Abstract
The fir engraver, Scolytus ventralis, is a bark beetle that infests true firs (Abies) in western North America. The beetle is known to carry a symbiotic fungus, Trichosporium symbioticum, in pit mycangia located on the heads of adult beetles. We investigated whether this fungus is associated with the beetle across its geographic range and the frequency of its occurrence with egg galleries, larvae, pupae and adult beetles in two major tree hosts. We also used morphology and DNA sequencing to determine the correct taxonomic placement of the fungus. We found the fungus is consistently associated with the beetle across its range and in all life stages. We also determined the fungus resides in the Ascomycota order Ophiostomatales and re-designated it as Ophiostoma symbioticum (Wright) Six nov. comb. We suggest O. symbioticum may be a nutritional mutualist with S. ventralis and that future studies should investigate this possibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Scolytus ventralis Le Conte (fir engraver) is an ecologically and economically important bark beetle that colonizes true firs (Abies) (Wood 1982). Like many bark beetles, S. ventralis is associated with a symbiotic fungus although little is known of its distribution or its effects on its host beetle. Despite the beetle’s designation as a tree-killer, it has a life history and population dynamics distinct from other conifer-killing bark beetles. While many conifer-killing bark beetles use an aggregation pheromone-mediated mass attack to overwhelm tree defenses, S. ventralis avoids defensive trees, relying instead on locating weakened hosts via volatile chemical profiles (Macias-Samano et al. 1998, 2004). Outbreaks only develop during periods of drought when weak trees are abundant (Struble 1957; Otrosina and Zarnoch 2012). Trees that are drought stressed or infected with root disease are most attractive, potentially due to unique kairomonal cues (Macias-Samano et al. 1998). Scolytus ventralis is intolerant of, and its fungus highly inhibited by, tree resin, and so an ability to avoid healthy defensive hosts may be crucial for successful establishment (Raffa and Berryman 1987; Macias-Samano et al. 1998).
In healthy or moderately stressed trees, attacks occur in low densities and are often unsuccessful. When successful, broods develop in small, localized patches on the mainstem. The beetles’ excavate transverse galleries that deeply score the sapwood. Their location and shape allows the beetles to avoid resin while inoculating a large area of tree tissue with their symbiotic fungus (Macias-Samano et al. 1998). Tree death is not required for successful reproduction and repeated low-density attacks on living trees over several years can result in characteristic ‘bubbled’ bark surrounding areas of past beetle brood development. Old attacks heal over and become buried in the wood and are visible in felled trees as deeply stained inclusions (Struble 1957). In very weak trees, beetles colonize in high densities resulting in tree death and high fecundity and brood success.
The common association of S. ventralis with a single fungus was first noted by Struble (1931, unpubl. cited in Wright 1935). Subsequently, the fungus was formally described by Wright (1935) as Trichosporium symbioticum and placed in the Dematiaceae (a polyphyletic group of asexual fungi with dark hyphae). The fungus imparts a brown color to the phloem and sapwood surrounding beetle galleries. Adult male and female beetles possess pit mycangia (fungal transport structures) on their heads that contain secretory canals that may produce substances that protect the spores during transport. The mycangia support vertical transmission of the fungus from parents to offspring (Livingston and Berryman 1972).
The taxonomic position of T. symbioticum has long been in question. Harrington et al. (2010) noted that Trichosporium tingens, another Scolytinae-associated fungus, resides in the Ophiostomatales (Ascomycota) and our initial examination of T. symbioticum suggested it should reside in the genus Ophiostoma, in particular. Morphologically, it produces the typical Hyalorhinocladiella-like anamorph of Ophiostoma and it is readily cultured on cycloheximide-amended malt extract agar, a selective medium tolerated by many Ophiostomatales, but not by most other Ascomycota (Harrington 1981).
The common occurrence of the fungus with the beetle, its presence in mycangia, and its apparent affiliation with Ophiostomatales (the most common group of fungi associated with conifer-infesting bark beetles including many mutualistic species) suggests this fungus may be an obligate mutualist with the beetle. All bark beetles with specific fungal partners thus far investigated benefit from nutritional provisioning and beetle hosts can be dependent upon the fungus to meet their requirements for nitrogen (N) and phosphorus (P) (Six and Elser 2019, 2020). The fungi, unlike the beetle larvae, are not confined to feeding in the phloem, but are able to grow into the sapwood of the tree where they have access to a greater pool of nutrients. The obligate mutualist fungi of several bark beetles translocate nutrients from sapwood to the phloem making them available to larvae as they feed on phloem containing the fungal mycelium and to new adults that feed on fungal spores in beetle pupal chambers (Six and Paine 1998; Six and Elser 2019, 2020).
If a fungus is obligate for beetle survival, it must be an extremely consistent partner. Past work suggests this is the case for T. symbioticum with S. ventralis. Wright (1935, 1938) conducted extensive sampling and isolations of the T. symbioticum from galleries and adult S. ventralis collected from A. concolor in California, USA, and was able to isolate the fungus in almost all cases. The fungus also has been isolated from pupal chambers in A. grandis (grand fir) in Oregon, USA (Filip et al. 1989), and from A. grandis at another, but undescribed, location (Raffa et al. 1985). However, the distribution of the fungus with the beetle across its geographic range and with various beetle life stages remained uninvestigated.
Our objectives were to investigate whether this fungus is consistently associated with the beetle across the western USA, its frequency with various beetle life stages, and to use morphology and DNA sequences of diagnostic gene regions to determine the fungus’ proper taxonomic placement.
2 Materials and methods
2.1 Collection sites
Beetles, wood, and insects used for isolations of fungi associated with S. ventralis were collected from infested trees at several locations: Victoria (11 km northwest of Victoria, British Columbia, Canada), Echo Lake (8 km northeast of Bigfork, Montana, USA), FLBS (Flathead Lake Biological Station, Polson, Montana, USA), Lost Creek (8 km north of Lozeau, Montana, USA), Oakhurst (Oakhurst, California, USA), Sandia Crest Road (near Tijeras, NM, USA), Laird Park (16 km north of Potlatch, Idaho, USA), Enaville (2 km north of Enaville, ID, USA), and Moscow Mountain (10 km northeast of Moscow, Idaho, USA). Collections were made from two primary host tree species: Abies grandis (grand fir) in Canada, Montana, and Idaho, and A. concolor (white fir) in California and New Mexico.
2.2 Isolations for descriptions of prevalence and geographic distribution
Slivers of xylem and phloem were taken from egg galleries, larval mines, stained and unstained phloem directly ahead of larval galleries, and pupal chambers. Larvae, pupae, and parent and emerging new adults were also collected from infested trees. Tree tissues and insects were then placed individually onto water agar in Petri dishes. Hyphae growing out from wood or insects were then subcultured onto 2% malt extract agar (MEA) for growth and identification. Sample sizes are provided in Tables 1, 2, 3 and 4.
2.3 Isolations for DNA sequencing, taxonomic reclassification, and growth experiments
Cultures of the symbiotic fungus from A. grandis at FLBS, Lost Creek, and Enaville were purified by subculturing onto cycloheximide-amended 2% MEA and then onto 2% MEA using single spore isolations. Three isolates from each of the three sites were used for DNA sequencing, growth experiments, and for morphological measurements and descriptions.
2.4 Growth of the symbiotic fungus at various temperatures
Isolates were grown on 2% MEA by placing 3 mm plugs of actively growing cultures face down onto the center of the medium in 90 X 15 mm Petri dishes. Three replicates of each isolate were held at 15C, 20C, 25C, 30C, and 35C for 2 wk. Growth outward from the edge of the plug was traced with a felt-tipped pen on the underside of the Petri dish and then the amount of growth after two weeks was estimated by averaging radial growth measured at four equidistant points.
2.5 Morphology
The same isolates used for sequencing and temperature assays were grown on potato dextrose agar (PDA) and 2% MEA to describe cultural and morphological characteristics after 2 wk growth. Micro-morphological characteristics were described from mycelium and conidiophores mounted in lactophenol blue or water and viewed using a Zeiss Axioscop compound microscope. Fifty measurements were made of each structure type.
2.6 DNA extraction, PCR, and phylogenetic analysis
For extractions of genomic DNA, a small amount of mycelium and conidia were scraped from the surface of cultures of each of the three purified isolates. The fungal material was then macerated in 200ul Prepman Ultra (Applied Biosciences, USA) in a 1.5 microcentrifuge tube using a micropestle. The solution was incubated at 95 °C for 10 min, centrifuged, and the supernatant containing the extracted DNA removed and used in PCR. PCR amplification was carried out using the primer pairs ITS3 (White et al. 1990) and LR3 (Vilgalys and Hester 1990) (partial ribosomal RNA encoding region: partial 5.8S, ITS2, and partial 28S), Bt2b (Glass and Donaldson 1995) and T10 (O'Donnell and Cigelnik 1997) (β-tubulin gene), and EF1f and EF2r (Jacobs et al. 2004) (elongation factor 1α gene).
Each PCR reaction mixture consisted of 25 μl Master Mix (Promega, USA), 0.5 μl of each primer (10 pmol concentration), 23 μl molecular grade water, and 1 μl of DNA template. PCR conditions were one cycle of denaturation at 92 °C for 2 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, an extension at 72 °C for 1 min, and one final cycle of extension at 72 °C for 8 min. Amplicons were purified with a High Pure PCR Product Kit (Roche). Sequencing was performed on an ABI 3130xl genetic analyzer at the Core Genomics Facility at the University of Montana, Missoula, MT, USA.
Contigs of forward and reverse sequences obtained with the various primers pairs were aligned using MEGA6 (Tamura et al. 2013). The closest matching sequences retrieved from GenBank (https://www.ncbi.nlm.nih.gov/genbank/) using the BLASTn algorithm were compared with sequences for the isolates of the fungus in this study. The phylogenetic position of the fungus within Ophiostoma was determined by developing individual maximum-likelihood trees for each of the three gene regions (337 bp for EF1α, 342 bp for β-tubulin, and 795 bp for ITS) using MEGA6 (Table 5).
3 Results
The fungus was obtained at all collection sites. Not all stages of the insect were present at all sites at the time collections were made, but the fungus was consistently isolated from S. ventralis egg galleries and all of the beetle stages that were present (Tables 1, 2, 3 and 4).
3.1 Prevalence of the symbiotic fungus with egg galleries of S. ventralis
The fungus was isolated from 92 of 102 egg galleries (Table 1). Five galleries from Laird Park, ID, that did not produce cultures of the symbiotic fungus yielded colonies of ubiquitous environmental fungi in Aspergillus, Penicillium and Trichoderma (listed as other filamentous fungi, Table 1).
A yeast was isolated from 23 egg galleries and was mainly present in phloem samples taken from within 2 cm of egg galleries associated with fresh attacks. We did not attempt to identify this yeast and there may have been more than one yeast in our isolations although colonies were similar in growth. Fungi other than the symbiotic fungus and yeast were isolated from 29% of egg galleries. There was a very low incidence of other filamentous fungi in new egg galleries under active construction or in galleries where egg laying had recently stopped and the female was still alive. Of the 47 galleries sampled while under construction, only four yielded other filamentous fungi. Conidiophores and perithecia of Ceratocystiopsis minuta and Ophiostoma minus, commensals common with many bark beetles and with beetle-associated mites, were also occasionally found in older egg galleries.
3.2 Prevalence of the symbiotic fungus with S. ventralis larvae and larval mines
The fungus and the yeast were consistently found with larvae (Table 2). Of the 13 larvae from which the fungus was not isolated, eight were from Laird Creek site and had just hatched and had not yet begun to mine the phloem. Larval samples in California also yielded few isolates of the fungus. These were taken from a log that was very dry and most larvae had died prior to sampling. The dry nature of the substrate may have contributed to the low incidence of the fungus in these samples.
Both the fungus and the yeast were isolated from brown-stained phloem tissue immediately adjacent to larval mines. However, the yeast was never isolated from the stained phloem immediately in front of advancing larvae where the fungus appeared to grow alone. This is not surprising as most yeasts cannot grow into intact tree tissues but are dependent on beetle tunneling for their spread. During later phases of beetle development, when almost all phloem was been consumed by the beetle, the yeast was found in all samples regardless of location. It was only during late stages of larval tunneling that the bark beetle and phoretic mite generalist fungi, O. minus and C. minuta, were also sometimes isolated from larval mines, albeit rarely.
3.3 Fungi associated with S. ventralis pupae and their chambers
Pupal chambers were coated with white linings of fungal material. This lining was not found in larval mines nor in the pupal chamber after adult emergence and is apparently develops only during the pupal stage and likely fed upon by the teneral (pre-emergence, pre-sexual maturity) adult stage. This lining consisted of a mat of interwoven hyphae bearing a profuse layer of conidia.
As the pupae are in direct contact with the fungal layer it was not surprising that they yielded colonies of the fungus. Of the eight pupae from which isolations were made, seven yielded the fungus and six yielded the yeast (Table 3).
3.4 Fungi associated with S. ventralis adults
Male and female adult beetles consistently carried the fungus and the yeast (Table 4). All beetles (males and females) that were actively engaged in gallery construction yielded the fungus and the yeast. Aspergillus, Penicillium, and Trichoderma were also sometimes isolated from adults (other filamentous fungi, Table 4). Because S. ventralis possesses specialized mycangia on its head (Livingston and Berryman 1973), the heads of 52 additional beetles were used for additonal isolations. Of these, all yielded the fungus and 12 also yielded other filamentous fungi.
3.5 Comparison of DNA sequences of isolates from S. ventralis with sequences in GenBank
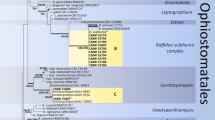
DNA sequences of the nine isolates were identical for all three gene regions. The sequences for the neotype (see below) were deposited in GenBank (Table 5). The fungus clearly falls within Ophiostoma and in each of the maximum likelihood trees generated for the three gene regions, the fungus resolved distinct from other species within Ophiostoma (Fig. 1).
3.6 Morphology and growth of the symbiotic fungus at different temperatures
The fungus grown on phloem alone or on 2% MEA or PDA developed luxuriant growth of hyphae in a loose mycelial mat from which arose numerous Hyalorhinocladiella-like conidiophores bearing many small sub-globose to oblong conidia (Fig. 2).
Observations of pupal chambers, most containing pupae or teneral adults, contained mats of hyphae with simple conidiophores (Hyalorhinocladiella-like). However, a very few yielded synnemata. In these instances, the white lining that was normally present was missing. Spores on small synnema formed in ball-like masses, while spore masses on large synnema produced three-to-four short thick ribbons on their tips branching out like petals of a flower. When spores from synnema were placed into a drop of water, they immediately became dislodged and dispersed. All cultures produced from these spores were identifiable as the symbiotic fungus.
The fungus also produced a yeast-like stage. The fungus grew as a filamentous fungus at temperatures from 5–30 °C (Fig. 3), with growth optimal at 25C whereas it grew only as a yeast at 35C. The yeast stage recovered hyphal growth when sub-cultured to new media and grown at a lower temperature. No sexual structures were observed in pupal chambers or in culture.
3.7 Reclassification of T. symbioticum
The results of our morphological observations and phylogenetic analyses revealed the fungus previously described as T. symbioticum forms a lineage within the Ophiostomataceae in Ophiostoma distinct from other described species in the genus. We were unable to locate the type specimen, but the morphological characteristics and ecological attributes of our isolates (including its close association with S. ventralis) matched perfectly with the original description by Wright (1935). We therefore transfer T. symbioticum to Ophiostoma while retaining the original species epithet.
Ophiostoma symbioticum (Wright) Six comb. nov.
Mycobank No.: 279646.
Basionym: Trichosporum symbioticum E. Wright, E. Journal of Agricultural Research 50: 525–538 (1935).
Typification: Type not found. Neotype DLSSVFLBS1, USA, Montana, Polson, Flathead Lake Biological Station; Coords; 47.876562, -114.032974, Elevation; 892 m. From Scolytus ventralis adult collected from Abies grandis 22 June 2019. Collected and isolated by Diana L. Six.
Distribution: Only found in symbiosis with the scolytine bark beetle, S. ventralis. California, Idaho, Montana, New Mexico, USA, and Victoria, British Columbia, Canada. Host tree species: Abies grandis and A. concolor.
Material examined: Ex-neotype DLSSVFLBS1 Flathead Lake Biological Station, Polson, Montana, USA (CBS 149,614). Isotypes DLSSVFLBS2 Flathead Lake Biological Station, Polson, Montana, USA; DLSSVLC1 Lozeau, Montana, USA (CBS 149,615); DLSSVENA1 Enaville, Idaho, USA (CBS149640).
Description: Grown on 2% MEA: Sexual morph: Not observed. Asexual morph: Hyalorhinocladiella-like. Hyphae: Submerged hyphae brown, septate, 4.2 (3.2–6.1) μm in diameter. Fine aerial hyphae hyaline, septate, 1.6 (1.0–2.1) μm in diameter (Fig. x). Conidiophores: Hyaline, septate, single or branched with conidia arising singly or in clusters at tips 1.6 (0.8–2.0) μm in diameter (Fig. 2), occasionally forming into loose synnemata. Conidia: Smooth, hyaline, and oblong to sub-globose 2.2 (1.5–4.0) μm in diameter (Fig. 2). Cultural characteristics: On 2% MEA: Upper surface dark brown with sparse hyaline aerial hyphae bearing conidiophores and condial masses (Fig. 2). On PDA: Dark grey underside. Upper surface covered in dense white hyphae (Fig. 2) and conidiphores, synemmata sometimes forming near point of inoculation (area of oldest growth).
4 Discussion
Morphological observations and phylogenetic analyses using three gene regions show that T. symbioticum resides in Ophiostoma in the Ophiostomataceae. Thus, we re-designated the fungus as Ophiostoma symbioticum. Ophiostoma symbioticum was widespread across the geographic and host range of S. ventralis and was found consistently with all life stages of the beetle. Its highly consistent nature with the beetle suggests it is a mutualist and supports previous work indicating that it is efficiently vertically transmitted within the pit mycangia of the beetle (Livingston and Berryman 1972).
Ophiostoma are common associates of conifer-colonizing bark beetles and some are known to be nutritional mutualists including O. montium with Dendroctonus ponderosae (mountain pine beetle) (Bleiker and Six 2007) and O. ips with Ips pini (pine engraver) (Six and Elser 2020). Future work on the S. ventralis-O. symbioticum symbiosis should include investigations into whether O. symbioticum functions as a nutritional mutualist.
Data Availability
Data is available upon request.
References
Bleiker K, Six DL (2007) Dietary benefits of fungal associates to an eruptive herbivore: potential implications of multiple associates on host population dynamics. Environ Entomol 36:1384–1396. https://doi.org/10.1093/ee/36.6.138
Filip GM, Christiansen E, Parks, CA (1989) Secondary resin production increases with vigor of Abies grandis inoculated with Trichosporium symbioticum in northeastern Oregon. USDA Forest Service Pacific Northwest Research Station Research Note PNW-RN-489, Portland. pp. 1–10
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330. https://doi.org/10.1128/aem.61.4.1323-1330.1995
Harrington TC (1981) Cycloheximide sensitivity as a taxonomic character in Ceratocystis. Mycologia 73:1123–1129. https://doi.org/10.1080/00275514.1981.12021447
Harrington TC, Aghayeva DN, Fraedrich SW (2010) New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the redbay ambrosia beetle, Xyleborus glabratus. Mycotaxon 111:337–361. https://doi.org/10.5248/111.337
Jacobs K, Bergdahl DR, Wingfield MJ, Halik S, Seifert KA et al (2004) Leptographium wingfieldii introduced into North America and found associated with exotic Tomicus piniperda and native bark beetles. Mycol Res 108:411–418. https://doi.org/10.1017/s0953756204009748
Livingston RL, Berryman AA (1972) Fungus transport structures in the fir engraver, Scolytus ventralis (Coleoptera: Scolytidae). Can Entomol 104:1793–1800. https://doi.org/10.4039/ent1041793-11
Macias-Samano JE, Borden JH, Gries R, Pierce HD, Gries G (1998) Lack of evidence for pheromone-mediated secondary attraction in the fir engraver, Scolytus ventralis (Coleoptera: Scolytidae). J Entomol Soc British Columbia 95:117–125
Macias-Samano JE, Borden JH, Gries R, Pierce HD, Gries G, Skip King GG (2004) Primary Attraction of the Fir Engraver, Scolytus ventralis. J Chem Ecol 24:1049–1075. https://doi.org/10.1023/a:1022354503443
O’Donnell K, Cigelnik E (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molec Biol Evol 71:103–116. https://doi.org/10.1006/mpev.1996.0376
Otrosina WJ, Zarnoch SJ (2012) Response of different white fir geographic provenances to Trichosporium symbioticum inoculation in California. Can J for Res 42:1178–1183. https://doi.org/10.1139/x2012-059
Raffa KF, Berryman AA (1987) Interacting selective pressures in conifer-bark beetle systems: A basis for reciprocal adaptations? Am Nat 129:234–262
Raffa KF, Berryman AA, Simasko J, Teal W, Wong BL (1985) Effects of grand fir monoterpenes on the fir engraver, Scolytus ventralis (Coleoptera: Scolytidae) and its symbiotic fungus. Environ Entomol 14:552–556
Six DL, Paine TD (1998) The effects of mycangial fungi on development and emergence of Dendroctonus ponderosae and D. jeffreyi. Environ Entomol 27:1393–1401. https://doi.org/10.1093/ee/27.6.1393
Six DL, Elser JJ (2019) Extreme ecological stoichiometry of a bark beetle-fungus mutualism. Ecol Entomol 44:543–551. https://doi.org/10.1111/een.12731
Six DL, Elser JJ (2020) Mutualism is not restricted to tree-killing bark beetles and fungi: the ecological stoichiometry of secondary bark beetles, fungi and a scavenger. Ecol Entomol 45:1134–1145. https://doi.org/10.1111/een.12897
Struble GR (1957) The fir engraver: A serious enemy of western true firs. USDA Forest Service Production Research Report No. 11. California Forest and Range Experiment Station, Berkeley. pp 1–17
Tamura K, Stecher G, Petersen D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis ver. 6.0. Molec Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990
White T, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innes MA, Gelfand DH, Snisky JJ, White TJ (eds) PCR Protocols: A Guide to Methods and Applications. Academic Press, New York, pp 315–322
Wood SL (1982) The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Naturalist Memoirs, Number 6, pp 1–1359
Wright E (1935) Trichosporium symbioticum, n. sp., a wood-staining fungus associated with Scolytus ventralis. J Agric Res 50:525–538
Wright E (1938) Further investigations of brown-staining fungi associated with engraver beetles (Scolytus) in white fir. J Agric Res 57:759–773
Acknowledgements
We thank Morgan Wilson for her exceptional help in the lab and field, Jim Elser, Director of the Flathead Lake Biological Station for access for the collection of fungi at the station, and Bruce Erickson of the USDA Forest Service for his help locating S. ventralis-infested trees at Lost Creek, Montana.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Six, D.L., Livingston, R.L. Distribution and taxonomic reclassification of the mycangial fungus of the fir engraver, Scolytus ventralis. Symbiosis 89, 123–131 (2023). https://doi.org/10.1007/s13199-023-00894-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-023-00894-z






