yue Y, Wu X, Chixotkin VF (2016) Decreasing coalbed methane formation damage using microfoamed drilling fluid stabilized by silica nanoparticles. J Nanomater 2016:52
Chen Z, Khaja N, Valencia KL, Rahman SS (2006) Formation damage induced by fracture fluids in coalbed methane reservoirs. SPE Asia Pacific Oil Gas Conf Exhib. https://doi.org/10.2118/101127-MS
Gürdal G, Yalçın MN (2001) Pore volume and surface area of the Carboniferous coals from the Zonguldak basin (NW Turkey) and their variations with rank and maceral composition. Int J Coal Geol 48(1–2):133–144
He J, Okere CJ, Guandong S, Pengjie H, Zhang L, Xiong W, Li Z (2021) Formation damage mitigation mechanism for coalbed methane wells via refracturing with fuzzy-ball fluid as temporary blocking agents. J Nat Gas Sci Eng 90:103956. https://doi.org/10.1016/j.jngse.2021.103956
Hu YL, Wu XM (2014) Research on coalbed methane reservoir water blocking damage mechanism and anti-water blocking. J China Coal Soc 39(6):1107–1111
Jia Q, Liu D, Cai Y, Fang X, Li L (2020) Petrophysics characteristics of coalbed methane reservoir: a comprehensive review. Frontiers of Earth Science 15:1–22
Lau HC, Li H, Huang S (2017) Challenges and opportunities of coalbed methane development in China. Energy Fuels 31(5):4588–4602
Li L, Liu D, Cai Y, Wang Y, Jia Q (2020) Coal structure and its implications for coalbed methane exploitation: a review. Energy Fuels 35(1):86–110
Li Z, Ren T, Li X, Cheng Y, He X, Lin J, Yang X (2023) Full-scale pore structure characterization of different rank coals and its impact on gas adsorption capacity: A theoretical model and experimental study. Energy 277:127621
Lin J, Dong C, Lin C, Duan D, Ma P, Zhao Z, Liu B, Zhang X, Huang X (2024) The impact of tectonic inversion on diagenesis and reservoir quality of the Oligocene fluvial sandstones: the upper Huagang formation, Xihu Depression, East China Sea Shelf Basin. Marine and Petroleum Geology, 106860.
Liu S, Wei J, Ma Y, Liu H, Liu X, Yan B (2021) Numerical simulation investigations of coalbed methane drainage performance with multilateral well. J Petrol Explor Product 11:1303–1321. https://doi.org/10.1007/s13202-021-01108-2
Liu L, Wang J, Su P, Huang W, Zhang B, Zhang X, Li M (2022) Experimental study on interlayer interference of coalbed methane reservoir under different reservoir physical properties and pressure systems. J Petrol Explor Product Technol 12(12):3263–3274. https://doi.org/10.1007/s13202-022-01513-1
Mohamed T, Mehana M (2020) Coalbed methane characterization and modeling: review and outlook. Energy Sources, Part a: Rec, Util Environ Eff 5:1–23
Pashin JC, Pradhan SP, Vishal V (2018) Formation damage in coalbed methane recovery. Formation damage during improved oil recovery. Elsevier, pp 499–514. https://doi.org/10.1016/B978-0-12-813782-6.00013-0
Perera MS, Ranjith PG, Choi SK, Airey D, Weniger P (2012) Estimation of gas adsorption capacity in coal: a review and an analytical study. Int J Coal Prep Util 32(1):25–55
Siemons N, Busch A, Bruining H, Krooss B, Gensterblum Y (2003) Assessing the kinetics and capacity of gas adsorption in coals by a combined adsorption/diffusion method. SPE Annual Tech Conf Exhib. https://doi.org/10.2118/84340-MS
Siriwardane H, Haljasmaa I, McLendon R, Irdi G, Soong Y, Bromhal G (2009) Influence of carbon dioxide on coal permeability determined by pressure transient methods. Int J Coal Geol 77:109–118. https://doi.org/10.1016/j.coal.2008.08.006
Wang X, Ward C (2009) Experimental investigation of permeability changes with pressure depletion in relation to coal quality. University of Alabama, Tuscaloosa, Alabama, International Coalbed Methane Symposium, p 31
Wang Z, Liu S, Qin Y (2021) Coal wettability in coalbed methane production: a critical review. Fuel 303:121277. https://doi.org/10.1016/j.fuel.2021.121277
Wang K, Ren H, Wang Z, Wei J (2022) Temperature-pressure coupling effect on gas desorption characteristics in coal during low-variable temperature process. J Petrol Sci Eng 211:110104. https://doi.org/10.1016/j.petrol.2022.110104
Wang F, Xu H, Liu Y, Meng X, Liu L (2023) Mechanism of low chemical agent adsorption by high pressure for hydraulic fracturing-assisted oil displacement technology: a study of molecular dynamics combined with laboratory experiments. Langmuir 39(46):16628–16636. https://doi.org/10.1021/acs.langmuir.3c02634
Warwick PD (2005) Coal systems analysis: a new approach to the understanding of coal formation, coal quality and environmental considerations, and coal as a source rock for hydrocarbons. In: Warwick PD (ed) Coal Systems Analysis: Geological Society of America Special Paper, 387. Boulder Co, USA, pp 1–8. https://doi.org/10.1037/0033-295X.92.3.389
Yan J, Feng X, Guo Y, Wang W, Wu L, Tan Z (2021) Desorption effects and laws of multiscale gas-bearing coal with different degrees of metamorphism. ACS Omega 6(34):22114–22125
Yang HL, Wang WY, Tian ZL (2014) Reservoir damage mechanism and protection measures for coal bed methane. J China Coal Soc 39(1):158–163
Zhang S, Tang S, Tang D, Pan Z, Yang F (2010) The characteristics of coal reservoir pores and coal facies in Liulin district, Hedong coal field of China. Int J Coal Geol 81:117–127. https://doi.org/10.1016/j.coal.2009.11.007
Zhang X, Zhang B, Zhang J, Deng Z, Guo D (2022) A new desorption-induced permeability dynamic model and its application in primary coalbed methane recovery. J Petrol Explorat Product Technol. https://doi.org/10.1007/s13202-021-01393-x
Zhang J, Lin C, Tang H, Wen T, Tannant DD, Zhang B (2024) Input-parameter optimization using a SVR based ensemble model to predict landslide displacements in a reservoir area–A comparative study. Appl Soft Comput 150:111107
Zhong H, Yang T, Yin H, Lu J, Zhang K, Fu C (2020) Role of alkali type in chemical loss and ASP-flooding enhanced oil recovery in sandstone formations. SPE Res Eval Eng 23(02):431–445. https://doi.org/10.2118/191545-PA
Funding
This study was supported by Science and Technology Research Program of Chongqing Municipal Education Commission (Grant No. KJQN202203201); and supported by Natural Science Foundation of Chongqing (Grant No. cstc2021jcyj-msxmX0959); and supported by Doctoral Research Fund of Chongqing Industry Polytechnic College (Grant No. 2023GZYBSZK1-11); and supported by Guizhou Provincial Science and Technology Foundation (Grant No.Qian Ke He Zhi Cheng [2023] Yiban 482).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, P., Tang, X., Wen, L. et al. Geological characteristics and coalbed methane adsorbability of shallow coal rock in Qinshui Basin, China. J Petrol Explor Prod Technol (2024). https://doi.org/10.1007/s13202-024-01869-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13202-024-01869-6