Abstract
Piriformospora indica, an endophytic fungus of Sebacinales, colonizes the roots of a wide range of host plants and provides various benefits to the plants. Cardamom (Elettaria cardamomum Maton) is an economically valuable spice crop of the tropics. In this work, we describe differentially expressed transcripts responding to P. indica root colonization in small cardamom for elucidation of molecular basis of growth and development. During the study, a wild genotype of cardamom was propagated under in vitro conditions using full strength Murashige and Skoog medium supplemented with 1 mg l−1 BAP (for shoot induction) and basal MS liquid medium (for root induction). Cardamom plantlets were co-cultivated with P. indica. Microscopic observation confirmed the presence of P. indica inside the roots of cardamom plantlets. Growth parameters of control and P. indica colonized plantlets were observed for three months at an interval of 15 days. P. indica colonization resulted in a significant increase in the morpho-physiological traits of the host plant. The growth enhancement was visible after 15 days of co-culture. There was a significant increase (p < 0.05) in the number and length of leaves, height of the plant and chlorophyll content in P. indica colonized plants compared to non-colonized control plant. In addition to this, the expression levels of auxin, nitrate reductase, vegetative storage protein and phosphate transporter genes were upregulated by 3.45, 3.26, 1.62 and 1.19 times respectively by the co-cultivation of P. indica in cardamom plantlets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Small cardamom (Elettaria cardamomum (L.) Maton), also known as the “Queen of spices” is one of the most important spice crops in the world. It is the third expensive spice globally after vanilla and saffron (Tangjang and Sharma 2018). It belongs to family Zingiberaceae and has believed to be originated in the moist evergreen forests of the Western Ghats region of Southern India. Cardamom is a shade-loving crop plant cultivated at an altitude of 600–1200 m above mean sea level (MSL) with an annual rainfall of 1500–4000 mm and a temperature of 10 to 35 °C (Madhusoodanan et al. 2002). Damp, loamy soil rich in humus is well suitable for its cultivation. As it’s a shade lover, 40 to 60% shade is requisite for their proper growth and flowering.
Parched ripe fruits (capsules) of cardamom have high economic value with a variety of medicinal properties. The essential oil and other bioactive molecules in the fruits have many curative effects including anti-inflammatory, anticancer, anti-oxidant, antibacterial, antifungal, antiviral, anti-diabetic, gastro-protective and analgesic activities (Al-Zuhair et al. 1996; Verma et al. 2009; Khan et al. 2011; Winarsi et al. 2014). Mishra et al. (1991) found out that antimicrobial activities of cardamom extract are mainly due to the terpenoids. In 2020, Ashokkumar et al. reviewed that terpenoids, flavonoids, anthocyanins, alkaloids and phenolics of capsules are responsible for controlling cardiovascular, pulmonary, kidney and lung associated disorders. Along with this, a variety of cardamom- flavoured food products are available in the markets including tea powder, biscuits, baked food items, confectionaries, etc.
The axenically cultivable root endophytic beneficial fungus Piriformospora indica, isolated from the desert soil of northwest India in the state of Rajasthan, interacts with many plant species and promotes their growth (Weiss et al. 2004; Varma et al. 2012). It also enhances plant resistance to biotic stresses such as bacterial, fungal, viral and nematode diseases and abiotic stresses like heavy metals, salinity and drought (Deshmukh and Kogel 2007; Sherameti et al. 2008a, b; Daneshkhah et al. 2013; Lakshmipriya et al. 2016; Li et al. 2017; Varkey et al. 2018; Athira and Anith 2020; Paul et al. 2021). Significant increase in growth and yield of many medicinal plant species was recorded on inoculation with P. indica (Rai et al. 2001; Kilam et al. 2015). Xu et al. (2017) reported that the co-cultivation with P. indica improved the growth of maize plants by enhancing phenotypic traits like plant height and leaf number. The mean number of leaves per plant and the leaf area per plant in the P. indica inoculated Piper nigrum recorded an increase compared to that of the control plants starting from the first month, to one year after transplanting (Anith et al. 2018). Bacopa monnieri co-cultivated with P. indica exhibited an enhanced growth, elevated bacoside endogenous level, antioxidant activity and nuclear hypertrophy (Prasad et al. 2013).
Over 100 years ago, Haberlandt conceptualised the idea of plant tissue culture and laid the foundation for the production of plant cells, tissues, and organs in culture. In addition to its utility as a research tool, plant tissue culture techniques have increasingly gained significant industrial importance in the fields of plant improvement, disease eradication, secondary metabolite production, and plant propagation (Hussain et al. 2012). Under controlled conditions, a single explant can be reproduced into many thousand plants in a relatively short amount of time and space, regardless of the season or weather, all year long. Because of the rapid rate of multiplication and low requirements for the initial plant population and available space, endangered, threatened, and unusual species have been successfully cultivated and conserved through micropropagation (Idowu et al. 2009).
Micropropagation opens up a lot of possibilities for rapid vegetative propagation of elite clones that are free of systemic diseases like viruses. Cardamom elite clones can be selected and rapidly multiplied using tissue culture technique (Hemavathy and Balaji 2010). Micro propagation technology has gained traction in India, reclaiming the global monopoly in cardamom production. The first report of cardamom tissue culture was published in 1982, claiming that callus cultures could regenerate plants (Rao et al. 1982). In vitro methods for the clonal propagation of cardamom from vegetative buds have been standardised (Reghunath and Gopalakrishnan 1991). Many commercial laboratories are using micropropagation techniques for large scale production of cardamom. The field evaluation of tissue cultured cardamom showed that the micropropagated plants performed on par with suckers (Chandrappa et al. 1997). The cardamom shoot growth patterns were studied on 45 media treatments, with the best shoot proliferation reported on Murashige and Skoog (MS) medium (Murashige and Skoog 1962) supplemented with and 2.32 M kinetin (Kn) for root induction and 4.4 M 6-benzylaminopurine (BAP) for shoot induction and 5.8 shoots regenerated per explant. On full strength MS basal medium, regenerated shoots rooted well, creating 3.5 roots per explant with an average root length of 4.3 cm in 4 weeks (Malhotra et al. 2021). Mixture of garden soil, sand and vermiculite in equal proportions was determined to be the best for planting, with 92% establishment rate (Babu et al. 1999).
A comparative study was undertaken to realise the role of P. indica in the growth response of cucumber (Cucumis sativus), okra (Abelmoschus esculentus), egg plant (Solanum melongena) and chilli (Capsicum annuum) under in vitro condition. These P. indica-colonised plants exhibited a considerable improvement (P < 0.001) in root lengths, shoot number and shoot length. Additionally, a concomitant rise in chlorophyll concentration was seen, suggesting a potential improvement in photosynthetic efficiency (Shukla et al. 2022). P. indica was found to have prospective uses in the banana tissue culture sector. In comparison to controls, P. indica colonised banana seedlings had greater plant height, more and longer roots, increased shoot fresh weight, greater leaf breadth and length, and larger stem circumference at 4 weeks following inoculation. The chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid levels of banana leaves were likewise markedly improved by the P. indica treatments (Li et al. 2019). Colonization by P. indica is expected to improve the overall growth and yield characteristics of cardamom as reported in other studies (Rai et al. 2001; Kilam et al. 2015; Xu et al. 2017; Weiss et al. 2004; Varma et al. 2012). Present study reports for the first time the genetic basis involved in the growth and development of E. cardamomum induced by the symbiotic fungus P. indica.
Very little is known about the exact mechanisms behind growth promotion in plants upon P. indica colonization. High-affinity phosphate (PO43−) transporter (PiPT) found in P. indica is found to enhance plants’ ability to absorb phosphate (Pederson et al. 2013), but without P. indica similar enhancement could not be observed (Bakshi et al. 2017). According to some recent research, P. indica modifies metabolic pathways during colonisation, such as a rapid rise in auxin levels during early host recognition, which is crucial for reprogramming the root development (Conchillo et al. 2021; Meents et al. 2019).
In this study, differential expression of four growth-related genes namely AUX1, NIA1, VSP and PHT1 were analyzed in P. indica colonized cardamom plants in comparison with the non-colonized control plants. These four genes were selected based on the observations made in previous studies reported elsewhere. In root and shoot tissues, AUX1 activity is necessary for both polar and phloem-based IAA transport routes (Leyser 2006). It was previously reported that co-cultivation with P. indica resulted in a massive transfer of nitrogen from the agar plates to the plants’ aerial parts and this action was linked to the activation of the NADH-dependent nitrate reductase (NIA1), a critical enzyme in the uptake of nitrate in plants (Sherameti et al. 2008a, b). Lin et al. (2019) reported that the expression of VSP (Vegetative storage protein gene) marked an increment after infection of the roots with Ralstonia solanacearum post infection in comparison with that of uncolonized controls. Plants have numerous transporters, including the PHT1, PHT2, PHT3, and PHT4, which are involved in inorganic phosphate (Pi) uptake by the root and remobilization in plants for being adapted to low phosphate environments. Among the four major phosphate transporters, PHT1 is responsible for phosphate uptake from soil by root cells, which is the first and most important stage in the Pi assimilation process (Liu et al. 2011). Considering these findings, genes namely AUX1, NIA1, VSP and PHT1 were selected for the present study.
The major objective of the study was to identify differentially expressed transcripts responding to Piriformospora indica root colonization in small cardamom for elucidation of molecular basis of growth and development.
2 Materials and methods
2.1 Source of culture
Initiated in vitro cultures of cardamom (wild genotype from the natural forest area in Edamalayar, Ernakulam district of Kerala, India.) were received from the Plant Tissue Culture laboratory of Biotechnology and Bioinformatics Division, Jawaharlal Nehru Tropical Botanic Garden and Research Institute, at the 1st subculture cycle and repeatedly subcultured to produce enough plantlets.
2.2 Propagation of in vitro cardamom culture
Initiated cultures (3 weeks old) were repeatedly subcultured to fresh Murashige and Skoog (MS) supplemented with 1 mgl−1 BAP (pH 5.8) for further shoot multiplication and elongation. Each subculture passage lasted for 3 weeks. After 2 weeks, shooted plantlets were transferred to full strength MS basal liquid medium (pH 5.8) for root induction. The cultures were kept in sterile conical flasks at 23 ± 2ºC provided with 16 h/8 h photoperiod, light intensity of 25 µmol m−2 s−1 using white fluorescent tubes and 55–65% relative humidity.
2.3 Fungal culture
P. indica was originally gifted by Dr. Ajit Varma; former Professor, School of Life Sciences, Jawaharlal Nehru University, New Delhi, India. It was maintained by sub culturing on Potato Dextrose Agar (PDA) at pH 7.0 and incubated in the dark at 27 °C. For mass multiplication, fungal hyphal discs were transferred to Potato Dextrose Broth (PDB) and shaken at 110 rpm in a shaker with maintenance of the same growth conditions as above.
2.4 Co-cultivation
2.4.1 In vitro co-culture
Mycelial discs of 0.8 cm diameter from 20 days old P. indica maintained on PDA plates were placed near roots of three weeks old E. cardamomum plantlets maintained in MS liquid culture under aseptic condition. It was then incubated for 35 days and provided 16 h: 8 h light/dark photoperiod, 23 ± 2ºC temperature, 55–65% humidity and a light intensity of 25 µmol m−2 s−1. In vitro plantlets maintained under identical conditions in MS liquid medium without P. indica co-cultivation served as control.
2.4.2 Ex vitro co-culture
Potting mixture containing sand, soil and vermiculite in 1:1:1 ratio (pH 6.5) was mixed with P. indica mycelium at the rate of 1% (w/v) (Anith et al. 2015). Primary hardening of 40 days old plantlets was done in small plastic cups (8 × 6) filled with the above medium. Inoculated plants were kept inside a mist house. After three weeks of primary hardening, the plants were transferred to larger plastic pots (18.5 × 14) containing sand, soil, and vermiculite in a 2:2:1 ratio and kept for secondary hardening in a mist house with 85% humidity. The plants were observed for growth parameters such as leaf numbers, leaf length and plant height for three months at an interval of every 15 days. Plants maintained under identical conditions in potting mixture containing sand, soil and vermiculite (without P. indica) served as control.
2.5 Confirmation of root colonization
2.5.1 Microscopic observation
Roots of P. indica inoculated and control in vitro grown plants were collected and were carefully cleaned in tap water before being chopped into one-cm pieces. They were boiled 100 °C in made 10% KOH solution for 5 min to soften them. The root pieces were washed in distilled water, then acidified with 1 M HCl for 5 min, followed by staining for 15 min in 0.02% lactophenol-trypan blue. Before viewing under a compound bright field microscope (Olympus CX43, Japan), the staining was removed using lactophenol solution. Chlamydospores in the root cortex cells were interpreted as a sign of root colonization.
2.6 Estimation of chlorophyll content
Chlorophyll content of cardamom plantlets maintained under in vitro condition (after 30 days of co-cultivation) and ex vitro condition (after 90 days of co-cultivation) was determined. Leaf sample (250 mg) was macerated with 10 mL of 80% acetone using a pestle and mortar. The extract was centrifuged at 3000 rpm for 10 min. The supernatant was transferred into a 25 mL volumetric flask and final volume was made up to 25 mL using 80% acetone. Color intensity of the green pigment was read at 645 nm, 663 nm and 652 nm. Chlorophyll a, chlorophyll b and total chlorophyll were calculated and expressed in mg of pigments/gram of fresh weight (Arnon 1949).
2.7 Gene expression analyses
2.7.1 RNA extraction and cDNA synthesis
RNA was isolated from 100 mg of young leaf (6th leaf) of both control and P. indica treated in vitro plantlets (9 weeks old after second subculture) using a modified CTAB approach in combination with the RNeasy Plant Mini Kit method (Nadiya et al. 2015). The integrity and quality of RNA were checked using Agarose gel (1.2%) electrophoresis. The N50 NanoPhotometer (IMPLEN, Germany) was used to determine both the concentration and purity of RNA (A260/A280 and A260/A230). Takara PrimeScript™ RT reagent Kit (Takara Bio Inc., Japan) was used to synthesize cDNA from RNA. The first strand cDNA synthesis was carried out according to the manufacturer’s instructions. A total of 10 µL reaction mixture (Prime script buffer (5 X) − 2 µL; Primescript RT − 0.5 µL; OligodT primer (50 µM) − 0.5 µL; Random hexamer (100 µM) − 0.5 µL; RNA (100 ng/µL) − 2.5 µL; RNase free water − 4 µL) was made and incubated for 15 min at 37 °C.
2.7.2 Primers used in the study
Out of the four selected genes studied, primer sequences of three genes such as NIA1 and PHT1 (Bandyopadhyay et al. 2022) and VSP (Lin et al. 2019) were taken from previous studies (Table 1). UBCE and AUX1gene sequences were developed from the transcriptome sequences of cardamom deposited in NCBI (Accession ID SRX1141276 and SRX1141272). These sequences were aligned using the software ‘MEGAX’ (Kumar et al. 2018). Primers for the real time PCR analyses were designed using IDT’s primer quest tool (http://www.idtdna.com). Then, using Net Primer and NCBI Primer BLAST, the quality and specificity of the primers were tested. Running a normal PCR with cardamom cDNA as template allowed for the preliminary selection of primers for qPCR experiments. Using gradient PCR, the annealing temperature for all of these primers was standardised at 60 °C.
2.7.3 Real-Time PCR analysis
Using the StepOnePLus™ Real Time PCR equipment (Applied Biosystems, USA), the expression patterns of AUX1 (Auxin responsive gene), NIA1 (gene involved in nitrate transport-Nitrate reductase 1), PHT1 (gene involved in phosphate uptake - Phosphate transporter1) and VSP (Vegetative storage protein gene) were studied. The relative change in gene expression was calculated using the Comparative Cq method utilising the Ct values obtained from Real time analysis. The real time gene expression data was normalized using the house keeping gene ubiquitin C (UBC).
2.8 Statistical analysis
For analysis of growth parameters, experiments were laid out in Completely Randomized Design (CRD). The mean values were compared using Two Sample Student’s t test (p < 0.05), and analysis of data was carried out using the Grapes 1.0.0 web application designed by Kerala Agricultural University (https://www.kaugrapes.com/).
3 Results
3.1 Colonization of P. indica in cardamom root
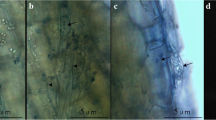
Intracellular hyphae were observed at 10 days and sporulation at 20 days after inoculation. Morphologically, the hyphae were straight and hyaline, and the surface of the hyphal walls was smooth. Pear shaped chlamydospores were observed within the cortical cells of root tissues of the inoculated in vitro grown plants when detection was carried out at 20 days after inoculation (Fig. 1). No colonization was observed in the uninoculated control plants.
3.2 Growth performance of P. indica co-cultured cardamom plants
The symbiotic association of cardamom with P. indica resulted in a significant increase in the morpho-physiological traits of the host plant. The growth enhancement was visible even from 15th day of co-culture. There was a significant increase (p < 0.05) in the number of leaves, length of leaves and height of the plant of colonized plants compared to control plant (Table 2; Figs. 2 and 3). Three months after transplanting, the P. indica inoculated plants recorded a mean leaf number of 20.6 per plant; average leaf length of 21.9 cm and mean plant height of 38.1 cm whereas that of the control plants were 12.8, 13.9 and 27.7 cm respectively.
Accumulation of different growth- related gene transcripts in cardamom as a result ofP. indica co-cultivation. ***C-NIA1 - Control-Nitrate reductase, T-NIA1 - Treated-Nitrate reductase, C-VSP - Control-Vegetative Storage Protein, T-VSP - Treated-Vegetative Storage Protein, C-AUX1 - Control-Auxin, T-AUX1 - Treated-Auxin, C-PHT1 - Control-Phosphate Transporter, T-PHT1 - Treated-Phosphate Transporter. The error bars indicate ΔCт standard error values
3.3 Effect of P. indica on chlorophyll content
A significant enhancement (p < 0.05) was obtained in the chlorophyll a, chlorophyll b and total chlorophyll contents in P. indica colonized plants when compared to non-colonized control plants (Table 3). The percent increase in photosynthetic pigment content in P. indica colonised plants above control was 19% (chlorophyll a), 60% (chlorophyll b), and 22% (total chlorophyll) in in vitro conditions. Similarly, the increase in photosynthetic pigment content in P. indica colonised plants compared to control was 92% (chlorophyll a), 86% (chlorophyll b), and 84% (total chlorophyll) in ex vitro conditions.
3.4 Real-time PCR analyses for the validation of gene expression
The relative enhancement in the transcript levels of the aforementioned genes in both the control and P indica co-cultured samples is shown in Fig. 3. The most over-expressed genes were NIA1, which plays an irreplaceable role in nitrogen assimilation in plant cells as well as serving as a key enzymatic source of nitric oxide, which governs plant growth and AUX1 which is involved in the auxin transport. Samples treated with P indica showed a 3.45 and 3.26 times increases in the transcript levels of NIA1 and AUX1. The gene which encodes VSP marked a slight up-regulation by 1.19 times in transcript level. Similarly, PHT1, which is involved in Pi uptake by the root and remobilization in plants, marked a 1.16 times expression at transcript level.
4 Discussion
Endophytes are ubiquitous which grow in plant tissues and offers many growth promoting effects to the host plants (Alikulov et al. 2022; Choudhury et al. 2023; Urumbil and Anilkumar 2021). The effects of root colonization by P. indica on growth have been reported in spice crops like black pepper, chilli (Anith et al. 2011; Nandana and Anith 2020; Lekshmi et al. 2022). However, in cardamom, there is no report on the co-cultivation with P. indica and its effects on growth promotion. In the present study we used in vitro raised cardamom plantlets as the planting material for growth evaluation studies, and colonization by the root endophyte was confirmed through microscopic detection method. All the planting materials used were clonally propagated from a single mother plant and thus it was ensured that the genetic base of the plants remained the same, but due to the microbial treatment, growth pattern of the control and treated plants differed. Assessment of plant growth promotion was done by taking observations on the number of leaves, length of leaves and the plant height. Plant growth promotion was evidenced by the production of more number of leaves, increased leaf length and plant height in the P. indica inoculated plants compared to the control plants. Chlorophyll (Chl) is a crucial photosynthetic pigment for the plant, and it plays a significant role in determining photosynthetic capacity and, consequently, plant growth (Li et al. 2018). Plant metabolism is directly impacted by the amount of chlorophyll in leaves through photosynthesis, growth, and development (Dai et al. 2009). Furthermore, formation and growth of plant structures in higher plants is influenced by light harvesting such as accumulation of food reserves in leaf base cells (Olive et al. 2007). With this perspective, it can be concluded that the increased leaf chlorophyll content may facilitate more harvesting of light thereby directly promote plant growth. The chlorophyll content in the leaves of P. indica inoculated cardamom plants recorded an increase over the control plants under both in vitro and ex vitro conditions. Similar observations have been reported earlier on Arabidopsis (Sherameti et al. 2008a, b); cucumber, okra, chilli (Jisha et al. 2019); black pepper (Anith et al. 2018) by the co-cultivation of P. indica. Decrease in chlorophyll content in plant fresh leaves is considered as an indicator of photosynthetic incapability of plant machinery (Sakuraba et al. 2018). Primarily, chlorophyll a and b are necessary for the primary photosynthetic reaction (Croft et al. 2017). Abdelaziz et al. (2021) reported that P. indica supports chlorophyll metabolism by inducing biosynthesis genes (protochlorophyllide oxidoreductase [POR], chlorophyll a oxygenase [CAO]) and downregulating selected chlorophyll degradation genes (PPH, pheophorbide a oxygenase [PAO], and red chlorophyll catabolite reductase [RCCR]). These improvements in chlorophyll content and leaf greening indicates that P. indica colonization indirectly slows leaf senescence.
The growth enhancing capacity of P. indica has been demonstrated in many crop plants (Kundu et al. 2022). Previous reports suggest that P. indica enhanced the nutrient uptake in plants, especially nitrogen, phosphorous, sulphur, magnesium (Sherameti et al. 2005; Yadav et al. 2010; Kumar et al. 2011; Cruz et al. 2013; Bakshi et al. 2017; Narayan et al. 2021; Kushwaha et al. 2022). The broad impression of P. indica colonisation suggests numerous beneficial interactions with the host plants, implicating their role in general recognition and triggering of intracellular signalling pathways (Jogawat et al. 2020). Understanding the molecular mechanisms behind the growth enhancement would be useful for developing genetic markers which remains a research gap in cardamom. Here, we have observed the differential expression of four growth- related gene namely AUX1, NIA1, VSP and PHT1 at the transcript level. It was previously reported that co-cultivation with P. indica resulted in a massive transfer of nitrogen from the agar plates to the plants’ aerial parts and this action is linked to the activation of the NADH-dependent nitrate reductase (NIA1), a critical enzyme in the uptake of nitrate in plants (Sherameti et al. 2008a, b). In this study, we found out that the relative expression of the NIA1 gene got upregulated by 3.45 times in cardamom plants colonized by P. indica than that of control plants. Numerous studies have reported the positive influence of P. indica on auxin transport. Sirrenberg et al. (2007) demonstrated the production of IAA in a liquid culture of P. indica, which may induce growth promotion, as shown by other plant beneficial fungi such as Trichoderma virens, which increases biomass production in Arabidopsis thaliana through an auxin-dependent mechanism (Contreras-Cornejo et al. 2009). In this work, the expression of auxin (AUX1) was significantly upregulated by 3.26 times in P. indica colonized cardamom plants compared with the non-colonized controls which points out the hormone dependent growth of cardamom plants on co-cultivation with P. indica. In root and shoot tissues, AUX1 activity is necessary for both polar and phloem-based IAA transport routes (Leyser 2006).
In terms of plant growth and development, phosphorus (P), which makes up 0.5% of plant cell biomass, is the second most important limiting macronutrient after nitrogen (N) (Schachtman et al. 1998). Functional characterization of P. indica roots revealed a high affinity PiPT transporter (Pht1 family) gene that transports phosphate (Pi) from the fungus to the host plant. In P-deficient conditions, PiPT are activated, increasing the absorption of Pi against the gradient in concentration between inner plant tissue and soil solution. These results suggest that PiPT plays a crucial role in P transfer at the P. indica-plant root interface, which enhances overall plant development and yield (Muchhal et al. 1996). P. indica is now considered as one of the important contributors in defining alternate P absorption mechanisms by terrestrial plants. This symbiotic endophyte benefits plants by enhancing P absorption and translocation and facilitating the movement of other nutrients. Plants have numerous transporters, including the PHT1, PHT2, PHT3, and PHT4, which are involved in Pi uptake by the root and remobilization in plants for being adapted to low phosphate environments. Among the four major phosphate transporters, PHT1 is responsible for phosphate uptake from soil by root cells, which is the first and most important stage in the Pi assimilation process (Liu et al. 2011). In this study, E. cardamomum on P. indica co-cultivation did not show significant up-regulation in PHT1 transcript level expression when compared with the non-colonized controls. In fact, there are conflicting reports on the role of P. indica in phosphate transfer and host plant enhancement. P. indica increases phosphate uptake 2–3 times in Arabidopsis seedlings, according to Shahollari et al. (2005), implying that P. indica stimulates Arabidopsis growth in a similar way to mycorrhizal fungi. On the other hand, it has been found that P. indica does not cause a considerable increase in leaf P and N, and that phosphate has no function in improved growth of Nicotiana attenuata (Barazani et al. 2005).
As expected, the expression of VSP did not show significant up-regulation in transcript level under the influence of P. indica. Plants benefit from the accumulation of store protein because it helps them survive (Fujiwara et al. 2002). Lin et al. (2019) reported that the expression of JA-responsive genes like VSP (Vegetative storage protein gene) marked an increment after infection of the roots with R. solanacearum at 12 h, 1, 2 days, and this increase was stronger for the colonised Anthurium plants than that of uncolonized controls. Considering these findings, it can be speculated that the accumulation of VSPs take place in conjunction with some exogenous abiotic or biotic stress. Based on the coherent results, it can be concluded that the growth enhancement in cardamom plants under the influence of P. indica is directly correlated with the elevated expressions of NIA1 and AUX1, whereas an upregulated transcript levels of PHT1 and VSP might be seen in the later stages of growth of the cardamom plant.
To the best of our knowledge, this work represents the first report on the co-cultivation of in vitro E. cardamomum with beneficial root endophyte P. indica and the expression of essential genes participating in the process of growth enhancement in plants. This could be further extended to find out whether P. indica promotes early flowering and thereby induce early fruiting. Earliness in flowering on P. indica inoculation was reported in barley (Achatz et al. 2010), Coleus forskohlii (Das et al. 2012), Arabidopsis (Kim et al. 2017; Pan et al. 2017), black pepper (Anith et al. 2018). Varma et al. (2014) reported that P. indica culture filtrate promotes plant growth and seed germination in Helianthus annus and Phaseolus vulgaris. Considering this, cardamom plantlets primed with P. indica can be used in fields for faster cultivation, better performance and improved yield. Amendment of P. indica during the hardening of tissue cultured cardamom plants would result in better acclimatization and survival under normal climatic conditions. It is known that the growth of cardamom plant is altitude specific. Thus our study is extending to evaluate the performance of cardamom under the influence of P. indica on higher and lower altitudes. We have already initiated research on this line and hope to get early results. Early flowering and fruiting, if occur in field grown cardamom, will definitely be a breakthrough, as first flowering in normal cardamom plants occurs only after 2–3 years of planting.
5 Conclusion and future prospects
The study recommends the use of P. indica as a potential means for enhancing the growth and overall performance of cardamom and other crop plants. The study’s findings also suggest that this symbiotic association could be used as a model for further research into the molecular and physiological pathways involved in symbiotic association and plant growth promotion. P. indica serves as a model organism for studying beneficial plant microbe interactions as well as a novel tool for improving plant production systems due to its simplicity of cultivation. Growth enhancement caused by P. indica colonisation minimises the need for fertiliser in the soil, lowering the risk of fertiliser overuse and contamination of the environment.
Data Availability
Data availability The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdelaziz ME, Atia MA, Abdelsattar M, Abdelaziz SM, Ibrahim TA, Abdeldaym EA (2021) Unravelling the role of Piriformospora indica in combating water deficiency by modulating physiological performance and chlorophyll metabolism-related genes in Cucumis sativus. Horticulturae 7(10):399
Achatz B, von Rüden S, Andrade D, Neumann E, Pons-Kühnemann J, Kogel KH, Franken P, Waller F (2010) Root colonization by Piriformospora indica enhances grain yield in barley under diverse nutrient regimes by accelerating early plant development. Plant Soil 333:59–70
Al-Zuhair H, El-Sayeh B, Ameen HA, Al-Shoora H (1996) Pharmacological studies of cardamom oil in animals. Pharmacol Res 34(1–2):79–82
Alikulov B, Shurigin V, Davranov K, Ismailov Z (2022) Plant growth-promoting endophytic bacteria associated with Halocnemum strobilaceum (pall.) M.Bieb and their plant beneficial traits. Plant Sci Today 8(sp1):44–50. https://doi.org/10.14719/pst.1605
Anith KN, Aswini S, Varkey S, Radhakrishnan NV, Nair DS (2018) Root colonization by the endophytic fungus Piriformospora indica improves growth, yield and piperine content in black peer (Piper Nigurm L). Biocatal Agric Biotechnol 14:215–220
Anith KN, Faseela KM, Archana PA, Prathapan KD (2011) Compatibility of Piriformospora indica and Trichoderma Harzianum as dual inoculants in black pepper (Piper nigrum L). Symbiosis 55:11–17
Anith KN, Sreekumar A, Sreekumar J (2015) The growth of tomato seedlings inoculated with co-cultivated Piriformospora indica and Bacillus pumilus. Symbiosis 65(1):9–16
Arnon D (1949) Copper enzyme in isolated chloroplast and chlorophyll expressed in terms of mg per gram. Plant Physiol 24:15–23
Ashokkumar K, Murugan M, Dhanya MK, Warkentin TD (2020) Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton]-A critical review. J Ethnopharmacol 246:112244
Athira S, Anith KN (2020) Plant growth promotion and suppression of bacterial wilt incidence in tomato by rhizobacteria, bacterial endophytes and the root endophytic fungus Piriformospora indica. Indian Phytopathol 73:629–642
Babu M, Radhika M, Sehgal PK (1999) Cellular proliferation on desamidated collagen matrices. Comp Biochem Physiol C Toxicol Pharmacol 124(2):131–139
Bakshi M, Sherameti I, Meichsner D, Thürich J, Varma A, Johri AK, Yeh KW, Oelmüller R (2017) Piriformospora indica reprograms gene expression in Arabidopsis phosphate metabolism mutants but does not compensate for phosphate limitation. Front Microbiol 8:1262
Bandyopadhyay P, Yadav BG, Kumar SG, Kumar R, Kogel KH, Kumar S (2022) Piriformospora indica and Azotobacter chroococcum Consortium facilitates higher Acquisition of N, P with Improved Carbon Allocation and enhanced plant growth in Oryza sativa. J Fungi 8:453
Barazani O, Benderoth M, Groten K, Kuhlemeier N, Baldwin IT (2005) Piriformospora indica and Sebacina Vermifera increase growth performance at the expense of herbivore resistance in Nicotiana attenuate. Oecologia 146:234–243
Chandrappa HM, Shanthaveerabhadraiah SM, Jagannath B (1997) Response of cardamom (Elettaria cardamomum Maton) to NPK under uniform shade. J Spices Aromat Crops 6(2):115–118
Choudhury D, Tarafdar S, Parvin N, Rit R, Roy S, Sadhu S, Dutta S (2023) Endophytic microbes and their diverse beneficial aspects in various sectors: a critical insight. Plant Sci Today 10(1):96–107. https://doi.org/10.14719/pst.1877
Conchillo LB, Haro R, Benito B (2021) K+ nutrition exchange in the serendipita-Arabidopsis symbiosis: study of the fungal K+ transporters involved front. Ecol Evol 9:876
Contreras-Cornejo HA, Macias-Rodriguez L, Corte-Penagos C, Lopez-Bucio J (2009) Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol 149:1579–1592
Croft H, Chen JM, Luo X, Bartlett P, Chen B, Staebler RM (2017) Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob Chang Biol 23:3513–3524
Cruz C, Fegghi Z, Martins-Loução MA, Varma A (2013) Plant nitrogen use efficiency may be improved through symbiosis with Piriformospora indica. In: Varma A, Kost G, Oelmüller R (eds) Piriformospora indica: sebacinales and their biotechnological applications; Soil Biology, vol 33. Springer, Berlin (Heidelberg), pp 285–293
Dai Y, Shen Z, Liu Y, Wang L, Hannaway D, Lu H (2009) Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma Hemsleyanum Diels et Gilg. Environ Exp Bot 65(2–3):177–182
Daneshkhah R, Cabello S, Rozanska E, Sobczak M, Grundler FMW, Wieczorek K, Hofmann J (2013) Piriformospora indica antagonizes cyst nematode Infection and development in Arabidopsis roots. J Exp Bot 64(12):3763–3774
Das A, Kamal S, Shakil NA, Sherameti I, Oelmüller R, Dua M, Tuteja N, Johri AK, Varma A (2012) The root endophyte fungus Piriformospora indica leads to early flowering, higher biomass and altered secondary metabolites of the medicinal plant, Coleus Forskohlii. Plant Signal Behav 7(1):103–112
Deshmukh SD, Kogel KH (2007) Piriformospora indica protects barley from root rot caused by Fusarium Graminearum. J Plant Dis Prot 114(6):263–268
Fujiwara T, Nambara E, Yamagishi K, Goto DB, Naito S (2002) Storage proteins. The Arabidopsis Book/American Society of Plant Biologists 1
Hemavathy AT, Balaji K (2010) In vitro propagation studies in cardamom. Crop Res (Hisar) 40(1/3):217–220
Hussain A, Qarshi IA, Nazir H, Ullah I (2012) Plant tissue culture: current status and opportunities. Recent Adv Plant vitro Cult 6(10):1–28
Idowu PE, Ibitoye DO, Ademoyegun OT (2009) Tissue culture as a plant production technique for horticultural crops. Afr J Biotechnol 8(16):3782–3788
Jisha S, Sabu KK, Manjula S (2019) Multifunctional aspects of Piriformospora indica in plant endosymbiosis. Mycol 10(3):182
Jogawat A, Meena MK, Kundu A, Varma M, Vadassery J (2020) Calcium channel CNGC19 mediates basal defense signaling to regulate colonization by Piriformospora indica in Arabidopsis roots. J Exp Bot 71:2752–2768
Khan AU, Khan QJ, Gilani AH (2011) Pharmacological basis for the medicinal use of cardamom in Asthma. Bangladesh J Pharmacol 6(1):34–37
Kilam D, Saifi M, Abdin MZ, Agnihotri A, Varma A (2015) Combined effects of Piriformospora indica and Azotobacter chroococcum enhance plant growth, antioxidant potential and steviol glycoside content in Stevia rebaudiana. Symbiosis 66:149–156
Kim D, Abdelaziz ME, Ntui VO, Guo X, Al-Babili S (2017) Colonization by the endophyte Piriformospora indica leads to early flowering in Arabidopsis thaliana likely by triggering gibberellin biosynthesis. Biochem Biophys Res Commun 490:1162–1167
Kumar M, Yadav V, Kumar H, Sharma R, Singh A, Tuteja N, Johri AK (2011) Piriformospora indica enhances plant growth by transferring phosphate. Plant Signal Behav 6:723–725
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547
Kundu A, Mishra S, Kundu P, Jogawat A, Vadassery J (2022) Piriformospora indica recruits host-derived putrescine for growth promotion in plants. Plant Physiol 188(4):2289–2307
Kushwaha A, Goswami L, Lee J, Sonne C, Brown RJ, Kim KH (2022) Selenium in soil-microbe-plant systems: Sources, distribution, toxicity, tolerance, and detoxification. Crit Rev Environ Sci Technol 52(13):2383–2420
Lakshmipriya P, Nath V, Veena SS, Anith KN, Sreekumar J, Jeeva ML (2016) Piriformospora indica, a Cultivable Endophyte for Growth Promotion and Disease Management in Taro (Colocasia esculenta L). J Root Crops 42(2):107–114
Lekshmi RS, Sora S, Anith KN, Soniya EV (2022) Root colonization by the endophytic fungus Piriformospora indica shortens the juvenile phase of Piper nigrum L. by fine tuning the floral promotion pathways. Front Plant Sci 13:954693
Leyser O (2006) Dynamic integration of auxin transport and signaling. Curr Biol 16:424–433
Li Y, He N, HouJ, Xu L, Liu C, Zhang J, Wang Q, Zhang X, Wu X (2018) Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front Ecol Evol 6:64
Li D, Mensah RA, Liu F, Tian N, Qi Q, Yeh K, Xuhan X, Cheng C, Lai Z (2019) Effects of Piriformospora indica on rooting and growth of tissue-cultured banana (Musa acuminata Cv. Tianbaojiao) seedlings. Scientia Hort 257:108649
Li X, Wei Y, Acharya A, Jiang Q, Kang J, Brummer EC (2017) A saturated genetic linkage map of autotetraploid alfalfa (Medicago sativa L.) developed using genotyping-by-sequencing is highly syntenous with the Medicago truncatula genome. Genes Genom Genet G3(10):1971–1979
Lin HF, Xiong J, Zhou HM, Chen CM, Lin FZ, Xu XM, Oelmüller R, Xu WF, Yeh KW (2019) Growth promotion and Disease resistance induced in Anthurium colonized by the beneficial root endophyte Piriformospora indica. BMC Plant Biol 19:40
Liu F, Chang X, Ye Y, Xie W, Wu P, Lian X (2011) Comprehensive sequence and whole-life-cycle expression profile analysis of the phosphate transporter gene family in rice. Mol Plant 4:1105–1122
Madhusoodanan KJ, Kumar KP, Ravindran PN (2002) Botany, crop improvement and biotechnology of cardamom. In: Taylor and Francis (eds.), Cardamom-The genus Elettaria. Taylor & Francis Inc, London, pp 11–68
Malhotra EV, Kamalapriya M, Bansal S, Meena DPS, Agrawal A (2021) Improved protocol for micropropagation of genetically uniform plants of commercially important cardamom (Elettaria cardamomum Maton). In Vitro Cell Dev Biol P 57(3):409–417
Meents AK, Furch AC, Almeida-Trapp M, Özyürek S, Scholz SS, Kirbis A, Lenser T, Theißen G, Grabe V, Hansson B, Mithöfer A (2019) Beneficial and pathogenic Arabidopsis root-interacting fungi differently affect auxin levels and responsive genes during early Infection. Front Microbiol 10:380
Mishra AK, Dwivedi SK, Kishore N, Dubey NK (1991) Fungistatic properties of essential oils of cardamom. Int J Pharmacogn 29:259–262
Muchhal US, Pardo JM, Raghothama KG (1996) Phosphate transporters from the higher plant Arabidopsis thaliana. Proc Natl Acad Sci 93(19):10519–10523
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with Tobacco tissue cultures. Physiol Plant 15:473–497
Nadiya F, Anjali N, Gangaprasad A, Sabu KK (2015) High-quality RNA extraction from small cardamom tissues rich in polysaccharides and polyphenols. Anal Biochem 485:25–27
Nandana MS, Anith KN (2020) Growth promotion in Chilli (Capsicum annuum L.) on inoculation with co-cultured Piriformospora indica and Pseudomonas fluorescens. Int J Curr Microbiol Appl Sci 9:2015–2027
Narayan OP, Verma N, Jogawat A, Dua M, Johri AK (2021) Sulfur transfer from the endophytic fungus Serendipita indica improves maize growth and requires the sulfate transporter SiSulT. Plant Cell 33:1268–1285
Olivé I, Brun FG, Vergara JJ, Pérez-Lloréns JL (2007) Effects of light and biomass partitioning on growth, photosynthesis and carbohydrate content of the seagrass Zosteara Noltii Hornem. J Exp Mar Biol Ecol 345(2):90–100
Pan R, Xu L, Wei Q, Wu C, Tang W, Oelmüller R, Zhang W (2017) Piriformospora indica promotes early flowering in Arabidopsis through regulation of the photoperiod and gibberellin pathways. PLoS ONE 12:e0189791. https://doi.org/10.1371/journal.pone.0189791
Paul T, Nysanth NS, Yashaswini MS, Anith KN (2021) Inoculation with bacterial endophytes and the fungal root endophyte, Piriformospora indica improves plant growth and reduces foliar Infection by Phytophthora capsici in black pepper. J Trop Agric 59:224–235
Pedersen BP, Kumar H, Waight AB, Risenmay AJ, Roe-Zurz Z, Chau BH, Schlessinger A, Bonomi M, Harries W, Sali A, Johri AK (2013) Crystal structure of a eukaryotic phosphate transporter. Nature 496(7446):533–536
Prasad R, Kamal S, Sharma PK, Oelmueller R, Varma A (2013) Root endophyte Piriformospora indica DSM 11827 alters plants morphology, enhances biomass and antioxidant activity of medicinal plant Bacopa monniera. J Basic Microbiol 53:1016–1024
Rai M, Acharya D, Singh A, Varma A (2001) Positive growth responses of the medicinal plants Spilanthes calva and Withania somnifera to inoculation by Piriformospora indica in a field trial. Mycorr 11:123–128
Rao NKS, Narayanaswamy S, Chacko EK, Swamy RD (1982) Regeneration of plantlets from callus of Elettaria cardamomum Maton. Proc Indian Acad Sci (Plant Sci) 91:37–41
Reghunath BR, Gopalakrishnan PK (1991) Successful exploitation of in vitro culture techniques for rapid clonal multiplication and crop improvement in cardamom. Proc. of 3rd Kerala Sci. Cong., held at Kozhikode, Kerala: 70–71
Sakuraba Y, Kim D, Paek NC (2018) Salt treatments and induction of Senescence. In: Guo Y (ed) Plant Senescence; methods in Molecular, Biology, vol 1744. Humana Press, New York, NY, USA, pp 141–149
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116(2):447–453
Shahollari B, Varma A, Oelmuller R (2005) Expression of a receptor kinase in Arabidopsis roots is stimulated by the basidiomycete Piriformospora indica and the protein accumulates in Triton X- 100 insoluble plasma membrane microdomains. J Plant Physiol 162:945–958
Sherameti I, Shahollari B, Venus Y, Altschmied L, Varma A, Oelmüller R (2005) The endophytic fungus Piriformospoa indica stimulates the expression of nitrate reductase and the starch degrading enzyme glucan-water dikinase in Tobacco and Arabidopsis roots through a homodomain transcription factor that binds to conserved motif in the promoters. J Biol Chem 280:26241–26247
Sherameti I, Tripathi S, Varma A, Oelmuller R (2008a) The root colonizing endophyte Pirifomospora indica confers drought tolerance in Arabidopsis by stimulating the expression of drought stress related genes in leaves. Mol Plant Microbe Interact 21:799–807
Sherameti I, Venus Y, Drzewiecki C, Nitz I (2008b) PYK10, a β -glucosidase located in the endoplasmatic reticulum, is crucial for the beneficial interaction between Arabidopsis thaliana and the endophytic fungus Piriformospora indica. Plant J 54:428–439
Shukla J, Mohd S, Kushwaha AS, Narayan S, Saxena PN, Bahadur L, Mishra A, Shirke PA, Kumar M (2022) Endophytic fungus serendipita indica reduces arsenic mobilization from root to fruit in colonized tomato plant. Environ Poll 298:118830
Sirrenberg A, Goebel C, Grond S, Pawlowski K (2007) Piriformospora indica affects plant growth by auxin production. Plant Physiol 131:581–589
Tangjang A, Sharma A (2018) Marketing pattern of large cardamom (Amomum Sabulatum) in Tirap District of Arunachal Pradesh, India. Int J Curr Microbiol App Sci 7(05). http://www.ijcmas.com
Urumbil SK, Anilkumar M (2021) Metagenomic insights into plant growth promoting genes inherent in bacterial endophytes of Emilia sonchifolia (Linn.) DC. Plant Sci Today 8(sp1):6–16
Varkey S, Anith KN, Narayana R, Aswini S (2018) A consortium of rhizobacteria and fungal endophyte suppress the root-knot nematode parasite in tomato. Rhizosphere 5:38–42
Varma A, Sree KS, Arora M, Bajaj R, Prasad R, Kharkwal AC (2012) Functions of novel symbiotic fungus - piriformospora indica. Proc Indian Natl Sci Acad 80:429–441
Varma A, Sree KS, Arora M, Bajaj R, Prasad R, Kharkwal AC (2014) Functions of novel symbiotic fungus - piriformospora indica. Proc Indian Natl Sci Acad 80:429–441
Verma K, Jain V, Katewa SS (2009) Blood pressure lowering, fibrinolysis enhancing and antioxidant activities of cardamom (Elettaria cardamomum). Indian J Biochem Biophys 46:503–506
Weiss M, Selosse MA, Rexer KH, Urban A, Oberwinkler F (2004) Sebacinales: a hitherto overlooked cosm of heterobasidiomycetes with a broad mycorrhyzal potential. Mycol Res 108:1003–1010
Winarsi H, Sasongko ND, Purwanto A, Nuraeni I (2014) Effect of cardamom leaves extract as antidiabetic, weight lost and hypocholesterolemic to alloxan induced Sprague Dawley diabetic rats. Int Food Res J 21(6):2253
Xu L, Aiai W, Jun W, Qiao W, Wenying Z (2017) Piriformospora indica confers drought tolerance on Zea mays L. through enhancement of antioxidant activity and expression of drought-related genes. Crop J 3:251–258
Yadav V, Kumar M, Deep DK, Kumar H, Sharma R, Tripathi T, Tuteja N, Saxena AK, Johri AK (2010) A phosphate transporter from the root endophytic fungus Piriformospora indica plays a role in phosphate transport to the host plant. J Biol Chem 285:26532–22654
Acknowledgements
Authors acknowledge A. S. Hemanthakumar, Technical Officer, JNTBGRI for developing initial in vitro cultures of cardamom.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest/competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anjana, H.N., Anith, K.N. & Sabu, K.K. Growth promoting effects of endophytic fungus Piriformospora indica in small cardamom (Elettaria cardamomum Maton). Symbiosis 91, 79–89 (2023). https://doi.org/10.1007/s13199-023-00949-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-023-00949-1