Abstract
This report aimed to evaluate the ability of the dominant indigenous strains of bradyrhizobia obtained from different locations in the Philippines in terms of their symbiosis, N-fixation and nodulation with various soybean cultivars harboring different Rj genotypes. This was done to select the most efficient and effective strain that can be used as an inoculant under the Philippines’ local condition. Two soybean cultivars from the Philippines and three cultivars from Japan and Brazil were used and inoculated with 12 indigenous strains and the reference strain Bradyrhizobium diazoefficiens USDA110. Culture pots were grown inside a growth chamber for four weeks with 33 °C for 16 h as daytime and 28 °C for 8 h as night time. All the strains formed nodules on all soybean cultivars, except for B. elkanii BO-4, which only formed nodules on Philippines’ cultivars. Among the indigenous strains, B. elkanii IS-2 is significantly the most efficient and effective N-fixer than the other strains for the Philippines’ cultivars. In contrast, B. diazoefficiens SK-5 was found to be the most efficient and effective N-fixer for cultivars from Japan and Brazil. Additionally, a positive correlation was observed between the symbiotic efficiency and the nitrogenase activity indicating an efficient N-fixation by the indigenous strains. Thus, we were able to identify the most promising indigenous bradyrhizobia that could be used as an inoculant to increase the soybean yield in the Philippines and provided insights on soybean inoculation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The Philippines is a tropical archipelago located in the Southeast Asia with a land area of about 298 km2. From the approximately 7 M hectares used for agriculture, only about 1000 ha. are allocated to soybean. This is due to the low yield (≤1.0 ton/ha) of soybean and also low government support to soybean planters. Although the Government’s program entitled “Philippine Soybean Development Program” launched in 2011 was able to increase the production area from ≈1000 ha. to a record high of 5280 has., soybean yield is still low at about 1.0 ton/ha. Thus, projects and studies on how to increase soybean yield in a sustainable manner prompted researchers to venture into development of plant varieties with high-yielding ability. However, breeding of high-yielding soybean varieties alone does not guarantee that it would attain its optimal yield. Other factors such as soil management and cultural practices has to be improved too.
Soybean (Gycine max [L.] Merill) can establish a symbiotic relationship with rhizobia, a general term for diazotrophic bacteria in the soil that are able to convert the atmospheric N into ammonia and renders it available for the plant’s growth and development. In turn, the plant provides food for the rhizobia from the product of photosynthesis. Nitrogen fixation takes place inside the root nodule, a specialized organ that was formed from the infection of the rhizobia. An efficient and effective symbiotic relationship between the plant (soybean) and the bacteria (rhizobia) could lessen the chemical inputs, particularly the nitrogenous fertilizers that are applied to the soil. It is a proven knowledge that soils with low N content respond better with rhizobia inoculation so, this technique is very helpful for soil restoration activities. If this technique is properly utilized, farmers will have better profit from buying lesser chemical fertilizers and decreased soil degradation from using chemicals.
Previous studies reported that aside from various agro-environmental factors, competition with the native rhizobia is a hindrance for successful inoculation (Yamakawa et al. 2003; Grönemeyer et al. 2014). Therefore, it is essential to select and evaluate the symbiotic competitiveness of the indigenous strains which are native and existing in high density.
In soybean, there are genetic loci which are known as Rj(s) or rj(s) that were reported to restrict the nodulation between a certain rhizobia strains and legume plants. A non-nodulating line of soybean was first identified by Williams and Lynch (1954) and reported that it was due to a single recessive gene in the host plant, rj1. Thereafter, the discovery of dominant Rj genes allowed the nodulation between certain rhizobia as summarized by Hayashi et al. (2012). The recessive rj genes (rj1, rj5, rj6, rj7) restrict the nodulation with all strains while the dominant Rj genes (Rfg1, Rj2, Rj3, Rj4) restrict the nodulation only with certain strains. In one study about soybean preference for nodulation using the B. diazoefficiens USDA110, it was observed that the Rj2Rj3Rj4 line of soybean were superior than the other Rj lines in relation to the inoculation with a type A strain (Yamakawa et al. 2003).
In the Philippines, the first study about the diversity of soybean bradyrhizobia was reported by Mason et al. (2017) where the dominance of B. elkanii strains found among Kumamoto and Okinawa in Southern Japan and in Nueva Ecija, Philippines was compared. It was then stated that the difference in temperature and similarity in soil pH were the driving force for the presence of the particular strains on those three locations. Additionally, Mason et al. (2018) reported the pioneer work about the diversity and distribution of indigenous bradyrhizobia collected from the northern to the southern areas in the Philippines. This report was able to identify the strains of bradyrhizobia that are dominant and abundant in specific locations and at the same time, identified the agro-environment conditions that influenced the prevalence of certain species. In the latter report, indigenous strains clustered under the B. elkanii were identified to be dominant in the non-flooded soils whereas B. japonicum and B. diazoefficiens were prevalent in flooded soils of the country. Many strains of B. elkanii were reported to be relatively inefficient microsymbionts of soybean and can induce chlorosis in soybean plants (Devine et al. 1988), and they lack nosZ gene (Sameshima-Saito et al. 2006) that encodes the N2O reductase responsible for reducing N2O into N2. Meanwhile, the strain B. diazoefficiens USDA110 is globally used in soybean research as it has a high symbiotic efficiency and nitrogen fixation ability (Champion et al. 1992; Soe et al. 2012; Chibeba et al. 2017), as well as it possesses a complete denitrification ability that allows the release of N2 into the atmosphere as the end product (Sameshima-Saito et al. 2006; Itakura et al. 2013; Shiina et al. 2014; Akiyama et al. 2016), rather than N2O which is the end product of certain B. japonicum strains (Sameshima-Saito et al. 2006). This led us to hypothesize that the B. diazoefficiens indigenous strains from the Philippines might be useful as potential inoculant in the country to increase the yield of soybean rather than the other Bradyrhizobium species. Consequently, the present study aimed to confirm this by conducting a single-strain inoculation test on tropical and temperate soybean cultivars harboring various Rj genotypes using the most dominant strains in specific locations against the B. diazoefficiens USDA110 as the positive control.
2 Materials and methods
2.1 Selection and preparation of inoculant strains
In our previous experiment (Mason et al. 2018), we were able to identify the most dominant bradyrhizobia at a particular location in the Philippines which are listed in Table 1. The identification of the strains was based on the Restriction Fragment Length Polymorphism (RFLP) treatment and sequence analysis of the 16S rRNA gene, internal transcribed spacer (ITS) region of the 16S–23S rRNA gene and the rpoB housekeeping gene (Mason et al. 2018). The selection process was based on the most abundant strain in the total population of bradyrhizobia at a specific location. For example, in Ilagan, Isabela, 80 isolates were collected from which 50% of the population (40 out of 80) belonged to B. elkanii, then IS-2 was selected to represent the 40 isolates. The most prevalent strain in the specific location was selected in the present study for a single-strain inoculation test, with an exemption for Sultan Kudarat (SK) where two strains were selected, SK-5 and SK-12. Sultan Kudarat is the only location where B. yuanmingense was isolated of which SK-12 was phylogenetically similar.
Each strain was grown in yeast-extract mannitol agar (YMA, Vincent 1970) with Congo Red until a single colony appears then, the single colony was cultured in YM broth (Vincent 1970) for about 1 week at 28 °C in a dark shaker with continuous agitation at 120 rpm. The culture was diluted with sterile distilled water at a rate of 1 × 106 ml−1 as an inoculant.
2.2 Cultivation in culture pots, maintenance and harvesting
For the soybean plant, two cultivars from the Philippines, PSB-SY2 (Rj4: Ph-SY2) and Collection 1 (non-Rj: Ph-Col1), and three cultivars from Japan, Orihime (Rj3: Ja-Ori), Akisengoku (Rj4: Ja-Aki), and Brazil, IAC-2 (Rj2Rj3: Br-IAC2), were used in cultivation. For easier referral in this manuscript, the two cultivars from the Philippines are referred to as tropical cultivars while the three cultivars from Japan and Brazil are referred to as temperate cultivars from henceforth. The IAC-2 from Brazil is adapted at climatic condition 17 °C to 24 °C which is lower than the Philippines (28 °C - 34 °C). The soybean seeds were surface-sterilized by soaking in 70% ethanol and sodium hypochlorite solution as formerly described (Saeki et al. 2006) and planted in 1-l culture pots (n = 4). Next, the culture pots were filled with vermiculite then, N-free nutrient solution was added (Saeki et al. 2004) at 40% (vol/vol) distilled water content and were autoclaved for 20 min at 121 °C. The seeds were then sown on the vermiculite, and the pot was weighed. Afterwards, the plants were grown for 4 weeks inside the growth chamber (33 °C for 16 h, day; 28 °C for 8 h, night), and were supplied weekly with sterile distilled water until the initial weight of the pot was reached.
For reference, B. diazoefficiens USDA110 was inoculated as a positive control and a pot without inoculated strain served as the negative control. All treatments were conducted with four replications.
2.3 Data collection and analysis
After 4 weeks, data gathering was conducted as follows: 1) the chlorophyll content or the greenness of the plant was taken with the use of SPAD 502 Plus Chlorophyll Meter (KONICA MINOLTA, Inc., Tokyo, Japan) at three points in the youngest fully expanded leaf of each plant and the average was recorded; 2) plants were gently uprooted and were washed with distilled water until the roots were free from vermiculite; 3) plant height was measured from the base of the plant to the tip of the longest and youngest leaf; 4) clean roots containing the nodules were immediately separated and placed in 100 mL Erlenmeyer flask with a rubber cork then were used for the Acetylene Reduction Assay (ARA) using gas-chromatography (GC-8A, Shimadzu Co. Ltd., Kyoto, Japan) which was described by Hardy et al. (1968); 5) then, nodules were collected from the roots, counted and the fresh biomass was recorded along with the shoot and root fresh weight; 6) shoot, root, and nodules were then placed in an oven to dry at 70 °C for 48 h; then finally, the dry biomass of the shoot, root, and nodules were read and recorded. The oven-dried shoots were ground up to about 2 mm and were used for Total N analysis using an Automatic NC Analyzer (Sumigraph NC-220F, Sumika Chemical Analysis Service. Ltd., Tokyo, Japan).
The symbiotic efficiency was computed with the formula adapted from Risal et al. (2010) which is: (amount of N fixed / dry weight of nodule) × 100. The amount of N fixed was obtained from the difference between the N content of the inoculated treatments against the uninoculated pots. The nitrogenase activity was expressed as the concentration of ethylene produced and computed with the following formula: (CE x SE) / (KE x E) where CE and KE are the ethylene concentration (μl/L) in the sample and standard, respectively, and E (sample) and SE (standard) are the respective areas obtained after the chromatographic analyses (Unkovich et al. 2008).
Statistical analysis was conducted using R software v.3.4.0 and the comparison among means was analyzed by Tukey’s Honest Significant Difference (HSD) test. For the nitrogenase activity, the analysis was performed only with 2 replications so statistical test was not applied.
3 Results
3.1 Nodule number, oven dry weight of shoot and nodules
Shown in Table 2 is the influence of the single-strain inoculation test on the tropical and temperate soybean cultivars in terms of the number of nodules, and the dry biomass of the shoot and nodules and graphical representations are provided as Supplementary Materials (Fig. S1a-c). All the indigenous strains from the Philippines were able to form nodules on all the soybean cultivars, regardless of the Rj genotype, except for strain BO-4. USDA110 showed the highest number of nodules significantly in comparison with all the indigenous strains, except with Akisengoku (Rj4), wherein IS-2 showed the highest nodule number. For the tropical cultivars, strain BO-4 has the least nodulation ability, regardless of the Rj genotype. The PSB-SY2 (Rj4) showed similar preference with strains IS-2, GI-4, BA-23, NE1–6, SO-1 and LT-3 while Collection 1 (non-Rj) seemed to prefer the strains GI-4, NR-2, and SK-5. For the temperate cultivars, the strain BO-4 did not form any nodule with all cultivars, regardless of the Rj genotype. For Orihime (Rj3), both the strains NR-2 and SK-5 showed the highest nodulation ability among the rest. In the case of Akisengoku (Rj4), the strain IS-2 showed the highest nodule number, followed similarly by the strains GI-4, BA-24, NE1–6, NE2–37, SO-1, LT-3, NR-2, and SK-5. Meanwhile, IAC-2 (Rj2Rj3) seemed to have an almost similar preference with all the indigenous strains, with the lowest nodule numbers obtained from the strains LT-3 and SC-3.
However, it is visible that on both the tropical and temperate cultivars, the indigenous strains have better nodulation compatibility with the cultivars that harbor the Rj4 genotype, except for BO-4. This shows that BO-4 might not be compatible with the temperate cultivars as it failed to nodulate any of the three cultivars. Furthermore, it also has the least nodulation compatibility with the tropical cultivars.
For the nodule dry weight, although USDA110 showed the highest number of nodules, it did not possess the highest dry nodule biomass, indicating that the indigenous strains were able to form larger nodules than the ones formed by the inoculation with USDA110. For the temperate cultivars, it is noticeable that the strains NE1–6 and SK-5 had the highest nodule biomass regardless of the Rj genotype, while for the tropical cultivars, the strains NE1–6 and NE2–37 obtained the highest nodule biomass with Collection 1 (non-Rj) and PSB-SY2 (Rj4), respectively.
In terms of the shoot dry weight, the tropical cultivars generally had lower biomass than the temperate cultivars, which could be attributed to the fact that the tropical cultivars were also generally smaller than the temperate cultivars. Although the data on plant height was not shown in this report, the plant height of the tropical cultivars generally ranged from 18 to 22 cm, whereas the temperate cultivars generally ranged from 23 to 27 cm.
3.2 Symbiotic performance and nitrogen fixation ability of bradyrhizobia from the Philippines
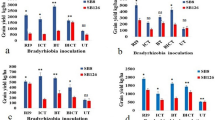
Presented in Fig. 1a is the symbiotic efficiency of the indigenous bradyrhizobia strains isolated from the Philippines against that of the USDA110. It is evident that USDA110 had significantly the highest symbiotic efficiency, regardless of the origin of the cultivars or the Rj genotypes. For the tropical cultivars, it is noticeable that the most efficient indigenous strain was the IS-2 while the least efficient was LT-3, regardless of the Rj genotype. On the other hand, the highest symbiotic efficiency with the temperate cultivars from the indigenous strains was obtained with the inoculation of SK-5, while the least was obtained with BO-4. Additionally, the strain LT-3 showed a comparably low efficiency with BO-4 on the temperate cultivars harboring the Rj3 and Rj4 genotypes.
a Effect of the single-strain inoculation of twelve (12) indigenous Philippines bradyrhizobia and B. diazoefficiens USDA110T on the symbiotic efficiency of the two tropical and three temperate soybean cultivars. Error bar = SE. b Effect of the single-strain inoculation of indigenous Philippines bradyrhizobia and B. diazoefficiens USDA110T on the amount of N fixed of the two tropical and three temperate soybean cultivars. Error bar = SE
Meanwhile, the symbiotic performance of the possible novel species GI-4 and NE2–37 also merit further evaluation as these two, which were classified as Bradyrhizobium sp. were comparably efficient with both the tropical and temperate cultivars. Unlike the strains IS-2 ad SK-5, these two strains did not show any preference with the origin of the cultivars. The two isolates BA-24 and SC-3 which were classified under B. japonicum also showed better performance with the temperate cultivars, in particular with the Rj4 plants. In contrast to the strain IS-2, NE1–6, which is also a B. elkanii strain, showed preference to the temperate cultivars than with the tropical cultivars.
With regard to the amount of Nitrogen fixed in the shoot, it is again the USDA110 that showed the highest performance (Fig. 1b). For the indigenous strains, IS-2, GI-4, NE2–37, SK-5, and SK-12 have comparable N-fixing ability with Collection 1 (non-Rj) while the lowest was obtained with the inoculation of LT-3. Similarly, LT-3 had the lowest amount of fixed N with PSB-SY2 (Rj4) while the highest was obtained with the inoculation of IS-2. Meanwhile, SK-5 showed the highest N-fixation ability with the temperate cultivars while the lowest was obtained again with the inoculation of LT-3 for the Rj3 and Rj4-harboring cultivars, and NR-2 had the least amount of N fixed with the Rj2Rj3-harboring cultivar.
3.3 Chlorophyll content and nitrogenase activity of bradyrhizobia from the Philippines
The chlorophyll content or the greenness of the leaves is presented in Fig. 2a. The plants inoculated with USDA110 have the greenest leaves regardless of the cultivars and the Rj genotypes. However, it was not significantly different with some of the indigenous strains. For tropical cultivars, the plants inoculated with the strains IS-2 and NE2–37 have the greenest leaves or relatively higher chlorophyll content similar with the USDA110. The tropical plants inoculated with LT-3 showed the lowest chlorophyll content. For the temperate cultivars, the plants that were inoculated with SK-5 had the highest chlorophyll content similar with the USDA110, regardless of the Rj genotypes. The lowest chlorophyll content was observed on the Rj3 and Rj4 plants inoculated with LT-3 while for the Rj2Rj3 plants, both the strains NR-2 and SK-12 provided the lowest chlorophyll content. Most of the readings obtained from the SPAD-502 m showed a consistent result with the amount of N fixed in the shoot of the plants.
a Effect of the single-strain inoculation of twelve (12) indigenous Philippines bradyrhizobia and B. diazoefficiens USDA110T on the leaves’ greenness of the two tropical and three temperate soybean cultivars. Error bar = SE. b Nitrogenase activity by Acetylene Reduction Assay (ARA) of the twelve (12) indigenous Philippines bradyrhizobia and B. diazoefficiens USDA110T on the two tropical and three temperate soybean cultivars
Meanwhile, the nitrogenase activity as obtained from the ARA (Fig. 2b) showed an almost similar result with the N fixation ability (Fig. 1b) on most of the strains. Moreover, a positive correlation between the symbiotic efficiency and the nitrogenase activity is observed (Fig. 3) which confirmed the N fixation ability of the indigenous strains. It is noticeable that USDA110 obtained the highest activity for both the tropical and the temperate cultivars. For the indigenous strains, IS-2 had the highest nitrogenase activity with the tropical cultivars whereas SK-5 was the highest with the temperate cultivars. Again, the strains LT-3 and BO-4 showed the least activity with the tropical cultivars and temperate cultivars, respectively.
As an alternative information, Principal Component Analysis (PCA) plots of each cultivar showing all the plant parameters obtained are provided as supplementary material (Fig. S2). The plots showed that the nodulation differences are not positively or negatively correlated with the other plant parameters from each cultivar but may have been due to the compatibility between the Rj genotype and the strains. This observation has been reported previously (Ishizuka et al. 1991; Yamakawa et al. 2003; Shiro et al. 2012).
4 Discussion
4.1 Nodulation and N-fixation ability of the indigenous bradyrhizobia
As reported by Ishizuka et al. (1991), type A strains are generally preferred by non-Rj genotypes but is also compatible with Rj2Rj3 and Rj4 genotypes. In our study, it is observed that the number of nodules did not differ significantly with each cultivar or Rj genotype, regardless whether the cultivar was from the tropical or the temperate region. In a study by Yamakawa et al. (2003), it was reported that the presence of rhizobia strain (Type A, B, or C) can be enhanced or repressed by the Rj genotypes, especially from Rj2Rj3Rj4 genotypes, which came from the Rj4 parent cultivar. These results generally suggests the host specificity of rhizobia on specific Rj genotypes. Our results showed that all the indigenous strains from the Philippines were able to form nodules on all the cultivars that harbor different Rj genotypes, except BO-4. So, it is proposed that these indigenous and dominant strains isolated from the Philippines be considered as type A strains which can nodulate a wide variety of Rj genotypes in soybean. This observation is first obtained from this report and will provide the foundation for future research in the country.
In relation to the ability of the indigenous strains to fix N, it is an interesting finding that the strains IS-2 and SK-5 showed a certain pattern in their performance. The strain SK-5, which is classified as B. diazoefficiens (Mason et al. 2018), seemed to perform better along with the temperate cultivars than with the tropical cultivars. On the other hand, IS-2, which was classified under the B. elkanii species, showed the highest N fixation ability with the tropical cultivars, although it has a comparably similar performance with the other strains for the non-Rj plants.
4.2 Symbiotic performance of the indigenous bradyrhizobia
All the indigenous bradyrhizobia isolated from the Philippines showed lesser performance in comparison to the B. diazoefficiens USDA110 but better performance than the uninoculated treatment. Although using USDA 110 as a reference strain for symbiotic association with soybean has been established in many reports, a study noted that the effect of inoculating USDA110 in the presence of native rhizobia was not significantly higher under ambient and elevated CO2 especially in field condition (Sanz-Sáez et al. 2015). Thus, our result might change if the indigenous strains would be inoculated under natural conditions where they exist in higher population than the USDA110.
In Brazil, it was reported that some novel Bradyrhizobium species even showed a better symbiotic performance than the commercial inoculant B. elkanii SEMIA 5019 which was approved by their Ministry of Agriculture, Livestock and Supply (Ribeiro et al. 2015). Additionally, similar observation was obtained from another report conducted at Mozambique where the relative efficiency of native bradyrhizobia were higher than the B. diazoefficiens USDA110 (Chibeba et al. 2017). Meanwhile, a report stated that some native bradyrhizobia isolates from Mozambican soils were symbiotically efficient and can be used as potential inoculants (Gyogluu et al. 2017) and the same research group reported that many native bradyrhizobia which were phylogenetically similar with B. elkanii species are the preferred dominant microsymbiont of soybean in Mozambican soils (Gyogluu et al. 2018). Hence, it is better to harness and use the locally-adapted strains for inoculation.
This is the first time that the USDA110 was used to compare with the indigenous bradyrhizobia isolated from the Philippines and we were able to show that the selected strains IS-2 and SK-5 could be further studied as potential inoculants. Henceforth, it was shown in this report that the symbiotic efficiency of indigenous bradyrhizobia from the Philippines have different preference and performance according to the cultivar to be used. For the tropical cultivars from the Philippines, it is evident that the strains with the highest potential to become inoculant would be IS-2, followed by the GI-4 and NE2–37.
We have elucidated in our two previous reports (Mason et al. 2017, 2018) that B. elkanii was the most dominant and have the widest distribution in Philippines soil so it is suggested that among all the indigenous strains used in this study, IS-2 have the highest potential to be used as a candidate inoculant. Although we have hypothesized that the indigenous strains which were classified under the Bd110 cluster would be good inoculant in the Philippines, their symbiosis with the local soybean cultivars proved to be lower than IS-2, which is a strain of B. elkanii species. This observation is quite similar with previous reports (Chibeba et al. 2017; Gyogluu et al. 2017, 2018) wherein native bradyrhizobia isolated locally were symbiotically better than commercial inoculants which are non-native.
In conclusion, this study was able to provide the following observations: (1) the use of indigenous and locally-adapted bradyrhizobia for soybean production might be comparably efficient in symbiosis and N-fixation with the known model strain of bradyrhizobia, which is the B. diazoefficiens USDA110; (2) all the indigenous bradyrhizobia used in this study are classified as type A strains based on its nodulation ability with various Rj genotypes; and (3) the symbiotic performance and N-fixation ability of the indigenous strains are in this order for tropical cultivars, B. elkanii > Bradyrhizobium sp. > B. japonicum > B. diazoefficiens but is reversed for the temperate cultivars. Lastly, we recommend that for further research, if soybean yield is to be considered, we propose the use of IS-2 as a potential inoculant to increase soybean yield but if the objective is for climate change mitigation, SK-5 or LT-3 should be used.
For future prospect, it would be helpful if field trials using IS-2 strain in comparison or in combination with the commercially available inoculants would be conducted under different agro-environmental gradient in the country. Further on, evaluation of different carrier materials that would be suitable for the said strain should be conducted for mass production if IS-2 was proven in field trials to be competitive.
Data Availability
All data are included in this manuscript.
References
Akiyama H, Hoshino YT, Itakura M, Shimomura Y, Wang Y, Yamamoto A, Tago K, Nakajima Y, Minamisawa K, Hayatsu M (2016) Mitigation of soil N2O emission by inoculation with a mixed culture of indigenous Bradyrhizobium diazoefficiens. Sci Rep 6:322869. https://doi.org/10.1038/srep32869
Champion RA, Mathis JN, Israel DW, Hunt PG (1992) Response of soybean to inoculation with efficient and inefficient Bradyrhizobium japonicum variants. Crop Sci 32:457–463
Chibeba AM, Kyei-Boahen C, Guimarães MF, Nogueira MA, Hungria M (2017) Isolation, characterization and selection of indigenous Bradyrhizobium strains with outstanding symbiotic performance to increase soybean yields in Mozambique. Agric Ecosyst Environ 246:291–305
Devine TE, Kuykendall LD, O’Neill JJ (1988) DNA homology group and the identity of bradyrhizobial strains producing rhizobitoxine-induced foliar chlorosis on soybean. Crop Sci 28:939–941
Grönemeyer JL, Kulkarni A, Berkelmann D, Hurek T, Reinhold-Hurek B (2014) Identification and characterization of rhizobia indigenous to the Okavango region in sub-Saharan Africa. Appl Environ Microbiol 80:7244–7257
Gyogluu C, Mohammed M, Jaiswal SK, Kyei-boahen S, Dakora FD (2017) Assessing host range, symbiotic effectiveness, and photosynthetic rates induced by native soybean rhizobia isolated from Mozambican and south African soils. Symbiosis:1–10
Gyogluu C, Jaiswal SK, Kyei-Boahen S, Dakora FD (2018) Identification and distribution of microsymbionts associated with soybean nodulation in Mozambican soils. Syst Appl Microbiol 41:506–515
Hardy RWF, Holsten RD, Jackson EK, Burns RC (1968) The acetylene – ethylene assay for N2 fixation: laboratory and field evalution. Plant Physiol 43:1185–1207
Hayashi M, Saeki Y, Haga M, Harada K, Kouchi H, Umehara Y (2012) Rj (rj) genes involved in nitrogen-fixing root nodule formation in soybean. Breed Sci 61:544–553
Ishizuka J, Suemasu Y, Mizogami K (1991) Preference of Rj-soybean cultivars for Bradyrhizobium Japonicum for nodulation. Soil Sci Plant Nutr 7:15–21
Itakura M, Uchida Y, Akiyama H, Hoshino YT, Shimomura Y, Morimoto S, Tago K, Wang Y, Hayakawa C, Uetake Y, Sanchez C, Eda S, Hayatsu M, Minamisawa K (2013) Mitigation of nitrous oxide emissions from soils by Bradyrhizobium japonicum inoculation. Nat Clim Chang 3:208–212
Mason MLT, Matsuura S, Domingo AL, Yamamoto A, Shiro S, Sameshima-Saito R, Saeki Y (2017) Genetic diversity of indigenous soybean-nodulating Bradyrhizobium elkanii from southern Japan and Nueva Ecija, Philippines. Plant Soil 417:349–362. https://doi.org/10.1007/s11104-017-3263-4
Mason MLT, Tabing BLC, Yamamoto A, Saeki Y (2018) Influence of flooding and soil properties on the genetic diversity and distribution of indigenous soybean-nodulating bradyrhizobia in the Philippines. Heliyon 4(2018):e00921. https://doi.org/10.1016/j.heliyon.2018.e00921
Ribeiro PR, Santos JV, Costa EM, Lebbe L, Assis ES, Louzada MO, Guimaraes AA, Willems A, Moreira FM (2015) Symbiotic efficiency and genetic diversity of soybean bradyrhizobia in Brazilian soils. Agric Ecosyst Environ 212:85–93
Risal CP, Yokoyama T, Ohkama-Ohtsu N, Djedidi S, Sekimoto H (2010) Genetic diversity of native soybean bradyrhizobia from different topographical regions along the southern slopes of the Himalayan Mountains in Nepal. Syst Appl Microbiol 33:416–425
Saeki Y, Aimi N, Hashimoto M, Tsukamoto S, Kaneko A, Yoshida N, Akao S (2004) Grouping of Bradyrhizobium USDA strains by sequence analysis of 16S rDNA and 16S-23S rDNA internal transcribed spacer region. Soil Sci Plant Nutr 50:517–525
Saeki Y, Aimi N, Tsukamoto S, Yamakawa T, Nagatomo Y, Akao S (2006) Diversity and geographical distribution of indigenous soybean-nodulating bradyrhizobia in Japan. Soil Sci Plant Nutr 52:418–426
Sameshima-Saito R, Chiba K, Minamisawa K (2006) Correlation of denitrifying capability with the existence of nap, nir, nor and nos genes in diverse strains of soybean bradyrhizobia. Microb Environ 3:174–184
Sanz-Sáez A, Heath KD, Burke PV, Ainsworth EA (2015) Inoculation with an enhanced N2-fixing Bradyrhizobium japonicum strain (USDA110) does not alter soybean (Glycine max Merr.) response to elevated [CO2]. Plant Cell Environ 38:2589–2602
Shiina Y, Itakura M, Choi H, Saeki Y, Hayatsu M, Minamisawa K (2014) Relationship between soil type and N2O reductase genotype (nosZ) of indigenous soybean bradyrhizobia: nosZ-minus populations are dominant in andosols. Microbes Environ 29:420–426
Shiro S, Yamamoto A, Umehara Y, Hayashi M, Yoshida N, Nishiwaki A, Yamakawa T, Saeki Y (2012) Effect of Rj genotype and cultivation temperature on the community structure of soybean-Nodulating Bradyrhizobia. Appl Environ Microbiol 78:1243–1250. https://doi.org/10.1128/AEM.06239-11
Soe KM, Bhromsiri A, Karladee D, Yamakawa T (2012) Effects of endophytic actinomycetes and Bradyrhizobium japonicum strains on growth, nodulation, nitrogen fixation and seed weight of different soybean varieties. Soil Sci Plant Nutr 58:319–325
Unkovich M, Herridge D, Peoples M, Cadisch G, Boddey R, Giller K, Alves B, Chalk P (2008) Measuring plant-associated nitrogen fixation in agricultural systems ACIAR Monograph No 136, 258 pp.
Vincent JM (1970) A manual for the practical study of the root-nodule Bacteria. Blackwell Scientific, Oxford
Williams LF, Lynch DL (1954) Inheritance of a non-nodulating character in the soybean. Agron J 46:28–29
Yamakawa T, Hussain AKMA, Ishizuka J (2003) Soybean preference for Bradyrhizobium japonicum for nodulation. Soil Sci Plant Nutr 49:835–841
Funding
This research work was funded by the JSPS KAKENHI (Grant-in Aid for Scientific Research (C) No.18 K05376 from Japanese Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
Maria Luisa T. Mason – conceptualized research theme, did experiments, and wrote the manuscript; Baby Lyn T. De Guzman – did experiments and wrote manuscripts; Akihiro Yamamoto - did experiments and wrote manuscripts; Yuichi Saeki – conceptualized research theme and wrote manuscript.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
There is no conflict of interest among the authors.
Code availability
The software used in the analysis are all freely downloadable via the internet.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 6567 kb)
Rights and permissions
About this article
Cite this article
Mason, M.L.T., De Guzman, B.L.T., Yamamoto, A. et al. Symbiotic performance of indigenous soybean bradyrhizobia from the Philippines with soybean (Glycine max [L.] Merill) cultivars harboring different Rj genotypes. Symbiosis 83, 55–63 (2021). https://doi.org/10.1007/s13199-020-00731-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-020-00731-7