Abstract
Trophic exchanges constitute the bases of the symbiosis between the nitrogen-fixing actinomycete Frankia and its host plant Alnus, but the identity of the compounds exchanged is still poorly known. In the current work, previously published transcriptomic studies of Alnus nodules and of symbiotic Frankia were reexamined for TCA cycle related genes. The bacterial TCA enzyme genes were all upregulated, especially the succinyl-CoA synthase and the citrate synthase while on the plant side, none was significantly modified in nodules relative to non-inoculated roots. A preliminary metabolomics approach permitted to see that citrate, 2-oxoglutarate, succinate, malate and fumarate were all more abundant (FC (Fold change) = 5–70) in mature nitrogen-fixing nodules than in roots. In the evaluation of the uptake and metabolism of these organic acids, a significant change was observed in the morphology of nitrogen fixing vesicles in vitro: the dicarboxylates malate, succinate and fumarate induced the formation of larger vesicles than was the case with propionate. Moreover, the production of spores was also modified depending on the organic acid present. The assays showed that most C4 dicarboxylates were taken up while C6 tricarboxylates were not and citrate even partially blocked catabolism of reserve carbon. Tests were performed to determine if the change in membrane permeability induced by Ag5, a peptide previously shown to modify the membranes of Frankia, increased the uptake of specific organic acids. No effect was observed with citrate while an increase in nitrogen fixation was seen with propionate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Frankia is a nitrogen-fixing actinobacterium which establishes a nitrogen-for-carbon based root symbiosis with a diverse group of dicotyledonous plants collectively called actinorhizal. Its phylogeny has recently been reassessed using complete genomes and found to be at a basal position in the aerobic actinobacteria (Sen et al. 2014), evocative of a very ancient emergence. Although trophic exchanges are known to occur between the partners, the identity of the compounds that are exchanged is still unknown. A Frankia strain, CpI1, able to reinfect its hosts (Comptonia and Alnus) was first isolated in 1978 using a complex medium with mannitol, sucrose as well as yeast extract as carbon sources (Callaham et al. 1978). This strain was the first of several hundreds of isolates that were later obtained from a variety of host plants and biotopes and characterized for their biochemical abilities. The first system of classification proposed by Lechevalier (1984) stated that strains designated type-A isolated from Elaeagnaceae were able to grow on a large array of carbon sources (lipids, organic acids, sugars) while others designated type-B isolated from Alnus (Betulaceae) and Comptonia (Myricaceae) were only able to grow on a small number of carbon sources, predominant among which were organic acids. We now know most Alnus-infective strains form a cluster of related strains (Normand et al. 1996) that should be soon separated into species with minor physiological differences.
A study done on isolated vesicles clusters metabolic activities, has pointed to dicarboxylates, among them malate, as the compounds that could be provided as carbon sources to symbiotic Frankia (Huss-Danell 1997). A plant dicarboxylate transporter, AgDCAT1, has been shown to be specifically expressed in A. glutinosa nodules and to be localized in infected cells (Jeong et al. 2004). This transporter, which was immunolocalized to the perisymbiont membrane surrounding the nitrogen-fixing vesicles of the microsymbiont, was shown, when expressed in a dicarboxylate uptake-deficient Escherichia coli mutant to be able to transport several TCA intermediates, among them succinate, fumarate, malate and oxaloacetate (Jeong et al. 2004).
In vitro, some Alnus-infective Frankia strains have been shown able to grow on a range of organic acids comprising the monocarboxylates propionate, pyruvate, acetate and the TCA dicarboxylates succinate and malate (Murry et al. 1984), as well as on some lipids such as polysorbate 80 (Blom et al. 1980) but not on glucose (Stowers et al. 1986), or on complex sources such as pectin, or cellulose despite the presence of an extracellular cellulase (Igual et al. 2001). The spectrum of carbon sources used was shown to vary between Alnus-infective strains, with CpI1 able to grow well on succinate and fumarate while ACN1AG grew poorly on these (Tisa et al. 1983).
Frankia cells comprise three different types of structures. Besides hyphae, there are very large multilocular sporangia, produced in most strains only outside nodules in vitro, these can comprise hundreds of spores, which have the classic function of survival under unfavourable conditions. Several carbon sources (propionate, acetate, or trehalose) were shown to induce the formation of spores in some strains, in particular in ACN1AG, while succinate was shown to repress it (Tisa et al. 1983). Frankia also forms vesicles surrounded by several layers of hopanoid lipids that provide a barrier against oxygen to protect nitrogenase under aerobic conditions (Berry et al. 1993) while nitrogen fixation occurs in vesicles over a range of oxygen concentrations (Harris and Silvester 1992). The plant plays a pivotal role in shaping these vesicles since Frankia alni infects alders forming spherical vesicles and also infects bayberry (Morella cerifera) or sweetfern (Comptonia peregrina) where it forms club-shaped vesicles (Lalonde 1979). These vesicles are the target of some Alnus-produced cysteine rich plant defensins that modify their porosity and cause them in particular to release some amino acids such as glutamate and glutamine (Carro et al. 2015).
The TCA cycle is central to the physiology of Frankia as the bacterial genes for all the enzymes involved are up-regulated in symbiosis (Alloisio et al. 2010) showing its importance in nodules to provide energy to the bacterium for the energy-costly nitrogen fixation and for rapid cellular growth in host tissues. Besides energy, the TCA also provides carbon skeletons for some reactions. In particular, 2-oxoglutarate is essential for the assimilation of ammonium through the GS-GOGAT pathway. However, this function is thought to be exerted for the most part by the host plant, since plant GS is induced strongly in infected cells (Guan et al. 1996). Oxaloacetate may also be used by the aspartate transaminase and aspartate-fed Frankia has been shown to use this pathway (Zhang and Benson 1992) which would decrease the level of oxaloacetate and increase that of 2-oxoglutarate.
We undertook the present study to try to identify the organic acids present in nodules and to determine which ones could be used in vitro by Frankia alni strain ACN14a for growth and respiration. We also reexamined the transcriptome data of the plant (Hocher et al. 2011) and of the microbe (Alloisio et al. 2010) to analyse the expression levels of genes encoding TCA enzymes.
2 Materials and methods
2.1 Biological material and growth conditions
Frankia alni ACN14a (Normand and Lalonde 1982) was grown in liquid BAP medium without ammonium (BAP-) according to Alloisio et al. (2010). Seven day old cultures were used for in vitro analyses. Other carbon sources were tested using the same medium without sodium propionate, and adding the studied compound at a concentration of 5 mM (propionate, pyruvate, acetate, citrate, cis-aconitate, isocitrate, 2-oxoglutarate, succinate, fumarate, malate, oxaloacetate) with two induction steps where the compound to be studied was added at a final concentration of 5 mM on top of the 5 mM of propionate present in the BAP− medium. Besides this assay with individual carbon sources, a carbon mix containing 0.625 mM of each (citrate, fumarate, isocitrate, malate, oxaloacetate, 2-oxoglutarate, propionate and succinate) was also evaluated. OD600 measurements were performed on homogenized Frankia cultures before inoculation in order to follow the growth changes over the course of the experiment (3, 7, 10 and 14 days). Homogenization was achieved by syringing three times the Frankia cells through four needles of diminishing sizes (21G, 23G, 25G, 27G). These cultures were also used for the analysis of vesicles. All cultures in this work were grown at 28 °C with shaking and at a pH of 6.3.
Nodules of Alnus glutinosa were obtained from hydroponic cultures 56 days after inoculation with Frankia alni ACN14a. Nodule sections for vesicles observation were prepared as described before (Carro et al. 2015).
2.2 EST database and microarray expression analysis
A previously reported database of A. glutinosa ESTs and the microarray analysis of these transcripts (Hocher et al. 2011) which are available in the Gene Expression Omnibus database (www.ncbi.nlm.nih.gov/geo; accession number GSE24153) were analyzed. An extensive iterative BLAST search was made in order to find ESTs encoding TCA enzyme genes. Differential expression of all recovered genes was determined using the microarray dataset, according to Hocher et al. (2011). Briefly, a Student’s t-test was used to compare 21 dpi (days post-inoculation) nodules versus non-inoculated roots and average fold changes were calculated. Three biological replicates were analyzed for each condition.
All expression values for genes encoding TCA enzymes in alder were extracted and the 21dpi values expressed as the ratios of nodule/root values.
2.3 Frankia alni transcriptome analysis
F. alni transcriptomic data using microarrays from a previous study (Alloisio et al. 2010) are available in the Gene Expression Omnibus database (www.ncbi.nlm.nih.gov/geo; accession number GSE18190) with data from N-replete free-living cells (FLC), N-fixing FLC (N-fixFLC) and from Frankia cells from Alnus glutinosa nodules (Nod). These data were analyzed with a particular focus on genes encoding enzymes involved in carbon metabolism. Average fold changes were calculated and differential expression between Nod vs FLC or N-fixFLC vs FLC (three biological replicates per condition) were considered as significant based on the following 3 criteria: a P-value ≤ 0.05 using the Welch t-test with the multiple testing correction of Benjamini and Hochberg (1995), a fold-change ≥ 1.8 in all nine pairwise comparisons yielded by the three biological replicates of each conditions (3×3) and an average fold-change ≥ 2 (Alloisio et al. 2010).
2.4 Production of the Ag5 peptide
The mature Ag5 peptide (Carro et al. 2015) which is the peptide without the signal peptide as predicted by SignalP (http://www.cbs.dtu.dk/services/SignalP/), was synthesized by Proteogenix (Schiltigheim, France). This chemically synthesized peptide was purified by HPLC to a 96 % level and used at a 1 μM concentration.
2.5 Nitrogenase activity
Determination of nitrogenase activity of Frankia cultures was performed using the acetylene reduction assay with an acetylene concentration of 10 % vol/vol in 10 mL vials containing 1 ml of medium. Values were analyzed after different incubation times (3, 7, 10 and 14 days) in the presence of the different organic acids (propionate, pyruvate, acetate, citrate, cis-aconitate, isocitrate, 2-oxoglutarate, succinate, fumarate, malate, oxaloacetate, and carbon mix) in the growth medium at the concentrations given above. Analyses were done using a gas chromatograph (Girdel series 30, Suresne, France) equipped with a J&W PORA-Plot Q (Agilent, Santa Clara, CA) column at a H2 pressure of 1.2 bars, a dinitrogen pressure of 2.6 bars and an air pressure of 2.7 bars, a T° of 45 °C and with a flame ionization detector. Cultures of 3, 7, 10 and 14 days were incubated for three more days in the presence of acetylene (five biological replicates) to let the nitrogenase recover and stabilize after an initial decline (Tjepkema et al. 1988).
2.6 Respiration analyses, microscopy
To estimate the metabolically active biomass, respiration was measured by the tetrazolium/formazan INT (iodo-nitro-tetrazolium) assay (Prin et al. 1990) on cells grown in 1 mL vials with the OD490 measured. For light microscopy, samples were observed using 10× and 100× objectives on a Zeiss Axioskop (Zeiss, Jena, Germany) microscope. For measurements of vesicles diameter, photos of 100× INT-stained cells were scanned and an average of ≥24 vesicles diameters were computed.
Nodule sections were made as described before on 56 dpi nodules (Carro et al. 2015). The diameter of 50 vesicles was measured on photos.
Student’s t-test implemented in Excel (Microsoft, Redmond, WA) was used to compare diameters of Frankia cells following different treatments.
2.7 GS-MS metabolites analysis
Roots and 21 dpi nodules were harvested from hydroponics-grown plants and eight samples (of 10 mg each) were placed in Eppendorf tubes, frozen and ground in liquid nitrogen, kept at −80 °C and shipped to Umeå University (http://www.upsc.se/methodology.html) for further treatment. One ml of chloroform:methanol:H2O (20:60:20) mixture including internal standards + mixer beads was added, shaken for 3 min in mixer (30 Hz), and centrifuged 10 min at 14 000 g.
Derivatisation was done using methoxyamine (CH3-O-NH2) in pyridine to stabilize carbonyl moieties in the metabolites, thereby suppressing keto-enol tautomerism and the formation of multiple acetal- or ketal-structures. For methoxiamination, 30 μL of 15 mg/mL methoxyamine hydrochloride in dry pyridine is used at room temperature for 16 h. After methoxyamination, functional groups, such as -OH, −COOH, −SH or -NH groups, were converted into TMS-ethers, TMS-esters, TMS-sulfides or TMS-amines, respectively, using a trimethylsilyl (TMS) based-reagent BSTFA by the addition of 30 μL of N-methyl-N-trimethylsilyl-trifluoroacetamide (MSTFA + 1 % TMCS) for 1 h at room temperature.
One μL of the derivatized sample was injected splitless by an Agilent 7683 autosampler into an Agilent 6890 gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a 15 m x 0.18 mm i.d. fused silica capillary column with a chemically bonded 0.18 μm DB 5-MS stationary phase (J&W Scientific, Agilent). The injector temperature was 270 °C, the purge flow-rate was 20 ml.min−1 and the purge was turned on after 60 s. The gas flow rate through the column was 1 ml.min−1, the column temperature was held at 70 °C for 2 min, then increased by 40 °C min−1 to 320 °C, and held there for 1 min. The column effluent was introduced into the ion source of a Pegasus III time-of-flight mass spectrometer, GC/TOFMS (Leco Corp., St Joseph, MI, USA). The transfer line and the ion source temperatures were 250 °C and 200 °C, respectively. Ions were generated by a 70 eV electron beam at an ionization current of 2.0 mA, and 30 (15–30) spectra.s−1 are recorded in the mass range 60 to 800 m/z. The acceleration voltage was turned on after a solvent delay of 170 s. The detector voltage was 1500–1700 V. The values were expressed in arbitrary units. Statistical analyses were made using Excel.
3 Results
3.1 In vitro growth of Frankia alni ACN14a using TCA cycle intermediates and other acids as carbon sources
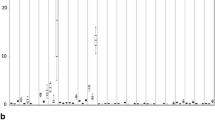
F. alni ACN14a is able to grow well in vitro with as carbon source propionate, acetate, succinate, fumarate and malate, and very poorly if at all with citrate, cis-aconitate, isocitrate, 2-oxoglutarate, oxaloacetate and pyruvate (Fig. 1). However, the growth dynamics were different with some of those carbon sources with an early stationary phase onset on propionate and acetate whereas the linear growth phase was maintained longer on malate, fumarate and succinate. Growth on isocitrate, oxoglutarate, or oxaloacetate was similar to that on medium without carbon. Growth did not occur on cis-aconitate or citrate, and the OD, respiration and ARA values were much below those on medium without carbon indicating inhibition of cells metabolism and possibly some cell lysis. When a mixture of different TCA cycle intermediates was provided, growth was inhibited at a level similar to that when citrate was the sole carbon source.
Evaluation of Frankia alni ACN14a development with different carbon sources in vitro. Growth was evaluated by OD600, capacity of nitrogen fixation in nmol C2H4/ml/OD600/day and respiration ability in nmol INTF/ml/OD600/day. Acetylene was added after 3, 7, 10, 14 days of growth and left for 72 h before measuring the ethylene produced and measuring the OD600 and then the INT.
To find out if growth was correlated with nitrogen fixation, the acetylene reduction assay (ARA) was performed on these cultures (Fig. 1). Evaluation of these data showed that the highest acetylene reduction values after 3 days of growth were obtained for propionate and acetate while after 14 days of growth the highest values were found with malate, fumarate and succinate.
Respiratory activity of cells was determined with the iodo-nitro-tetrazolium method, in which iodo-nitro-tetrazolium-formazan is formed when the respiration cell process occurs. The highest respiratory activity at 3 to 14 days was found during growth on succinate and propionate (Fig. 1). Respiration on pyruvate, 2-oxoglutarate and isocitrate at 3d was similar to that in medium without added carbon. However respiration fell at 7d without carbon presumably after reserves were consumed whereas it remained more stable on 2-oxoglutarate and isocitrate indicating very slow metabolism. Respiration on citrate was below the level of detection, similar to controls without cells.
The addition of the antimicrobial peptide Ag5 ASUP, did not change any of these activities when citrate or pyruvate were used as carbon source (Fig. 1). Preliminary test on several compounds in particular aspartate, 2-oxoglutarate and malate gave results similar to those with citrate (Fig. 2). The addition of Ag5 to propionate-grown cells increased all of these activities.
3.2 Effects of TCA on vesicles morphology and sporulation
The vesicle morphology was analyzed after several weeks of growth in the different media tested. Some carbon sources caused a significant change in the morphology of nitrogen-fixing vesicles in vitro, with a vesicle size in dicarboxylate-grown cells intermediate between that seen in acetate- or propionate-grown cells and those formed inside infected A. glutinosa nodule cells (Figs. 3 and 4).
After 3 days of growth, differences in vesicle size between Frankia cells grown on the different carbon sources tested were still minor. However marked differences in respiration ability could be observed between cells grown on the different carbon sources with more intense staining for succinate and propionate-grown cells (Fig. 5). Differences in size were observed after 7d of growth and these were maintained after 10d (Table 1). The size of the vesicles with succinate, fumarate and malate were 3.4–3.9 μm in diameter, smaller than that in nodules (5.2 μm) but significantly above the size of propionate or acetate-grown cells (2.3–2.9 μm). When expressed as volumes the difference is even more striking with 7–9 μm3 in acetate-grown cells as compared to 24–30 μm3 in succinate/fumarate/malate-grown cells and 75 μm3 in nodules.
Spore production was also significantly different between cultures grown on the different carbon sources for 7 days (Table 2 and Fig. 6). Propionate, pyruvate and 2-oxoglutarate medium yielded almost no sporulation, malate and isocitrate showed some small sporangia, while fumarate and oxaloacetate yielded numerous big sporangia.
3.3 Analysis of the expression levels of A. glutinosa TCA cycle genes in nodules vs roots: transcriptomic analysis
Symbiotic interactions with Frankia alni do not induce in nodules of its host Alnus glutinosa significant expression changes in 21 analyzed TCA related genes (Table 3). However gene expression could not be investigated in 5 other TCA related genes, due to incorrect probe sequences (Table 3). In addition, no gene coding for fumarase was found among our previously reported A. glutinosa ESTs (Hocher et al. 2011).
3.4 Analysis of the expression levels of Frankia TCA cycle genes in nodules vs in vitro: transcriptomic analysis
Conversely, the 13 Frankia TCA related genes were up-regulated in Alnus glutinosa nodules vs N-replete free-living (FL) cells (Table 4). Three of them, the succinyl-CoA synthetase alpha and beta subunits (FRAAL1156,1157) and the citrate synthase (FRAAL0111) were also among the 53 most up-regulated genes observed in nodule transcriptome vs N-replete FL cells (Alloisio et al. 2010). Up-regulation of TCA related genes was also seen in N-fixing FL cells vs N-replete FL cells but to a lower extent (Table 4).
3.5 Growth of ACN14a with Ag5 peptide: no influence on the uptake of carboxylates
Citrate and pyruvate provided as carbon sources in BAP medium resulted in little or no growth of F. alni ACN14a cells. Addition of Ag5 ASUP (Alnus glutinosa upregulated peptide) at a 1 μM concentration, previously reported to be the optimal concentration for nitrogen fixation (ARA test) and respiration (INT test) (Carro et al. 2015), to citrate- or pyruvate-containing medium did not lead to significant changes. As the Ag5 peptide interferes with the normal development of Frankia cells, their ability to fix atmospheric nitrogen and to respire were followed over time (Fig. 1). No differences could be detected between cultures with and without Ag5 when citrate or pyruvate were used as carbon source, therefore Ag5 did not improve growth, respiration or nitrogen fixation on these compounds. Conversely, an increase at 3d was seen in both ARA (25 %) and OD (7 %) of propionate-grown cells, this increase was not sustained beyond 3d and disappeared at 7d.
3.6 GC-MS analysis of TCA cycle intermediates in roots and nodules
There were five TCA cycle metabolites that could be identified in the roots and nodules of Alnus glutinosa analyzed. These were citrate, succinate, 2-oxoglutarate, fumarate and malate (Table 5). The most abundant among these was by far citrate in both roots and nodules. The concentration of citrate in nodules was increased 70fold over that in roots, while all others were increased by a factor ranging from 4 to 14. The other TCA intermediates (cis-aconitate, isocitrate, oxaloacetate) were present at significantly lower levels, below the detection level under the conditions used.
4 Discussion
Control of the microsymbiont is a key element of symbiotic interactions. The plant needs to prevent microbial cells proliferation outside dedicated organs and induce cell differentiation, while ensuring a two-way flow of metabolites that are the bases of trophic symbioses. Several control strategies have been uncovered, including synthesis of NCR peptides by Medicago truncatula that induce bacteroid formation in Sinorhizobium (Van de Velde et al. 2010), synthesis by Alnus glutinosa of defensins that modify the porosity of vesicles in Frankia (Carro et al. 2015), or the synthesis of tannins by Casuarina postulated to prevent Frankia from invading the stele (Laplaze et al. 1999). Control can also be metabolic as in Pisum sativum that controls the bacteria, imposing sanctions such as the limitation of resources levelled against inefficient nitrogen-fixing Rhizobium (Oono et al. 2011).
Glucose is used by many bacteria as carbon source including several Frankia strains; however, Alnus-infective Frankia strains are unable to grow on sugars as sole carbon source (Lechevalier 1984), presumably because they lack the corresponding transporters. When the genome of Frankia alni ACN14a was examined, all necessary enzymes to metabolize glucose were found to be present, as expected since gluconeogenesis that is essential when growth is on organic acids will replenish the glucose necessary for synthesis of wall peptidoglycan and of various hexoses and pentoses (Mort et al. 1983). The same situation is true for TCA and related organic acids that are all metabolizable by the bacterial energy machinery. The limiting step would thus appear to be the importation inside cells of these substrates and thus the presence in the genome of the appropriate transporters.
TCA transporters vary a lot between organisms for range, efficiency, and affinity. The genome of Frankia ACN14a contains several transporters that could internalize TCA intermediates; the best candidate may be FRAAL1390 which is labelled DctA (Dicarboxylate symporter) and would transport dicarboxylates transporter (succinate, malate, fumarate) but it is not up-regulated in symbiosis (Alloisio et al. 2010) relative to propionate grown cells as expected if the plant mainly provides the microbe with dicarboxylates. There are only a few monocarboxylate transporters described for actinobacteria in the literature, one is a homolog of FRAAL4816 labelled MctC (monocarboxylates transporter) in Corynebacterium glutamicum (Jolkver et al. 2009). It should transport acetate and propionate. However, the amino acid sequence similarity between MctC and FRAAL4816 is low (42 %) and the Frankia gene is down-regulated, though not significantly so, in symbiosis compared to in vitro growth on propionate. There are even recently described transporters that transport both mono- and dicarboxylates such as SATP (Succinate-Acetate Transporter Protein) in Escherichia coli (Sá-Pessoa et al. 2013). There are even cases where a transporter mutates to acquire the ability to transport a given compound (Reynolds and Silver 1983). A definite statement as to the specificity of the Frankia transporter(s) thus cannot be made.
The expression of all TCA cycle bacterial genes was markedly up-regulated in nodules vs FLC, especially those of the succinyl-CoA synthase and the citrate synthase, which is consistent with the proteome analysis of symbiotic Frankia performed by Mastronunzio and Benson (2010). This result is likely related to the high energy requirement of nitrogenase since up-regulation was also found in N-fixFLC vs FLC. Thus transcriptomic data do not provide clues to understand carbohydrate compound exchange between Alnus and Frankia.
Conversely, the expression of genes encoding plant TCA cycle enzymes was not significantly modified in nodules relative to non-inoculated roots, suggesting there is no marked increase in metabolic activity. However, there is a large abundance of citrate in nodules, a compound that F. alni ACN14a is not able to use or incorporate directly from the media with or without ASUPs. Moreover, this compound has been shown to inhibit bacterial growth (Korithoski et al. 2005; Watanabe et al. 2011), maybe through chelation of divalent cations. Most bacteria cannot use it as source of energy because they lack the enzymes required to transport it into the cell, even though it is often added as an iron-chelator in growth media. The abundance of citrate and the lower levels of the other TCA cycle intermediates may also indicate that there is compartmentalization in host cells, since only metabolites from the cytosol can be fed to symbiotic Frankia. Alternatively, it might mean that citrate has other functions, for instance it has been shown to accumulate in plant tissues as a response to different kinds of stress (de Vos et al. 1986; López-Bucio et al. 2000), and the nodule infection process could also be considered as stress-inducing for the plant, given the identification of superoxide dismutase proteins in actinorhizal nodules (Mastronunzio and Benson 2010). The accumulation of citrate in the cytosol has also been shown to be important in plant cells to maintain pH homeostasis during NO3- reduction (López-Bucio et al. 2000).
The idea of higher permeability of symbiotic Frankia vesicles for some TCA compounds was proposed more than 30 years ago (Akkermans et al. 1981). However, even though the Ag5 peptide has been shown to modify vesicle membranes (Carro et al. 2015) this ASUP was not capable of increasing the acquisition of citrate or pyruvate by Frankia cells. Ag5 however was found to increase nitrogen fixation (ARA) in propionate-fed cells but did not increase respiration (Carro et al. 2015), suggesting a specific permeabilization of Frankia cells for some compound. Vikman (1992) showed an increase in the respiration of Frankia symbiotic vesicles in comparison to vesicles formed in culture, when malate or glutamate where used as carbon sources, which may be linked to the particular mix of ASUPs present in nodules.
Another singularity observed in Frankia alni ACN14a cultures grown with succinate as carbon source, as well as in malate and fumarate fed cultures is the morphology of vesicles, whose size is significantly increased compared to the smaller ones observed in propionate-fed cultures. When symbiotic vesicles from Alnus nodules were observed under the microscope, their sizes were similar to those of 7 day old succinate, malate or fumarate-fed N2-fixing in vitro cultures. Morphological control by the host is a general feature of symbioses as seen in Medicago truncatula that causes bacteroids formation in Sinorhizobium meliloti (Van de Velde et al. 2010). Many undocumented changes do occur within endosymbionts in symbiosis and size is not the only difference between in vitro and symbiotic vesicles, septation also constituting a major difference (Torrey and Callaham 1982).
There are not many studies that have mentioned the sporulation process of Frankia in vitro in relation to the carbon source present. Symbiosis is also a situation where F. alni, that can sporulate in vitro, is completely inhibited although the physiological bases for this inhibition are unknown. In the course of this study, several differences in sporulation have been seen when different TCA intermediates were fed to F. alni. Contrary to what was expected, some cultures with abundant sporulation were not dormant but had high values for respiration, nitrogen fixation and growth (i.e. fumarate). In the case of propionate, the inhibition of sporulation could be related to long-term adaptation of this bacterium through repeated sub-culturing under laboratory conditions with BAP medium that would have thus selected actively growing hyphae rather than spores.
Step-wise improvements in Frankia growth conditions should help obtain larger biomass, which in turn should facilitate approaches such as genetic transformation or isolation of recalcitrant strains. Improved growth conditions should also help understand the physiological bases of the symbiosis. The use of ferric citrate, a strong growth inhibitor, in BAP medium is thus to be avoided. Sporulation and formation of vesicles can also be modulated through the compounds fed to Frankia. Whether these metabolites are the means through which the plant controls growth and cellular differentiation of its symbiont remains to be determined.
References
Akkermans ADL, Huss-Danell K, Roelofsen W (1981) Enzymes of the tricarboxylic acid cycle and the malate-aspartate shuttle in the N2-fixing endophyte of Alnus glutinosa. Physiol Plant 53:289–294. doi:10.1111/j.1399-3054.1981.tb04502.x
Alloisio N et al (2010) The Frankia alni symbiotic transcriptome. Mol Plant Microbe Interact 23:593–607. doi:10.1094/MPMI-23-5-0593
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B 57:289–300
Berry AM, Harriott OT, Moreau RA, Osman SF, Benson DR, Jones AD (1993) Hopanoid lipids compose the Frankia vesicle envelope, presumptive barrier of oxygen diffusion to nitrogenase PNAS 90:6091–6094
Blom J, Roelofsen W, Akkermans A (1980) Growth of Frankia AvcI1 on media containing tween 80 as C-source. FEMS Microbiol Lett 9:131–135
Callaham D, Del Tredici P, Torrey JG (1978) Isolation and cultivation in vitro of the actinomycete causing root nodulation in Comptonia. Science 199:899–902
Carro L et al (2015) Alnus peptides modify membrane porosity and induce the release of nitrogen-rich metabolites from nitrogen-fixing Frankia. ISME J. doi:10.1038/ismej.2014.257
de Vos CR, Lubberding HJ, Bienfait HF (1986) Rhizosphere acidification as a response to iron deficiency in bean plants. Plant Physiol 81:842–846. doi:10.1104/pp.81.3.842
Guan C, Ribeiro A, Akkermans AD, Jing Y, van Kammen A, Bisseling T, Pawlowski K (1996) Nitrogen metabolism in actinorhizal nodules of Alnus glutinosa: expression of glutamine synthetase and acetylornithine transaminase. Plant Mol Biol 32:1177–1184
Harris S, Silvester W (1992) Oxygen controls the development of Frankia vesicles in continuous culture. New Phytol 121:43–48
Hocher V et al (2011) Transcriptomics of actinorhizal symbioses reveals homologs of the whole common symbiotic signaling cascade. Plant Physiol 156:700–711. doi:10.1104/pp.111.174151
Huss-Danell K (1997) Actinorhizal symbioses and their N2 fixation. New Phytol 136:375–405. doi:10.1046/j.1469-8137.1997.00755.x
Igual JM, Velazquez E, Mateos PF, Rodrıguez-Barrueco C, Cervantes E, Martınez-Molina E (2001) Cellulase isoenzyme profiles in Frankia strains belonging to different cross-inoculation groups. Plant Soil 229:35–39
Jeong J et al (2004) A nodule-specific dicarboxylate transporter from alder is a member of the peptide transporter family. Plant Physiol 134:969–978
Jolkver E, Emer D, Ballan S, Kramer R, Eikmanns BJ, Marin K (2009) Identification and characterization of a bacterial transport system for the uptake of pyruvate, propionate, and acetate in Corynebacterium glutamicum. J Bacteriol 191:940–948. doi:10.1128/JB.01155-08
Korithoski B, Krastel K, Cvitkovitch DG (2005) Transport and metabolism of citrate by Streptococcus mutans. J Bacteriol 187:4451–4456
Lalonde M (1979) Immunological and ultrastructural demonstration of nodulation of the European Alnus glutinosa (L.) Gaertn. host plant by an actinomycetal isolate from the North American Comptonia peregrina (L.) Coult. root nodule. Bot Gaz 140(S):S35–S43
Laplaze L et al (1999) Flavan-containing cells delimit Frankia-infected compartments in Casuarina glauca nodules. Plant Physiol 121:113–122
Lechevalier M (1984) The taxonomy of the genus Frankia. Plant Soil 78:1–6
López-Bucio J, Nieto-Jacobo MF, Ramírez-Rodríguez V, Herrera-Estrella L (2000) Organic acid metabolism in plants: from adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci 160:1–13
Mastronunzio J, Benson D (2010) Wild nodules can be broken: proteomics of Frankia in field-collected root nodules. Symbiosis 50:13–26. doi:10.1007/s13199-009-0030-1
Mort A, Normand P, Lalonde M (1983) 2-O-methyl-D-mannose, a key sugar in the taxonomy of Frankia. Can J Microbiol 29:993–1002
Murry M, Fontaine M, Torrey J (1984) Growth kinetics and nitrogenase induction in Frankia sp. HFPArI3 grown in batch culture. Plant Soil 78:61–78
Normand P, Lalonde M (1982) Evaluation of Frankia strains isolated from provenances of two Alnus species. Can J Microbiol 28:1133–1142
Normand P, Orso S, Cournoyer B, Jeannin P, Chapelon C, Dawson J, Evtushenko L, Misra AK (1996) Molecular phylogeny of the genus Frankia and related genera and emendation of the family frankiaceae. IJSEM 46(1):1–9
Oono R, Anderson CG, Ford Denison R (2011) Failure to fix nitrogen by non-reproductive symbiotic rhizobia triggers host sanctions that reduce fitness of their reproductive clonemates. Proc Biol Sci 278:2698–2703
Prin Y, Neyra M, Diem H (1990) Estimation of Frankia growth using Bradford protein and INT reduction activity estimations: application to inoculum standardization. FEMS Microbiol Lett 69:91–96
Reynolds CH, Silver S (1983) Citrate utilization by Escherichia coli: plasmid- and chromosome-encoded systems. J Bacteriol 156:1019–1024
Sá-Pessoa J, Paiva S, Ribas D, Silva IJ, Viegas SC, Arraiano CM, Casal M (2013) SATP (YaaH), a succinate-acetate transporter protein in Escherichia coli. Biochem J 454:585–95
Sen A, Daubin V, Abrouk D, Gifford I, Berry AM, Normand P (2014) Phylogeny of the class Actinobacteria revisited in the light of complete genomes. The orders ‘Frankiales’ and Micrococcales should be split into coherent entities: proposal of Frankiales ord. nov., Geodermatophilales ord. nov., Acidothermales ord. nov. and Nakamurellales ord. Int J Syst Evol Microbiol 64:3821–3832. doi:10.1099/ijs.0.063966-0
Stowers M, Kulkarni R, Steele D (1986) Intermediary carbon metabolism in Frankia. Arch Microbiol 143:319–324
Tisa L, McBride M, Ensign JC (1983) Studies on the growth and morphology of Frankia strains EAN1pec, EuI1c, and ACN1AG. Can J Bot 61:2768–2773
Tjepkema JD, Schwintzer CR, Monz CA, (1988). Time course of acetylene reduction in nodules of five actinorhizal genera. Plant physiol 86(2):581–58
Torrey JG, Callaham D (1982) Structural features of the vesicle of Frankia sp. CpI1 in culture. Can J Microbiol 28:749–757. doi:10.1139/m82-114
Van de Velde W et al (2010) Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 327:1122–1126
Vikman P-Å (1992) The symbiotic vesicle is a major site for respiration in Frankia from Alnus incana root nodules. Can J Microbiol 38:779–784. doi:10.1139/m92-127
Watanabe R, Hojo K, Nagaoka S, Kimura K, Ohshima T, Maeda N (2011) Antibacterial activity of sodium citrate against oral bacteria isolated from human tongue dorsum. J Oral Biosci 53:87–92
Zhang X, Benson D (1992) Utilization of amino acids by Frankia sp. strain CpI1. Arch Microbiol 158:256–261
Acknowledgments
We acknowledge grants from French ANR (BugsInACell ANR-13-BSV7-0013-03), from the FR BioEnvironment and Health (Lyon) and a MEC postdoctoral fellowship from the Spanish government to LC (Programa Nacional de Movilidad de Recursos Humanos del Plan Nacional de I-D + i 2008–2011). We thank AME, PGE and DTAMB platforms for measurements. TP and KP acknowledge a grant from the Swedish research council FORMAS (229-2005-679) and support by the Carl Tryggers Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Lorena Carro, Tomas Persson, Petar Pujic, Nicole Alloisio, Pascale Fournier, Hasna Boubakri, Katharina Pawlowski and Philippe Normand declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Carro, L., Persson, T., Pujic, P. et al. Organic acids metabolism in Frankia alni . Symbiosis 70, 37–48 (2016). https://doi.org/10.1007/s13199-016-0404-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-016-0404-0