Abstract
Sap beetles (Coleoptera: Nitidulidae) are commonly associated with ophiostomatoid fungi, especially those belonging to the family Ceratocystidaceae. This coexistence of insects and fungi, usually on tree wounds, offers the ophiostomatoid fungi an effective means of dispersal. The selective advantage of this association to sap beetles is, however, confounded by the versatile life history strategies of these insects. In this study, we complemented field observations with rearing and feeding behavior experiments in the laboratory, to investigate the symbiology of interactions between sap beetles and co-occurring fungi, from the insect perspective. We determined that all predominant sap beetle vectors of the Ceratocystidaceae in a natural woodland feed on and use the mycelial mats of these fungi to nurse their offspring in tree wounds. When reared on fungal cultures in the laboratory, several of these insects successfully completed their life cycle. We were able to maintain Carpophilus hemipterus on this exclusive fungal diet over several generations. The feeding preference of this insect was generally consistent with the patterns of its fungal associations in the field as previously reported. There also appeared to be a correlation between the attractiveness of Ca. hemipterus to various fungi and its fitness benefits from feeding on these fungi. Overall, our results suggest that, from their partnership with ophiostomatoid fungi, sap beetles benefit from essential nutritional supplementation, enabling them to survive in saproxyly in woodland ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Few insects are metabolically equipped to break down wood structural polymers such as cellulose or lignin into nutritionally assimilable molecules (Martin 1983; Cragg et al. 2015; Ulyshen 2016). To circumvent this limitation, and to compensate for the low nutriment value of wood tissues, saproxylic beetles, which extract their food from wood while dwelling on dead or moribund trees, rely on a variety of symbioses with microorganisms, including fungi (Martin 1992; Douglas 2009). Some saproxylic beetles ingest or harbor fungi in their guts to exploit their lignocellulolytic activity for digestion (Vega and Dowd 2005; Delalibera et al. 2005; Geib et al. 2008); others maintain fungi externally as food gardens, from which they derive the bulk of their alimentation or some essential nutrients for their reproduction and development (Farrell et al. 2001; Klepzig and Six 2004; Harrington 2005; Mueller et al. 2005). Saproxylic beetles may also use fungal symbionts to neutralize ingested plant toxins or to overcome host defense when infesting living trees (Dowd 1992; Six and Wingfield 2011; Raffa et al. 2015). Fungal symbioses are consequently a key driver of the evolutionary diversification of saproxylic beetles, as well as a modulator of their physiological processes, ecological interactions and impact on natural and plantation forest ecosystems (Klepzig et al. 2009; Hulcr and Dunn, 2011; Six 2013; Ploetz et al. 2013).
Ophiostomatoid fungi (Wingfield et al. 1993) are major symbiont providers to saproxylic beetles (Kirisits 2007; Harrington et al. 2010; Khadempour et al. 2012; Mayers et al. 2015). Taxonomically, these fungi form a heterogeneous group, as some classify in the Microascales and others in the Ophiostomatales, two disparate lineages of Ascomycetes (de Beer et al. 2013). Yet, they are ecologically unified by the same affinity for fresh wood habitats. Furthermore, ophiostomatoid fungi have converged to similar morphologies, especially the formation of elongated fruiting bodies, enabling them to deliver sticky spores above the substrate, which adhere readily to the cuticle of insects (Malloch and Blackwell 1993; Klepzig and Six 2004). These morphological features, as well as the production of sweet, fruity odors by species in the Microascales that lure insects to infected substrates, are regarded as evolutionary novelties that facilitate symbiotic associations with insect vectors (Lin and phelan 1992; Malloch and Blackwell 1993).
Three groups of coleopteran insects are predominantly involved in the dispersal of ophiostomatoid fungi, including ambrosia beetles (Curculionidae: Scolytinae and Platipodynae), bark beetles (Curculionidae: Scolytinae) (Harrington 2005; Klepzig & Six 2004; Six 2012, 2013), and sap beetles (Nitidulidae) (Moller and De Vay 1968; Ambourn et al. 2002; Hayslett et al. 2008; Heath et al. 2009a; Mbenoun et al. 2016, 2017). These vectors can be further categorized as either tree wound creators or visitors and appear to differ in the nature of the relationships with fungal partners. Bark and ambrosia beetles create wounds by excavating breeding galleries in the phloem and sapwood layers of infested trees respectively (Six 2012; Kirkendall et al. 2015). They are strictly saproxylic and obligately dependent on the fungal flora introduced into the galleries for their nutrition (Harrington 2005; Six 2012, 2013), which makes these associations mutually beneficial (Klepzig and Six 2004; Harrington 2005; Six 2012). Sap beetles, on the other hand, do not create wounds; instead, they opportunistically infest freshly made wounds resulting from various forms of physical damage on trees (Juzwik et al. 2004; Heath et al. 2009a; Mbenoun et al. 2016, 2017). Even though some sap beetles have been documented to feed and rear their brood on the fungi they carry, these insects have generally been viewed as ecologically generalist, having only incidental interactions with fungi (Juzwik 1999). This perception probably explains why sap beetle-ophiostomatoid fungal associations have received comparatively little attention.
As their name suggests, sap beetles are assumed to feed on plant sap and therefore to be attracted for that resource to mechanically injured trees. In reality, life history strategies among the Nitidulidae are diverse and encompass feeding habits on fruits, foliage, flowers, detritus and decaying plant material, decomposing animal corpses and fungi, as well as sap feeding (Cline 2005; Jelinek et al. 2010). A few sap beetles have evolved to coexist commensally (inquilinism) with social insects such as bees and termites, while others have developed predatory behaviors towards other invertebrates (Cline 2005; Jelinek et al. 2010). Although narrow dietary specialization has been noted in this group, many sap beetles, including well known vectors of ophiostomatoid fungi, are polyphagous and occur across a broad-spectrum of trophic niches (Cline 2005; Jelinek et al. 2010). Such is for instance the case of the cosmopolitan dried-fruit beetle, Carpophilus hemipterus, which is frequently encountered on fresh and older tree wounds (Moller and De Vay 1968; Heath et al. 2009a; Mbenoun et al. 2017), flowers, fresh as well as overripe fruits (George et al. 1989; Hossain and Williams 2003; Rosi et al. 2019), but is also known as an important pest of ripening fruits and stored food products (Dobson 1954; James et al. 1997; Rosi et al. 2019 and references therein). This ecological plasticity has probably overshadowed the benefits of ophiostomatoid fungi to the ecological fitness of their sap beetle vectors in woodland ecosystems.
Sap beetles are most consistently associated with ophiostomatoid fungi belonging to the family Ceratocystidaceae (Microascales) as defined by de Beer et al. (2014). In that regard, these insects are best known for their role in the dispersal of virulent Ceratocystidaceae tree pathogens. In particular, the transmission of Bretziella fagacearum, the causal agent of oak wilt in the United States of America (USA) has been studied extensively (Cease and Juzwik 2001; Ambourn et al. 2002; Hayslett et al. 2008 and references therein). The principal sap beetle vectors implicated were observed to feed and reproduce on oak wilt fungus mats, with remarkable differences in their trophic interactions with B. fagacearum (Cease and Juzwik 2001). A similar pattern, characterized by variable interspecific affinities was uncovered in the interactions between sap beetle vectors and Ceratocystis albifundus causing wilt of Acacia mearnsii in South Africa (Heath et al. 2009a; Mbenoun et al. 2017). Despite these inconsistencies with the general theory regarding sap beetle relationships with ophiostomatoid fungi, there has been no study to purposefully investigate sap beetle interactions with the Ceratocystidaceae, in order to characterize the true nature of these associations from the insect perspective.
Our aim in this study was to further explore the symbiology of associations involving sap beetles and the Ceratocystidaceae, with a focus on how these associations influence the life cycle and ecological fitness of sap beetles. We hypothesized that: (i) all major sap beetle vectors of the Ceratocystidaceae are fungus-feeders; (ii) the patterns of their interactions with these fungi reflect feeding preferences; and (iii) there is a relation between fungus-feeding preference and ecological fitness of sap beetle vectors of the Ceratocystidaceae. To test these hypotheses, we carried out extensive field observations in a natural savanna woodland to characterize the interactions between sap beetles and Ceratocystidaceae fungal partners in their natural ecological niche on wounded trees. We also utilized Ca. hemipterus as a model to establish laboratory rearing and feeding choice experiments to study the fungus-feeding behaviors of sap beetles, in relation to their development and reproduction.
2 Materials and methods
2.1 Field studies
2.1.1 Characterization of sap beetle fungal interactions in tree wounds
Field studies of insect-fungus interactions were carried out on tree wounds arising from elephant browsing activity in the Kruger National Park (KNP) of South Africa, during surveys for ophiostomatoid fungi and their insect associations in natural savanna woodland ecosystems (Mbenoun et al. 2014, 2017). We especially focused on deep wood cracks and bark splits that provide optimal conditions for the growth and maintenance of these fungi. Infected wood cavities were further opened, using cutting tools such as axes, machetes or masonry chisels where necessary, to expose the fungal mats on fresh wood tissues. Fungal mats were inspected for infestation by adult and immature stages of sap beetles, as well as for the feeding signs of these insects. The flying and landing behaviors of visiting sap beetles were also recorded on very recently made wounds.
Insect specimens were collected and kept in 90% ethanol up till identification. Adult sap beetles were later identified as five species of Carpophilus (Ca. hemipterus, Ca. dimidiatus, Ca. bisignatus, Ca. apicipennis and Carpophilus sp.) and one species of Brachypeplus (Br. ater) based on morphological criteria (Mbenoun et al. 2017). The identity of the fungi was primarily determined on site based on phenotypic characteristics such as the typical ascomatal structures and fruity odors produced by the Ceratocystidaceae. Isolations were also made by transferring mycelial strands or ascopore masses from wood tissues onto 2% malt extract agar (Biolab, Midrand, South Africa; hereafter referred to as 2% MEA), supplemented with ∼0.01 g/L streptomycin sulphate (Sigma, Steinheim, Germany) to preclude bacterial growth. Purified fungal cultures were subsequently identified to the species level using multilocus DNA phylogenies based on the ITS, β-tub and tef1-α gene sequences, as previously reported in Mbenoun et al. (2014). Consistent with recent developments in the taxonomic treatment of the Ceratocystidaceae (de Beer et al. 2014; Nel et al. 2017), these fungi included three species of Huntiella (H. savanae, H. oblonga, H. cryptoformis), three species of Ceratocystis (C. albifundus, C. zambeziensis, C. thulamelensis), a Chalaropsis species and a Berkeleyomyces species.
2.1.2 Matching sap beetle larval broods and adults using DNA barcoding
Total genomic DNA was extracted from five representatives of each sap beetle morphospecies and 20 larvae from different fungal mats. Every insect specimen was processed separately, using the prepGEM™ Insect DNA extraction kit (zyGEM, New Zealand), following the manufacturer’s instructions. The resultant DNA solutions were utilized to PCR-amplify and sequence a portion of the cytochrome oxidase subunit 1 (CO1) gene, using the Cl-J-2183/TL2-N-3014 universal primer combination (Simon et al., 1994) and the same reaction conditions as described by Mbenoun et al. (2016). The sequences generated were queried against the NCBI-GenBank nucleotide database, using the BLASTn search tool. A nucleotide sequence dataset was assembled, including the queried sequences and 16 significant (≥ 90 sequence similarity) GenBank hits, supplemented with four previously unpublished sequences of African sap beetle specimens. Sequences representing two Curculio (Coleoptera: Curculionidae) wood-boring larvae from the KNP were used as outgroups (see Fig. 3 for all accession numbers).
A Multiple sequence alignment (MSA) was generated on the nucleotide sequence dataset using the web interface of the program MAFFT (http://mafft.cbrc.jp/alignment/server/). Phylogenetic relationships were inferred from the MSA based on the maximum likelihood (ML) criterion, with a gamma model of rate heterogeneity. The analyses were performed using the Vital IT RAxML web-server (http://embnet.vital-it.ch/raxml-bb/), where RAxML is executed with a rapid bootstrap algorithm (Stamatakis et al. 2008). Putative species were delineated on the CO1 ML phylogram based on the Poisson tree processes (PTP) model (Zhang et al. 2013), implemented with a Bayesian supplementation using the dedicated bPTP web-server (http://species.h-its.org/ptp/). Bayesian supports for the putatively delineated species nodes were generated based on 500,000 MCMC generations, including a 10% burn-in period. The convergence of the MCMC chain was checked by visually examining the likelihood trace plot.
2.2 Laboratory investigations
2.2.1 Establishing and maintaining a colony of Carpophilus hemipterus
For laboratory experiments, Ca. hemipterus was chosen as a model. This was based on the fact that, unlike all other sap beetle species recovered in the KNP, the rearing and developmental biology of Ca. hemipterus was well-documented. In addition, the availability of synthetic specific aggregation pheromones and the cosmopolitan distribution of Ca. hemipterus facilitated the collection of specimens using pheromone-trapping. A colony of Ca. hemipterus was initiated with three adult individuals captured in the KNP, using a wind-oriented funnel trap, baited with dough and a Ca. hemipterus pheromone blend (Great Lakes IMP). The beetles were transferred to the laboratory and introduced into a 90-mm Petri dish containing 2% MEA, overgrown with a culture of H. cryptoformis, one of the species of the Ceratocystidaceae most frequently associated with Ca. hemipterus in the KNP (Mbenoun et al. 2017). The plate was sealed with Parafilm tape and the lid was perforated with ventilation holes using a heated needle. After 5 days at 24 ± 1 °C and 12 L:12D photoperiod (hereafter referred to as standard conditions), several larvae emerged in the plate. The larvae and adult beetles grazed on the fungus mycelium and were transferred using a small paint brush to new plates whenever the fungus-feed was exhausted. As the number of insects increased, the Petri dish rearing was modified and scaled up by growing the fungus in 60-mm plates and fitting four of these into a breeding unit, made up of a 150x150x50-mm3 plastic food storage container, lined with moistened paper towel (Fig. 1a). Ventilation holes were made at each corner and center of the container cover. The efficiency of our modified Petri dish rearing method was later tested on various sap beetle species, including Ca. dimidiatus, Ca. bisignatus, Carpophilus sp. and Br. ater obtained from the KNP, as well as a Brachypeplus sp. from the Garden Route National Park (GRNP) of South Africa.
2.2.2 Effect of fungal diet on Carpophilus hemipterus survival and development
Ten ascomycete species were evaluated for their suitability as a food source for Ca. hemipterus. These included H. cryptoformis, C. albifundus, C. zambeziensis and Chalaropsis sp., which occur as tree wound colonists along with Ca. hemipterus and other sap beetle species in the KNP (Mbenoun et al. 2017); H. moniliformis and Thielaviopsis ethacetica, recorded in South Africa on eucalypts (Heath et al. 2009b) and sugarcane (Mbenoun et al. 2015) respectively, but also as common associates of sap beetles in cacao agroforests of Cameroon (Mbenoun et al. 2016); Ophiostoma sp., a common tree-wound colonist in the Garden Route National Park (GRNP) of South Africa; Fusarium sp. and Botryosphaeria sp., two endophytes occasionally isolated from tree wounds; and Penicillium sp., a late saprophyte invader of tree wounds. The fungi were maintained on 2% MEA for 10 days and arranged in breeding units. Ca. hemipterus was exposed to the fungi as eggs (except for Th. ethacetica for which 1-day-old larvae were used) and monitored for survival and development under standard conditions. The developmental time was measured as the number of days necessary for an individual egg to hatch and grow into an adult beetle (see Fig. 4).
2.2.3 Fungus-feeding choice behavior of Carpophilus hemipterus
To assess the fungus-feeding choice behavior of Ca. hemipterus, two sets of multichoice preference assays were performed. Firstly, the relative attractiveness of H. cryptoformis, C. albifundus, C. zambeziensis and Chalaropsis sp. to Ca. hemipterus was evaluated. A breeding unit was prepared for each fungus using 10-day-old cultures as described above. The four breeding units were arranged at right angles around a control unit containing sterile 2% MEA, interconnected through the control unit by means of plastic pipes (length = 10 cm, diameter = 1 cm) (Fig. 1b). Thirty unsexed adults of Ca. hemipterus were used in this experiment. These insects were initially maintained on a composite fungal diet to prevent them from becoming accustomed to any specific fungus. The experiment was started by releasing the insects into the control unit; the entire experimental arena was thereafter protected from light using a cardboard shield. Fifteen hours after the release, the distribution of the insects in the experimental arena was recorded. The experiment was replicated with a second set of fungi, including H. cryptoformis, H. moniliformis, Fusarium sp. and Ophiostoma sp. Five different new batches of insects were tested in the first experiment and four in the second experiment.
In the second assay, Ca. hemipterus was evaluated for feeding and oviposition substrate preference when presented with a choice between H. cryptoformis, H. moniliformis, Fusarium sp. and Ophiostoma sp. The fungi were cultured on 2% MEA for 10 days and arranged in composite breeding units containing all four fungal cultures. Twenty unsexed, adult individuals of Ca. hemipterus were introduced in each unit and maintained for 48 h, after which time the percentage of mycelium surface, grazed over by the insects, was visually estimated in each plate, facilitated by a grid drawn on the bottom, reverse side of the plate. A count of offspring was made in the plates after 72 h. The experiment was performed twice over time, using five different new batches of insects. To assess the potential effect of our laboratory rearing on the beetle choice behavior, each insect batch was preconditioned by feeding the insects for one week exclusively on one of five different fungal diets, including the same strains used as feeding treatments (H. cryptoformis, H. moniliformis, Fusarium sp., Ophiostoma sp.) and T. ethacetica which was included as a control.
2.2.4 Effect of fungal diet on Carpophilus hemipterus reproduction
The effect of fungal diet on Ca. hemipterus reproductive potential was considered using H. cryptoformis, H. moniliformis, Ophiostoma sp. and Fusarium sp. For each fungal diet, a breeding unit was set up with 10-day-old cultures on 2% MEA. Ten unsexed adult individuals of Ca. hemipterus, which had been reared on a composite fungal diet, were introduced into each breeding unit and monitored for brood production for 14 days, under standard breeding conditions. Fungal plates were renewed every five days and the old plates were kept for three additional days, after which a count of offspring was made in the plates. The experiment was performed four times with different new batches of insects.
2.2.5 Statistical analysis
Statistical analyses were performed using R statistical software (http://www.Rproject.org/). For all multichoice assays, i.e., the relative attractiveness of fungi, as well as the feeding and oviposition substrate preference of Ca. hemipterus, differences between treatments were analyzed using nonparametric Aligned Ranks Transformation analysis of variance (ART anova) (Wobbrock et al. 2011), followed where relevant by Tukey’s post-hoc pairwise comparisons. Fungal relative attractiveness (FRA) was measured as the percentage of effective number of insects attracted, as determined by the formula \( FRA=\frac{N}{T-C}\times 100\% \), where N is the number of insects recorded in the fungal diet unit, C is the number of insects recorded in the control unit, T is the total number of insects tested (sample size) and T-C is the effective number of insects tested (effective sample size). Feeding preference and oviposition substrate preference were considered in separate analyses, to test the effects of preconditioning fungus and feeding fungus (fixed factors), as well as their interaction on the surface grazed over and number of offspring laid (response variables), respectively. The reproductive potential of Ca. hemipterus, in terms of total number of offspring produced, was analyzed using one way analysis of variance (ANOVA) and Tukey’s pairwise comparisons. The conformity of offspring number data with ANOVA assumptions was checked beforehand using Shapiro’s test (w = 0.97, p = 0.83) for normality and Barlett’s test (χ2 = 3.53, df = 3, p = 0.32) for homogeneity of variances. For all statistical tests, the significance level was set to α = 0.05. Quantitative data were sumarized as mean ± standard deviation.
3 Results
3.1 Field studies
3.1.1 Patterns of sap beetle-fungal interactions in tree wounds
Fresh mycelial mats of the Ceratocystidaceae occurred generally on newly damaged trees (1–4-week-old wounds). Occasionally, we found fresh mycelial mats that had persisted for periods beyond one month in extensively deep wood cavities. A typical characteristic that distinguished a fresh mycelial mat from a dead one was that the former was associated with fruity odors, often concurrent with an infestation of sap beetles. The presence of sap beetles appeared to maintain the mycelial mats clean, apparently consisting of pure cultures of the Ceratocystisdaceae (Fig. 2), and signs of contamination by other fungi were uncommon. In contrast, contamination by Penicillium sp. was very common on abandoned or dead mycelial mats.
Ecological niche shared by sap beetles and ophiostomatoid fungi in the KNP woodland ecosystem: (a) extensive tree wounding resulting from elephant browsing, a major driver of saproxylic sap beetle population dynamics; (b) deep wood cracks infested by sap beetles, with a fungal lining building up on the cavity surfaces; (c) mature fungal mat under bark flap, (d) adult sap beetle actively feeding on fungal mat, (e, f) feeding marks of sap beetle larvae on fungal mat and inner bark tissue
Sap beetles were found indiscriminately as single species colonies in small mycelial mats, or in assemblies of colonies of different species in larger mycelial mats, although there was always a single dominant beetle species present. Various life stages of sap beetles occurred on mycelial mats, including adults and various larval instars, but pupae were never observed. The feeding marks of larvae were conspicuous, indicating in certain cases an exclusive fungal diet, and in other cases a composite diet including fungal material and inner bark tissue (Fig. 2). Although the feeding marks of adults were hardly noticeable, some individuals of various species were observed actively feeding on mycelial mats (Fig. 2). Where trees had just been damaged, sap beetles were observed landing on the wounds with a consistent behavioral pattern, consisting of frenetic movements, apparently in search of deep bark flaps or wood cracks where they eventually took refuge.
3.1.2 Relationship between adult sap beetles and immature larval stages on tree wounds
Thirty-five new nitidulid CO1 gene sequences of approximately 800 bp were generated in this study and made available in GenBank (see Fig. 3 for accession numbers), including 20 sequences for adult sap beetles and 15 sequences for immature larval stages. When queried against the GenBank database, only sequences of Ca. hemipterus emerged with a broad distribution of BLAST hits having a substantial degree of percentage identity (≥ 98%). Sequences representing Br. ater were most closely related to those of other Brachypeplus species previously reported from Africa, with sequence identity ranging between 90 and 98%. Similarly, Ca. bisignatus was most closely related to a morphologically identical Carpophilus sp. previously reported from Africa based on 92% sequence identity. For Ca. dimidiatus, Ca. apicipennis and the unnamed Carpophilus sp. from the KNP, there were no matches in the GenBank data base with ≥90% sequence identity.
Phylogenetic tree based on CO1 gene sequence showing the relationship between adults of predominant sap beetle vectors (pictures) of the Ceratocystidaceae and larval broods feeding on these fungi in the KNP. GenBank accession numbers of all sequences used are indicated as taxon labels. Sequences newly generated in this study are highlighted in boldface. Sequences obtained from larvae are marked with an asterisk. Bootstrap values (≥ 70%) are indicated below branches. PTP-delineated species are either terminal on branches or in red branching, with supporting posterior probabilities (≥ 0.8) above branches. The origins of beetle are indicated as KNP: Kruger National Park; CMR: Cameroon; CHN: China, RSA: South Africa
The ML phylogram (Fig. 3) discriminated adult sap beetle morphospecies from the KNP into discrete clusters with strong bootstrap support. These clusters intermingled adults and immature larval broods, which was indicative of a conspecific relationship or phylogenetic close relatedness between these life stages. The highest Bayesian supported PTP model revealed a greater species richness among the community of sap beetle vectors of ophiostomatoid fungi in the KNP than previously determined using morphological criteria, with nine putative species delineated (Fig. 3). Moreover, the weak Bayesian support (<0.8) of some of these delineated taxa suggested that they may include more than one species.
3.2 Laboratory investigations
3.2.1 Rearing and maintaing Carpophilushemipterus on fungal cultures
Using our modified Petri dish rearing method, it was possible to maintain a colony of Ca. hemipterus over a period of 10 months, producing 12 generations of beetles, and in sufficiently large numbers to provide for subsequent behavior experiments. The modified Petri dish rearing method was also effective with Ca. bisignatus, Carpophilus sp. and Br. ater from the KNP, as well as Brachypeplus sp. from the GRNP, which like Ca. hemipterus (Fig. 4) could complete their life cycle and be maintained under standard conditions. Only the offspring of Ca. dimidiatus failed to complete their life cycle under these conditions, although the two adults tested survived for an extended period of time.
3.2.2 Effect of fungal diet on survival and development of Carpophilus hemipterus
Among the eight fungi evaluated as food source for immature stages of Ca. hemipterus, only Penicillium sp. appeared to be unsuitable (Table 1). The eggs introduced onto Penicillium cultures, like in most other fungal cultures, hatched after ~2 days, but the larvae failed to grow past the first instar and died during the subsequent 24 to 48 h. The eggs introduced onto T. ethacetica cultures were also destroyed by what appeared to be a lytic activity on the egg membrane. However, when one-day-old larvae were provided with T. ethacetica, they all survived and developed into adults showing the largest body size (Table 1). All other fungi tested sustained the development of Ca. hemipterus from egg to adult in a comparable time frame, but producing adults having variable body size (Table 1). Overall, individuals reared on ophiostomatoid fungi exhibited larger body size as compared to individual raised on other fungi (Table 1).
3.2.3 Fungus-feeding choice behavior of Carpophilus hemipterus
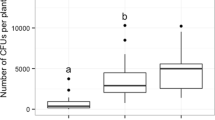
In assessing fungal relative attractiveness to Ca. hemipterus, 25–28 out of 30 total insects used in the experiment effectively participated in the test when only Ceratocystidaceae species cohabiting with Ca. hemipterus in the KNP were used. Of these, 46.7 ± 27.7% were attracted to H. cryptoformis, 20.4 ± 17.7% to C. zambeziensis, 19 ± 21.8% to C. albifundus and 13.9 ± 15.5% to Chalaropsis sp. However, although the relative attractiveness of H. cryptoformis was at least two times greater than that of any other fungus, no statistically significant difference (F = 1.11, df = 3, p = 0.38) was detected between the tested fungi (Fig. 5a). When fungi of disparate origin and families were compared, the effective number of insects tested varied between 25 and 29; of which H. moniliformis attracted 49.9 ± 10.7%, H. cryptoformis attracted 25.7 ± 11.2%, Ophiostoma sp. attracted 13.0 ± 7.1%, and Fusarium sp. attracted 11.4 ± 5.6%. A statistically highly significant variation (F = 12.2, df = 3, p = 0.0002) was detected in Ca. hemipterus response to the various fungi tested. Notably, H. moniliformis was found to be statistically more attractive than Fusarium sp. and Ophiostoma sp. but statistically comparable to H. cryptoformis, while H. cryptoformis was more attractive than Fusarium sp., but not Ophiostoma sp. (Fig. 5b, Table S1).
Comparison of fungal relative attractiveness to Carpophilus hemipterus as expressed by the percentage of insects attracted in a multichoice experimental setting: (a) the fungi tested are all members of the Ceratocystidaceae co-occurring with Ca. hemipterus in the KNP; (b) the fungi tested are from disparate taxonomic families and geographic origins. Letters on top of boxes, when different, indicate a significant difference between means (red crosses), based on Tukey’s pairwise comparison (α = 0.05)
In the feeding and oviposition substrate preference tests with Ca. hemipterus, the identity of the fungal food source (treatment) was the only statistically supported source of variation in Ca. hemipterus choice behavior (Table 2). The initial fungal diet (pre-treatment) had some effect on the number of offspring produced by the insects, but it did not affect their grazing activity (Table 2). There was no interaction between pre-treatment and treatment (Table 2), implying that preconditioning did not alter the choice behavior of Ca. hemipterus. Overall, H. moniliformis emerged as the most preferred food source with 80.5 ± 17.2% of the mycelial surface grazed over. This was statistically comparable to H. cryptoformis (69.44 ± 12.8%) but significantly different from Ophiostoma sp. (48.8 ± 17.9%) and Fusarium sp. (17.5 ± 13.8%) respectively (Fig. 6a, Table S2). In terms of oviposition, Ophiostoma sp. was statistically singled out as the favored substrate with 36.6 ± 16.1 offspring, while H. cryptoformis (16 ± 11.6 offspring), H. moniliformis (11.9 ± 11.1 offspring) and Fusarium sp. (5.5 ± 5.8 offspring) did not differ significantly from one another (Fig. 6b, Table S3).
Comparison of Carpophilus hemipterus feeding (a) and oviposition substrate (b) preference in a multichoice experimental setting with various fungi. Feeding is expressed in terms of percentage of surface grazed and oviposition in terms of number of offspring laid by 20 unsexed individuals in 24 h. Letters on top of boxes, when different, indicate a significant difference between means (red crosses), based on Tukey’s pairwise comparison (α = 0.05)
3.2.4 Effect of fungal diet on Carpophilus hemipterus reproductive potential
Carpophilus hemipterus exhibited statistically highly significant variation in reproductive potential when maintained on different fungal diets (F = 11.21, df = 3, p = 0.0009). Overall, the greatest reproductive potential was observed on H. moniliformis, with 138 ± 37 offspring produced by 10 unsexed individuals in 14 days. This value was statistically comparable to the 115 ± 33 offspring produced on H. cryptoformis, but significantly different from the 75 ± 20 and 33 ± 12 offspring obtained on Ophiostoma sp. and Fusarium sp. respectively (Fig. 7, Table S4). Pairwise comparisons also indicated that Ca. hemipterus performance on H. cryptoformis and Ophiostoma sp. were statistically comaparable, while H. cryptoformis provided a better oviposition substrate than Fusarium sp. (Fig. 7, Table S4).
Comparison of Carpophilus hemipterus reproductive potential, expressed in terms of number of offspring produced by 10 unsexed individuals, when maintained on various fungal diets for 14 days. Letters on top of boxes, when different, indicate a significant difference between means (red crosses), based on Tukey’s pairwise comparisons (α = 0.05)
4 Discussion
Several previous studies have documented the ecological association of sap beetles, notably members of the genera Carpophilus and Brachipeplus, with ophiostomatoid fungi in the Ceratocystidaceae on tree wounds in Africa (Heath et al. 2009a; Mbenoun et al. 2016, 2017; Misse et al. 2017; Lee et al. 2018). The focus of those studies was on the transmission and spread of some members of the Ceratocystidaceae associated with tree diseases. Consequently, their account of insect-fungus interactions placed most emphasis on the enhanced dispersal benefiting the fungi, while the insect perspective was largely overlooked. The results of the present study address this information gap, showing that sap beetles dispersing the Ceratocystidaceae in Africa selectively feed on and use these fungi to nurse their offspring in tree wounds. Similar patterns of life history have been reported for sap beetles elsewhere, including in the USA with sap beetle vectors of Ceratocystis fimbriata (Moller and De Vay 1968; Hinds 1972) and Bretziella fagacearum (Juzwik et al. 2004), as well as in Argentina in relation to the recently described Huntiella decorticans (de Errasti et al. 2015). While there is evidence that many other sap beetles interact only temporarily with tree wounds to feed on sap (Cline 2005), the results of the present study support the view that sap beetle vectors of the Ceratocystidaceae use their association with these fungi to foster a saproxylic lifestyle on freshly wounded trees. This relationship was postulated by Kirejtshuk (2003) but the present study is the first to consider it in any detail.
Within the context of saproxyly, sap beetles interact with tree wounds as early colonists. This implies that their appearance at the infestation site shortly after a tree has been injured (Juzwik et al. 2004; Mbenoun et al. 2016) is driven by the prospect of a convenient breeding nest. As they settle in, their movements enable multiple site inoculation of their fungal partners onto the wound surface. This promotes the formation of a mycelial mat, which creates a more sustainable living environment, especially for the young stages. Evidence from the present study suggests that the development of fungal mats could stimulate mating and oviposition in saproxylic sap beetles. After hatching, the larvae feed on this substrate until they reach the wandering stage. The absence of pupae from the wounds is consistent with the fact that wandering larvae ultimately fall off from the wounds to the ground, where pupation occurs, as has previously been reported by Moller and De Vay (1968) and Hinds (1972). Adult saproxylic sap beetles most probably also feed on fresh and fermented sap, which may be crucial during the early days of wound infestation. Despite the apparent cleanliness of infested fungal mats, we failed to observe any behavioral patterns of the insects that could represent measures to maintain a clean environment; and we are not aware of studies to suggest how the fungal mats are maintained without contamination. It was, however, evident that saproxylic sap beetles would abandon their nests whenever the fungal mats died or became heavily contaminated by other microbial invaders.
The results of our Petri plate assays suggest that saproxylic sap beetles meet their nutritional needs feeding on their Ceratocystidaceae fungal partners. We were able to breed Ca. hemipterus over several generations and achieved the completion of life cycle consistently with four other nitidulid species on cultures of H. cryptoformis in Petri dishes. Petri plate assays have been useful in investigating insect-fungus interactions in a variety of symbiotic associations, involving, e.g., bark beetles (Freeman et al. 2012), ants (Baker et al. 2017) and sap beetles (Cline et al. 2014). To the best of our knowledge, such assays have been utilized in only two previous instances involving sap beetle vectors of the Ceratocystidaceae (Moller and De Vay 1968; Hinds 1972). Those studies sought to better understand the transmission of C. fimbriata by insects. In the study of Moller and De Vay (1968), sap beetles could be reared from egg to adulthood on fungal cultures, whereas in the study of Hinds (1972), they seldom pupated and died and became overgrown by the fungi. This inconsistency in the results could have resulted from the fact that in the first study, only pure cultures of C. fimbriata were utilized, whereas in the second, a diverse microbial flora carried by the insects was allowed to grow in the plates. This could have included toxic species such as Penicillium sp., which was shown to be unsuitable as food for Ca. hemipterus in the present study. Our experiments, similar to those of Moller and De Vay (1968), were performed with pure fungal cultures, and under these conditions, of the five sap beetle species tested, only Ca. dimidiatus failed to thrive.
The elaborate feeding experiments conducted with Ca. hemipterus suggested that saproxylic sap beetles can survive and even proliferate on a relatively wide variety of fungi. These include members of the Ophiostomataceae, Nectriaceae and Botryosphaeriaceae, some of which (the Ophiostomataceae in particular) are common tree wound colonists and are vectored by sap beetles (e.g. Nkuekam et al. 2013). This raises questions regarding the factors driving the greater prevalence of sap beetle associations with the Ceratocystidaceae in natural environments. The fruity volatiles produced by these fungi, which give them a unique advantage over other fungi in attracting insect vectors, most likely play an important role. The corresponding adaptive character for saproxylic sap beetles might lie in their selective fungus-feeding behaviors, as shown in our results by a clear preference for the Ceratocystidaceae. Indeed, when simultaneously exposed to Ophiostoma sp. (Ophiostomataceae), Fusarium sp. (Nectriaceae) and two Huntiella species (Ceratocystidaceae), Ca. hemipterus exhibited significantly greater attractiveness to and feeding activity on Huntiella species. Interestingly, when Ca. hemipterus was exposed to a H. cryptoformis, C. albifundus, C. zambeziensis and a Chalaropsis sp., all members of the Ceratocystidaceae co-existing with Ca. hemipterus in the KNP, the Huntiella species remained the most attractive, although differences were less striking. This result is consistent with the prevailing associations of Ca. hemipterus with members of the genus Huntiella observed in South African savanna ecosystems (Heath et al. 2009a; Mbenoun et al. 2017).
The apparent correlation between the feeding attractiveness of various fungi and their effect on the brood production of Ca. hemipterus was noteworthy. This suggests that the feeding choice of this insect could be determined by a gain anticipated in reproductive success. It is well known that nutritional fungal associates enhance the fitness of saproxylic beetles with the dietary benefits they provide. These include amino acids, vitamins and sterols, which are essential nutrients for insect development and reproduction (Six 2012, 2013 and references therein). Moreover, fungi produce semiochemicals used by some insects as precursors or components of the pheromones modulating their mating and other social behaviors (Engl and Kaltenpoth 2018). As it relates to saproxylic sap beetles, we suspect that the Ceratocystidaceae feature a quantitatively and/or qualitatively better content of these elements as compared to other fungi. This would explain why Ca. hemipterus displayed greater brood production, but also larger body size and an aggregation behavior in association with Huntiella species, not found with Ophiostoma sp. or Fusarium sp.
Rather than to be solely linked to some physiological processes, the correlation between the feeding preference of Ca. hemipterus and its reproductive potential on various fungi could also reflect a selective behavior in the choice of oviposition sites. Such a relationship was documented by Schmidt (1935), who observed in his study that Carpophilus humeralis females chose to lay their eggs only when a suitable feeding substrate was supplied to them. That same study found that the nature of the medium was the ultimate factor significantly controlling the oviposition performance of Ca. humeralis during any given period. Ca. hemipterus could similarly be sensitive to the quality of the substrate in its oviposition behavior. This would explain the intriguing fact that this insect chose to deposit more eggs on Ophiostoma sp., while feeding more actively and showing greater reproductive potential on H. moniliformis and H.cryptoformis. In effect, overfeeding on Huntiella cultures depleted these favored treatments of their food provisions. This made them less attractive for oviposition than Ophiostoma cultures where less grazing had occurred. Besides, only Ophiostoma sp. regenerated readily in the plates after insect feeding (data not shown).
It is interesting that, in our experiments, H. moniliformis emerged over H. cryptoformis as a potentially better source of food for Ca. hemipterus, even though the differences between the two fungi were not statistically strong. This highlights the importance of contextual environmental factors in shaping patterns of saproxylic sap beetle fungal associations in nature. These factors may restrict the occurrence of favored fungal partners in certain environments, as appeared to be the case for H. moniliformis in the KNP. This fungus was never detected in our field surveys, in contrast to the highly prevalent H. cryptoformis and its close relatives (Mbenoun et al. 2017). This result echoes two previous studies conducted in South African savanna woodlands (Kamgan et al. 2008; Heath et al. 2009a) in suggesting that the savanna environment exerts a selective exclusion against H. moniliformis, all the more so considering that this fungus has been recorded in other South African ecosystems (Heath et al. 2009b). A similar argument was invoked to explain the differential association of Brachypeplus sap beetles with Huntiella fungal partners in Cameroonian cacao agroforests experiencing disparate environmental and agroecological conditions (Mbenoun et al. 2016).
Overall, the results of this study suggest that the partnerships between saproxylic sap beetles and ophiostomatoid fungi share some commonalities with fungal symbioses of bark and ambrosia beetles. These would include most notably the same foundation on mutual benefit, where the insects gain nutritional advantages and the fungi gain transport and access to ephemeral habitats (Six 2012; Hulcr and Stelinski 2017). This ecological convergence is even more conspicuous in the light of contemporary understanding of beetle-fungus symbioses, as dynamic assemblies of communities, which can change with context and involve multiple associates exchanging diverse services, with variable degrees of dependence and reciprocity (Klepzig and Six 2004; Six and Bentz 2007; Kostovcik et al. 2015). On that account, saproxylic sap beetles are authentic, mutualistic symbionts to ophiostomatoid fungi, notwithstanding the versatility of some of these insects in life history strategy. These partnerships are crucial for the survival of saproxylic sap beetles in the KNP and similar African ecosystems, especially during winter when extensive drought creates a scarcity of alternative food resources (Mbenoun et al. 2017). The perpetuation of such conditions may ultimately lead to a greater specialization of saproxylic sap beetles in their fungal interactions, possibly involving a corollary development of adaptive morphological structures. Bark and ambrosia beetles exemplify that specialization by their mycangia and sophisticated fungus-farming behaviors (Six 2012; Hulcr and Stelinski 2017). Recognising the symbiotic foundation of saproxylic sap beetle fungal associations will hopefully provide additional clues leading to a better understanding of sap beetle transmission of pathogenic ophiostomatoid fungi and enable the development of more effective management strategies for their associated diseases.
Data Availability
All DNA sequences generated in this study are available in the GenBank nucleotide database (accession numbers: KT936271–KT936307); all other datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Ambourn AK, Juzwik J, Moon RD (2002) Seasonal dispersal of the oak wilt fungus by Colopterus truncatus and Carpophilus sayi in Minnesota. Plant Dis 89:1067–1076
Baker CCM, Martins DJ, Pelaez JN, Billen JPJ, Pringle A, Frederickson ME, Pierce NE (2017) Distinctive fungal communities in an obligate African ant-plant mutualism. Proc R Soc B 284:20162501. https://doi.org/10.1098/rspb.2016.2501
Cease KR, Juzwik J (2001) Predominant nitidulid species. Coleoptera: Nitidulidae. Associated with spring oak wilt mats in Minnesota. Can J For Res 31:635–643
Cline AR (2005) Revision of Pocadius Erichson (Coleoptera: Nitidulidae). Dissertation, Louisiana State University
Cline AR, Skelley PE, Kinnee SA, Rooney-Latham S, Winterton SL, Borkent CB, Audisio P (2014) Interactions between a sap beetle, sabal palm, scale insect, filamentous fungi and yeast, with discovery of potential antifungal compounds. PLoS One 9:e89295. https://doi.org/10.1371/journal.pone.0089295
Cragg SM, Beckham GT, Bruce NC, Bugg TD, Distel DL, Dupree P, Etxabe AG, Goodell BS, Jellison J, McGeehan JE, McQueen-Mason SJ, Schnorr K, Walton PH, Watts JEM, Zimmer M (2015) Lignocellulose degradation mechanisms across the tree of life. Curr Opin Chem Biol 29:108–119
de Beer ZW, Seifert KA, Wingfield MJ (2013) The ophiostomatoid fungi: their dual position in the Sordariomycetes. In: Seifert KA, de Beer ZW, Wingfield MJ (eds) The Ophiostomatoid fungi: expanding frontiers. CBS-KNAW Fungal Biodiversity Centre, Utrecht, pp 1–19
de Beer ZW, Duong TA, Barnes I, Wingfield BD, Wingfield MJ (2014) Redefining Ceratocystis and allied genera. Stud Mycol 79:187–219
de Errasti A, de Beer ZW, Rajchenberg M, Coetzee MPA, Wingfield MJ, Roux J (2015) Huntiella decorticans sp. nov. (Ceratocystidaceae) associated with dying Nothofagus in Patagonia. Mycologia 107:512–521
Delalibera I, Handelsman J, Raffa KF (2005) Contrasts in cellulolytic activities of gut microorganisms between the wood borer, Saperda vestita (Coleoptera: Cerambycidae), and the bark beetles, Ips pini and Dendroctonus frontalis (Coleoptera: Curculionidae). Environ Entomol 34:541–547
Dobson RD (1954) The species of Carpophilus Stephens (Col. Nitidulidae) associated with stored products. Bull Entomol Res 45:389–402
Douglas AE (2009) The microbial dimension in insect nutritional ecology. Funct Ecol 23:38–47
Dowd PF (1992) Insect fungal symbionts: a promising source of detoxifying enzymes. J Ind Microbiol 9:149–161
Engl T, Kaltenpoth M (2018) Influence of microbial symbionts on insect pheromones. Nat Prod Rep 35:386–397
Farrell BD, Sequeira AS, O’Meara BC, Normark BB, Chung JH, Jordal BH (2001) The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 55:2011–2027
Freeman S, Protasov A, Sharon M, Mohotti K, Eliyahu M, Okon-Levy N, Maymon M, Mendel Z (2012) Obligate feed requirement of Fusarium sp. nov., an avocado wilting agent, by the ambrosia beetle Euwallacea aff. fornicata. Symbiosis 58:245–251
Geib SM, Filley TR, Hatcher PG, Hoover K, Carlson JE, Jimenez-Gasco MM, Nakagawa-Izumi A, Sleighter RL, Tien M (2008) Lignin degradation in wood-feeding insects. Proc Natl Acad Sci U S A 105:12932–12937
George AP, Nissen RJ, Ironside DA, Anderson P (1989) Effects of nitidulid beetles on pollination and fruit set of Annona spp. Hybrids Sci Hortic 39:289–299
Harrington TC (2005) Ecology and evolution of mycophagous bark beetles and their fungal partners in: Vega FE, Blackwell M (eds) insect-fungal associations: ecology and evolution. Oxford University Press, Oxford, pp 257–291
Harrington TC, Aghayeva DN, Fraedrich SW (2010) New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the redbay beetles Xyleborus glabratus. Mycotaxon 111:337–361
Hayslett M, Juzwik J, Moltzan B (2008) Three Colopterus beetle species carry the oak wilt fungus to fresh wounds on red oak in Missouri. Plant Dis 92:270–275
Heath RN, Wingfield MJ, van Wyk M, Roux J (2009a) Insect associates of Ceratocystis albifundus and patterns of association in a native savanna ecosystem in South Africa. Environ Entomol 38:356–364
Heath RN, Wingfield MJ, Wingfield BD, Meke G, Mbaga A, Roux J (2009b) Ceratocystis species on Acacia mearnsii and Eucalyptus spp. in eastern and southern Africa including six new species. Fungal Divers 34:41–67
Hinds TE (1972) Ceratocystis canker of aspen. Phytopathology 62:213–220
Hossain MS, Williams DG (2003) Phenology of carpophilus beetle populations (Coleoptera: Nitidulidae, Carpophilus spp.) in a fruit dump in northern Victoria. Aust J Exp Agric 43:1275–1279
Hulcr J, Dunn RR (2011) The sudden emergence of pathogenicity in insect-fungus symbioses threatens naive forest ecosystems. Proc R Soc Lond [Biol] 278:2866–2873
Hulcr J, Stelinski LL (2017) The ambrosia symbiosis: from evolutionary ecology to practical management. Annu Rev Entomol 62:285–303
James DG, Faulder RJ, Vogele B, Bartelt RJ, Moore C (1997) Phenology of Carpophilus spp. (Coleoptera: Nitidulidae) in stone fruit orchards as determined by pheromone trapping: implications for prediction of crop damage. Austral J Entomol 36:165–173
Jelinek J, Carlton C, Cline AR, Leschen RAB (2010) Nitidulidae Latreille, 1802. In: Beutel RG, Lawrence JF, Leschen RAB, Ślipiński a (eds) handbook of zoology. Coleoptera, beetles: morphology and systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim), Vol 2, Walter de Gruyter, New York, pp 390–406
Juzwik J (1999) Overland transmission of Ceratocystis fagacearum: extending our understanding. In: Ash CLR (ed) Shade tree wilt diseases. American Phytopathological Society Press, St Paul, pp 83–92
Juzwik J, Skalbeck TC, Newman MF (2004) Sap beetle species (Coleoptera: Nitidulidae) visiting fresh wounds on healthy oaks during spring in Minnesota. For Sci 50:757–764
Kamgan NG, Jacobs K, de Beer ZW, Wingfield MJ, Roux J (2008) Ceratocystis and Ophiostoma species, including three new taxa, associated with wounds on native south African trees. Fungal Divers 29:37–59
Khadempour L, LeMay V, Jack D, Bohlmann J, Breuil C (2012) The relative abundance of mountain pine beetle fungal associates through the beetle life cycle in pine trees. Micob Ecol 64:909–917
Kirejtshuk AG (2003) Subcortical space as an environment for paleoendemic and young groups of beetles, using mostly examples from sap-beetles (Nitidulidae, Coleoptera). In: Bowen PC (ed) Proceedings of the second pan-european conference on saproxylic beetles. People's Trust for Endangered Species, London, pp 50–56
Kirisits T (2007) Fungal associates of european bark beetles with special emphasis on the ophiostomatoid fungi. In: Lieutier F, Day KR, Battisti A, Grégoire JC, Evans HF (eds) Bark and wood boring insects in living trees in Europe, a synthesis. Springer, Dordrecht, pp 181–235
Kirkendall LR, Biedermann PHW, Jordal BH (2015) Evolution and diversity of bark and ambrosia beetles. In: Vega FE, Hofstetter RW (eds) Bark beetles: biology and ecology of native and invasive species. Academic Press, Cambridge, pp 85–156
Klepzig KD, Adams AS, Handelsman J, Raffa KF (2009) Symbioses: a key driver of insect physiological processes, ecological interactions, evolutionary diversification, and impacts on humans. Environ Entomol 38:67–77
Klepzig KD, Six DL (2004) Bark beetle-fungal symbiosis: context dependency in complex associations. Symbiosis 37:189–205
Kostovcik M, Bateman CC, Kolarik M, Stelinski LL, Jordal BH, Hulcr J (2015) The ambrosia symbiosis is specific in some species and promiscuous in others: evidence from community pyrosequencing. ISME J 9:126–138
Lee DH, Roux J, Wingfield BD, Wingfield MJ (2018) A microsatellite-based identification tool used to confirm vector association in a fungal tree pathogen. Australas Plant Path 47:63–69
Lin H, Phelan PL (1992) Comparison of volatiles from beetle-transmitted Ceratocystis fagacearum and four non-insect-dependent fungi. J Chem Ecol 18:1623–1632
Malloch D, Blackwell M (1993) Dispersal biology of the ophiostomatoid fungi. In: Wingfield MJ, Seifert KA, Webber JF (eds) Ceratocystis and Ophiostoma: taxonomy, ecology and pathogenicity. American Phytopathological Society, St Paul, pp 195–206
Martin MM (1983) Cellulose digestion in insects. Comp Biochem Physiol 75A:313–324
Martin MM (1992) The evolution of insect-fungus associations: from contact to stable symbiosis. Amer Zool 32:593–605
Mayers CG, McNew DL, Harrington TC, Roeper RA, Fraedrich SW, Biedermann PH, Castrillo LA, Reed SE (2015) Three genera in the Ceratocystidaceae are the respective symbionts of three independent lineages of ambrosia beetles with large, complex mycangia. Fungal Biol 119:1075–1092
Mbenoun M, Garnas JR, Wingfield MJ, Begoude Boyogueno AD, Roux J (2017) Metacommunity analyses of Ceratocystidaceae fungi across heterogeneous African savanna landscapes. Fungal Ecol 28:76–85
Mbenoun M, Wingfield MJ, Begoude Boyogueno AD, Nsouga Amougou F, Petchayo Tigang S, ten Hoopen GM, Mfegue CV, Dibog L, Nyasse S, Wingfield BD, Roux J (2016) Diversity and pathogenicity of the Ceratocystidaceae associated with cacao agroforests in Cameroon. Plant Pathol 65:64–78
Mbenoun M, Wingfield MJ, Begoude Boyogueno AD, Wingfield BD, Roux J (2014) Molecular phylogenetic analyses reveal three new Ceratocystis species and provide evidence for geographic differentiation of the genus in Africa. Mycol Prog 13:219–240
Mbenoun M, Wingfield MJ, Letsoalo T, Bihon W, Wingfield BD, Roux J (2015) Independent origins and incipient speciation among host-associated populations of Thielaviopsis ethacetica in Cameroon. Fungal Biol 119:957–972
Misse AC, Barnes I, Roets F, Mbenoun M, Wingfield MJ, Roux J (2017) Ecology and population structure of a tree wound-infecting fungus in a native south African forest environment. Fungal Biol 121:69–81
Moller WJ, De Vay JE (1968) Insect transmission of Ceratocystis fimbriata in deciduous fruit orchards. Phytopathology 58:1499–1508
Mueller UG, Gerardo NM, Aanen DK, Six DL, Schultz TR (2005) The evolution of agriculture in insects. Annu Rev Ecol Evol Syst 36:563–595
Nel WJ, Duong TA, Wingfield BD, Wingfield MJ, de Beer ZW (2017) A new genus and species for the globally important, multi-host root pathogen Thielaviopsis basicola. Plant Pathol 67:871–882
Nkuekam GK, Wingfield MJ, Roux J (2013) Ceratocystis species, including two new taxa, from Eucalyptus trees in South Africa. Australas Plant Path 42:283–311
Ploetz RC, Hulcr J, Wingfield MJ, de Beer ZW (2013) Destructive tree diseases associated with ambrosia and bark beetles: black swan events in tree pathology. Plant Dis 95:856–872
Raffa KF, Gregoire JC, Lindgren BS (2015) Natural history and ecology of bark beetles. In: Vega FE, Hofstetter RW (eds) Bark beetles: biology and ecology of native and invasive species. Academic Press, Cambridge, pp 1–40
Rosi MC, Pegna FG, Nencioni A, Guidi R, Bicego M, Belcari A, Sacchetti P (2019) Emigration effects induced by radio frequency treatment to dates infested by Carpophilus hemipterus. Insects 10:273. https://doi.org/10.3390/insects10090273
Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P (1994) Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am 87:651–701
Six DL (2012) Ecological and evolutionary determinants of bark beetle—fungus symbioses. Insects 3:339–366
Six DL (2013) The bark beetle holobiont: why microbes matter. J Chem Ecol 39:989–1002
Six DL, Bentz BJ (2007) Temperature determines symbiont abundance in a multipartite bark beetle-fungus ectosymbiosis. Microbial Ecol 54:112–118
Six DL, Wingfield MJ (2011) The role of phytopathogenicity in bark beetle-fungus symbioses: a challenge to the classic paradigm. Annu Rev Entomol 56:255–272
Schmidt CT (1935) Biological studies on the nitidulid beetles found in pineapple fields. Ann Entomol Soc Am 28:475–451
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57:758–771
Ulyshen MD (2016) Wood decomposition as influenced by invertebrates. Biol Rev 91:70–85
Vega FE, Dowd PF (2005) The role of yeasts as insect endosymbionts. In: Vega FE, Blackwell M (eds) Insect-fungal associations: ecology and evolution. Oxford University Press, Oxford, pp 211–243
Wingfield MJ, Seifert KA, Webber JF (1993) Ceratocystis and Ophiostoma: taxonomy, ecology and pathogenicity. APS Press, St Paul
Wobbrock JO, Findlater L, Gergle D, Higgins JJ (2011) The aligned rank transform for nonparametric factorial analyses using only ANOVA procedures. In: Proceedings of the CHI 2011 conference on human factors in computing systems. ACM Press, New York, pp. 143–146
Zhang J, Kapli P, Pavlidis P, Stamatakis A (2013) A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29:2869–2876
Acknowledgments
We thank Dr. Eston Mutitu for valuable advice in setting up laboratory rearing and feeding choice experiments with sap beetles. We thank the students at FABI who kindly provided additional insects and fungal cultures used in this study. We are also grateful to the two anonymous reviewers for their insightful comments and suggestions that helped improve the scientific quality of the submitted manuscript. This work is based on research undertaken as part of the first author’s postdoctoral fellowship at the University of Pretoria, with the financial support of the DST/NRF Centre of Excellence in Tree Health Biotechnology (CTHB) of South Africa.
Funding
This study was financially supported by the DST/NRF Center of Excellence in Tree Health Biotechnology (CTHB) of South Africa.
Author information
Authors and Affiliations
Contributions
Conceptualization: Michael Mbenoun, Jolanda Roux and Michael J. Wingfield; Methodology, investigation and formal analysis: Michael Mbenoun and Alain C. Misse; Writing - original draft preparation: Michael Mbenoun; Writing – review: Michael Mbenoun, Jolanda Roux and Michael J. Wingfield. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 98 kb)
Rights and permissions
About this article
Cite this article
Mbenoun, M., Wingfield, M.J., Misse, A.C. et al. Selective feeding behaviors illuminate patterns of sap beetle associations with ophiostomatoid fungi. Symbiosis 81, 287–302 (2020). https://doi.org/10.1007/s13199-020-00705-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-020-00705-9