Abstract
To our knowledge, there are no studies on the interactive effects of inoculation with arbuscular mycorrhizal fungi and cytokinin addition to plants under drought stress. We investigated the potential protective effect of arbuscular mycorrhizae on pomegranate plants, combined with exogenous cytokinin addition, under two contrasting soil water availability regimes. Our results showed that exogenous cytokinin addition enhances plant biomass, shoot to root ratio and water content, as well as increasing the anthocyanin content. However, a combination of AM fungal inoculation and cytokinin addition did not result in a synergistic protective effect against water stress. Plants were equally well protected against this stress by cytokinin spraying alone. The improvement of pomegranate growth was due mainly to exogenous cytokinin addition. Photosynthesis was promoted both by mycorrhizal inoculation alone and by exogenous cytokinin addition. The main protection against oxidative stress caused by drought was via enhanced accumulation of anthocyanins when the plants were sprayed with cytokinins. When cytokinins were used, the photosynthesis apparatus was also protected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plants often face different stressful situations caused by biotic or abiotic factors. Severity and exposure time to stressing factors produce a wide range of plant responses, such as the alteration of gene expression and cellular metabolism, or changes in growth rates and yields (Bray et al. 2000).
Cytokinins affect many physiological and developmental processes, including leaf senescence, nutrient mobilization, and chloroplast differentiation (Taiz and Zeiger 1998; Azcón-Bieto and Talón 2000). An association between cytokinins, plant senescence and intermittent drought response was observed by Pospísilová et al. 2000; Vomácka and Pospísilová 2003; Achard et al. 2006. An increase in endogenous CKs levels improved the photosynthesis and water efficiency in tobacco transgenic plants under severe drought conditions (Rivero et al. (2007). Although the application of exogenous CKs did not completely prevent senescence, they cause a short-term alleviation, particularly when directly sprayed on plants (Van Staden et al. 1988). Exogenous CKs can also increase the content of endogenous CKs (Hare et al. 1997; Pospísilová et al. 2000) and improve carbohydrates accumulation in grains of rice under salt stress (Javid et al. 2011). In the response of bentgrass to heat stress, CK application mitigated shoots and roots injury (Liu et al. 2002).
Arbuscular mycorrhizal fungi (AMF) enhance the nutrient status of the host plant by enhancing the uptake of minerals needed for photosynthesis (Marschner and Dell 1994). Several reports have shown an increase of CKs levels in roots and leaves of mycorrhizal plants (Baas and Kuiper 1989; Goicoechea et al. 1995, 1996, 1997; Barker and Tagu 2000; Vadassery et al. 2008). Interactions occur in the host roots where the AMF have the capacity to transport water more efficiently than non-mycorrhizal roots and consequently improves plant water status (Safir et al. 1972; Augé 2001; Kung’U et al. 2008). Water deficit in combination with other stress factors (e.g., high temperatures and high light intensity), disrupt photosynthesis and enhance photorespiration (Close and Beadle 2003; Miller et al. 2010), causing an increase in production of reactive oxygen species (ROS). These toxic molecules lead to oxidative damage to proteins, lipids and DNA (Mittler et al. 2004; Miller et al. 2010). Plants detoxify these molecules through ROS-scavenging enzymes such as catalase (CAT) and superoxide dismutase (SOD). The latter converts O2 − to O2 and H2O2 in chloroplasts, mitochondria and peroxisomes. While CAT, found in peroxisomes, detoxifies H2O2 to H2O (Mittler et al. 2004). Exogenous addition of CKs to plants facilitates scavenging of active oxygen species (Liu and Huang 2002) and increases the activities of antioxidant enzymes in mycorrhizal plants under drought condtions (Ruiz-Lozano et al. 1996; Zhu et al. 2011). During drought, mycorrhizal development (especially mycorrhizal colonization and arbuscules formation) may induce expression of antioxidant coding genes that are involved in alleviation of damage caused by ROS (Wu and Zou (2009).
Due to climate change and global warming several agricultural regions suffer an increase in temperature and therefore severe drought (Arndt et al. 2010). Arbuscular mycorrhizal inoculation could help to increase plant drought resistance in soils with low water availability leading to better plant survival (Stahl et al. 1998). Most experiments show that inoculated plants have higher yields than non-mycorrhizal ones confirming this role of AMF in host drought resistance (Augé 2001; Singh et al. 2011).
Mature plants of Pomegranate (Punica granatum L.) can tolerate long periods of drought with only 4 irrigations per year. However, effective water management is essential for 6 or 7 years after young plants are transplanted to the field. Pomegranates are grown in tropical and subtropical regions being a versatile crop adapted to marginal lands (Khattab et al. 2011a). In the north-center of Argentina, pomegranate crops have become more common during the last decade, due to the value of their fruits (juices, syrups, and jams) and as ornamental plant (Cecotto et al. 2007). Moreover, infusions of the fruit are popularly used as a medicinal plant for stomachaches (Martínez 2011).
Exogenous cytokinin addition and AMF inoculation could enhance resistance to drought stress in pomegranate and protect the plants against oxidative damage. The object of the present study was to investigate this and we hypothesized that the combination of AMF inoculation and exogenous CK addition in pomegranate plants could protect plants against the stress caused by variations in water regimes.
2 Materials and methods
2.1 Biological material and experimental design
A total of 150 young branch cuttings (8 cm length including 2–4 shoot tips) were macropropagated from a healthy and vigorous mother plant of Punica granatum L. cv. Plena (Voss) from the Departamento de Producción Vegetal (Facultad de Agronomía, FAUBA, Argentina, in order to eliminate genetic variability. A wound was made in each cutting and indole butyric acid (IBA) rooting hormone was added (concentration of 2,500 ppm, Knight et al. 2005). Rooting was performed in Neoform® PVC boxes with cover (25x17x12 cm) at 25 cuttings per box (three boxes inoculated and three boxes control) with 650 ml of a tyndallized perlite: vermiculite: soil mixture (1:1:1; v:v) per box. The soil characteristics were: pH 7.1, 12.08 g kg−1 total C, 1.1 g kg−1 N, 34.2 mg kg−1 P, 0.9 cmol kg−1 K, 7.5 cmol kg−1 Ca, 1.7 cmol kg−1 Mg, and 0.2 cmol kg−1 Na. Inoculation was performed at 1 cm deep with 50 ml of dry inoculum of Rhizophagus irregularis (Krüger et al. 2012) strain GA5 covering the box. The control treatment (C) received 50 ml of autoclaved mycorrhizal inoculum supplemented with a filtrate (<20 μm) of non-autoclaved mycorrhizal inoculum (to provide a microbial comunity). Boxes were initially irrigated with 100 ml distilled water (80 % soil capacity) followed by weekly watering repetitions in order to maintain the internal humidity (80 % soil water capacity). Plants have grown in a growing chamber (24 ± 2 °C day/night, 16/8 h light/dark, 87 nmol m−2 s−1 cold white light) for 60 days. A 70 % of rooting success was obtained under these conditions. These growing conditions and rooting were previously tested in our laboratory.
A strain of Rhizophagus irregularis (GA5) (GenBank accession number GU140042) was used as AMF model; a typical in vitro fast-growing strain (BGIV, http://www.bgiv.com.ar/strains/Rhizophagus-intraradices/ga5). GA5 was routinely propagated in Trifolium repens as plant host in 1.5 L pots with a tyndallized perlite: soil mixture (3:1; v:v, see above for soil characteristics and tyndallization). T. repens plants were grown for 4 months under greenhouse conditions (450 μE m−2 s−2, 400–700 nm; 16/8 h light/dark; 25/18 °C day/night; 60–70 % relative humidity). Then pots were maintained unwatered till dryness. After that time, there was enough mycorrhizal inoculum with many spores, intra and extraradical mycelium to use for the effective inoculation of host pomegranate plants. All plants were watered with Hewitt (1952) solution without phosphorous every 15 days.
One hundred cuttings of 60 days old rooted were transplanted to 1.5 L pots containing a tyndallized perlite: soil mixture (3:1; v:v; see above for soil characteristics) and were maintained for 30 days under nursery conditions. Thereafter, CK (6-benzylaminopurine, 5 mg L−1) was added to half of the experiment (50 plants) by spraying shoots (3 separated applications of 0.5 mL per plant during 15 days) and the other half were sprayed with distilled water in the same way (CK-). After CK application the experiment was continued until the plants were 105 days old. Half of the plants were maintained to soil field capacity (SFC, equivalent to 80 %) and the other half were maintained at 23.19 ± 2.04 % SFC (namely 23 % SFC) during 30 days. The SFC was calculated by weighing the substrate before and after drying at 105 °C for 24 h. These conditions were previously tested in our laboratory for pomegranate plants (unpublished results). All plants were irrigated once after the start of the experiment with nutritive solution lacking phosphate (Hewitt 1952).
2.2 Variables assessment
Mycorrhizal colonization was checked 30 days after fungal inoculation, a representative sample of the roots was stained using the method of Phillips and Hayman (1970) and a quantification of mycorrhizal infection was assessed as proposed by Giovanetti and Mosse (1980). The proportion of mycorrhizae (MI%), arbuscules (A%), and vesicles (V%) in each stained sample were determined separately (McGonigle et al. 1990). Plant survival per box (quantified as the percentage of rooted cuttings and at least a new bud / total cuttings) was measured before transplantation. The fresh and dry weights (at 70 °C until constant weight) of shoots and roots were quantified at the end of the experiment. Also, the water content of shoots and roots was calculated as the difference between shoot and root fresh and dry weight respectively. The shoot-to-root biomass ratio was evaluated.
Total polyphenol content (TPH) was measured with the method proposed by Capannesi et al. (2000). Four shoot samples per treatment were grounded in a mortar containing methanol-chloridric acid solution and centrifuged (10,000 g for 20 min). Supernatants were measured on a saturated solution of sodium carbonate (CO3Na2) plus Folin-Ciocalteu reagent (at 755 nm). A standard curve with gallic acid was made to express total polyphenol content as mg / 100 g of gallic acid equivalent (GAE) of dry mass.
Anthocyanin content (ACY) of four shoot samples per treatment was measured with the Fuleki and Francis (1968) protocol using the differential pH method, with buffer solutions (pH 1 and pH 4.5), and determined in a spectrophotometer at 510 nm and 700 nm, applying the extinction molar coefficient factor of cyanidin 3-glucoside (Ε = 29,600). Finally, results were expressed as mg of cyaniding 3-glucoside equivalents (Cy3-GE) / g fresh weight.
Chlorophyll content (Chl) was measured as proposed by Inskeep and Bloom (1985). Chl was extracted from fresh shoot sections of four plants per treatment with N,N-dimethylformamide (72 hs at room temperature). Pigment concentration (Chl a, b and a + b) was measured in a spectrophotometer at 647 nm and 663 nm, and expressed as mg / L. Also Chl a/b ratio was calculated.
Enzyme extraction and measurements: 1 g of fresh material (shoots and roots, n = 6 per treatment) was pulverized in a mortar with liquid nitrogen; 0.06 g polyvinylpolypyrrolidone (PVPP) were added per 6 mL of extraction buffer (KH2PO4 – K2HPO4 50 mM pH 7.8 plus 0.1 mM EDTA); then each sample was filtered through a nylon membrane in order to remove plant cell debris. Samples were centrifuged (20,000 g for 20 min) and supernatant was aliquoted in Eppendorf tubes and stored at −70 °C until use (Gogorcena et al. 1995).
Intracellular enzyme activities were also measured. Catalase (CAT) (EC 1.16.1.6): following a method based on absorbance dropping at 240 nm caused by H2O2 loss (Aebi 1984). Superoxide dismutase (SOD) (EC 1.15.1.1) was measured at 560 nm according to its capacity to inhibit the photochemical reduction of nitroblue tetrazolium in the presence of riboflavin (Beyer and Fridovich 1987). Enzyme activities were standarized by protein (PROT) quantified by the Bradford (1976) method.
2.3 Statistical analysis
The experiment was arranged in a completely randomized block factorial design. All data were subjected to analysis of variance (factorial ANOVA), with three factors: 1) AMF inoculation (GA5; C), 2) cytokinin addition (CK+; CK-) and 3) soil field capacity (SFC; 23 % SFC). Assumptions of homogeneity of variance and normality were checked. Comparisons of mean values among different treatments were made using the Tukey’s Honest Significant Difference (HSD) test using a significance level of p < 0.05 (Clewer and Scarisbrick 2001). Statistical procedures were carried out using the software STATISTICA 6.0 for Windows XP.
3 Results
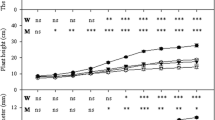
Inoculated plants survived better than control plants (98 ± 1.9 % for GA5 inoculated plants; 94.3 ± 0.1 % for control plants); however rooting was lower (68 ± 2.3 % for GA5 inoculated plants; 70.7 ± 1.3 % for control plants). Cytokinin addition resulted in a decrease in mycorrhizal and vesicle percentages at SFC (MI%: 12.71 ± 1.10 % in CK + treatment vs. 21.66 ± 5.25 % in CK- treatment; V%: 4.88 ± 1.13 % in CK + treatment vs. 15.19 ± 6.65 % in CK- treatment). However at 23 % SFC there were no differences (MI%: 19.63 ± 3.45 % in CK + treatment vs. 23.62 ± 6.84 % in CK- treatment; V%: 15.44 ± 4.73 % in CK + treatment vs. 9.02 ± 6.26 % in CK- treatment). The proportions of arbuscules were only significantly lower at 23 % SFC (SFC: 13.29 ± 3.65 % in CK- treatment and 10.54 ± 0.71 % in CK + treatment; 23%SFC: 9.68 ± 6.13 % in CK- treatment and 5.88 ± 2.99 % in CK + treatment). Since there was no triple interaction in growing parameters there was observed dual interaction. Exogenous cytokinin addition caused an improvement of fresh weight on shoots in non-inoculated plants regardless of water availability followed by inoculated plants at 23 %SFC (Fig. 1a). On the other hand the combination of CK addition and 23 %SFC improved shoot biomass (Fig. 1b). Shoot water content significantly decreased at 23 % SFC for all treatments except in the combination of CK addition and AMF inoculation. However, in control plant with CK addition the water content was significantly higher at SFC than the rest of the treatments (Fig. 1c). Regarding roots only inoculated plants without CK addition treatment caused a decrease in fresh, biomass and water content at 23 % SFC condition (Fig. 1d to f). Moreover, CK addition significantly increased shoot-to-root ratio at 23 % SFC in inoculated and non-inoculated plants (Fig. 2).
Fresh and dry weight, and water content in pomegranate shoots (a to c) and roots (d to f). Treatments: non-inoculated control (C), Rhizophagus irregularis strain inoculation (GA5), without cytokinin addition (CK-), cytokinin addition (CK+), soil field capacity condition (SFC), 23 % of soil field capacity condition (23 % SFC), fw (fresh weight), dw (dry weight), water content (g H2O). Mean ± S.E., n = 6. Different letters at each variable denote significant differences in Tukey’s test. Effects of factors according to three-way ANOVA at P < 0.05
Shoot-to-root ratio in pomegranate plants. Treatments: non-inoculated control (C), Rhizophagus irregularis strain inoculation (GA5), without cytokinin addition (CK-), cytokinin addition (CK+), soil field capacity condition (SFC), 23 % of soil field capacity condition (23 % SFC). Mean ± S.E., n = 6. Different letters at each variable denote significant differences in Tukey’s test. Effects of factors according to three-way ANOVA at P < 0.05
The combination of mycorrhizal inoculation and CK addition significantly increase TPH at 23 % SFC (6 ± 0.1 mg 100 g−1 GAE at SFC and 6.5 ± 0.03 mg 100 g−1 GAE at 23 % SFC) but no differences were observed in comparison to control plants CK- (5.7 ± 0.2 mg 100 g−1 GAE at SFC and 6.8 ± 0.07 mg 100 g−1 GAE at 23 % SFC). The opposite results were obtained at mycorrhizal inoculation without CK (6.5 ± 0.09 mg 100 g−1 GAE at SFC and 5.96 ± 0.1 mg 100 g−1 GAE at 23 % SFC) (Fig. 3a). ACY was significantly higher in treated plants regardless of water condition. Only inoculated plants without CK addition decreased ACY at 23 % SFC. However, these values were significantly higher than control plants (Fig. 3b).
Total polyphenol content (TPH) (a); anthocyanin content (ACY) (b); chlorophyll content (Chl): Chl a (c); Chl b (d); Chl a + b (e); Chl a/b (f) in pomegranate shoots. Treatments: non-inoculated control (C), Rhizophagus irregularis strain inoculation (GA5), without cytokinin addition (CK-), cytokinin addition (CK+), soil field capacity condition (SFC) and 23 % of soil field capacity condition (23 % SFC). Mean ± S.E., n = 4. Different letters at each variable denote significant differences in Tukey’s test. Effects of factors according to three-way ANOVA at P < 0.05
At 23 % SFC an increase in Chl a, b and a + b were observed in all treatments (Fig. 3c to e). Particularly Chl a in control plants with CK + at 23 % SFC was significantly lower than control plants CK- (Fig. 3c). Chl b was significantly lower at SFC in mycorrhizal plants regardless of CK addition and significantly higher in mycorrhizal plants without CK (Fig. 3d). On the other hand, Chl a + b was significantly lower at SFC in mycorrhizal plants regardless of CK addition. However, at 23 % SFC Chl a + b was significantly higher than the controls (Fig. 3e). Moreover, Chl a/b was significantly higher in treated plants at SFC condition but only mycorrhizal plants CK- exhibited lower Chl a/b than control plants (Fig. 3f).
The 23 % SFC condition significantly decreased shoot PROT at CK + in control and at CK- in mycorrhizal treatment, but at SFC a significant increase in PROT was observed at mycorrhizal CK- treatment followed by control CK + in comparison to control CK- plants (Fig. 4a). CAT activity of pomegranate shoots significantly decreased in treated plants under 23 % SFC in comparison to control plants CK- at the same condition. The lowest level of CAT activity was registered in mycorrhizal CK + plants. On the other hand, no differences in CAT activity were observed in control plants (Fig. 4b). At SFC a decrease in SOD enzyme was observed in treated shoots. At 23 % SFC this effect was reversed in control plants with CK addition. However, no differences were observed in comparison to 23 % SFC in mycorrhizal shoots regardless of CK addition (Fig. 4c). In pomegranate roots, PROT significantly decreased in control CK- at 23 % SFC but inverse results were obtained at CK+. On the other hand, no differences were observed in mycorrhizal plants (Fig. 4d). No differences in CAT activity at SFC condition were detected. While at 23 % SFC condition only in mycorrhizal plants CAT activity significantly decreased regardless CK addition, in control plants no differences were observed (Fig. 4e). CK addition significantly increased SOD activity at SFC but at 23 % SFC they decreased. However, there were no differences in comparison to control treatment at the same condition (Fig. 4f).
Enzyme activities and protein content in pomegranate shoots (a to c) and roots (d to f). Treatments: non-inoculated control (C), Rhizophagus irregularis strain inoculation (GA5), without cytokinin addition (CK-), cytokinin addition (CK+), soil field capacity condition (SFC) and 23 % of soil field capacity condition (23 % SFC), protein content (PROT), catalase (CAT), superoxide dismutase (SOD). Mean ± S.E., n = 6. Different letters at each variable denote significant differences in Tukey’s test. Effects of factors according to three-way ANOVA at P < 0.05
4 Discussion
Hormones mediate plant growth and adaptation to changing environments (Wolters and Jurgens 2009). Pospísilová et al. (2000) showed that exogenous cytokinin stimulates osmotic adjustment relieving stress effects and retarding senescence. Khattab et al. (2011b) found that stressful conditions decreased CK transport from roots to shoots and caused an increase in abscisic acid levels. This change in hormonal balance reduced growth and shoot expansion. Our experiments showed that exogenous cytokinin addition improved plant growth and maintained water content. However, under the tested conditions, the combination of mycorrhizal inoculation and CK addition did not have the synergistic effect that we expected. Perhaps there was a regulation of the endogenous cytokinin content. Several studies suggest that the establishment of arbuscular mycorrhizal symbiosis maintains endogenous levels of cytokinins in host plants (Goicoechea et al. 1995, 1997; Barker and Tagu 2000). However exogenous cytokinins improved the shoot-to-root ratio in concordance with experiments carried out on transgenic plants overexpressing CKs (Ainley et al. 1993; Wang et al. 1997).
Polyphenolic compounds (including flavonoids and anthocyanins) have been proposed as having a protective effect overcoming oxidative stress by absorbing and neutralizing free radicals, quenching singlet oxygen, or decomposing hydrogen peroxides (Sreenivasulu et al. 2000; Patumi et al. 2002; Ksouri et al. 2007). The synthesis and accumulation is generally stimulated in response to abiotic and biotic stresses (Ksouri et al. 2007). In this work, a decrease in total polyphenol content was observed in inoculated plants without cytokinin addition at 23 % SFC. However, these results were reversed by cytokinin addition. Even though we did not observed a synergistic effect as expected. Nacif De Abreu and Mazzafera (2005), in a study of the combined effects of water and temperature stress on Hypericum brasiliense, found a decrease in biomass was related to an increase in polyphenolic compounds. Also Aseri et al. (2008) observed an increase of total phenols in pomegranate plants inoculated with Rhizophagus fasciculatus or Funneliformis mosseae and a maximum with a dual inoculation with the phosphate-solubilizing bacteria Azotobacter chroococcum and F. mosseae. In our experiments, all polyphenolic levels were similar and the biomass was improved by the cytokinin addition. Thus antioxidative defense was not mainly caused by the level of phenolic compounds.
Anthocyanins are also natural anti-oxidative compounds that may reduce oxidative damage in plants (Kong et al. 2003; Castaneda‐Ovando et al. 2009). Synthesis, accumulation and catabolism of anthocyanins in plant tissues have been strongly suggested to be closely associated with environmental changes and auto-oxidative or enzymatic degradation (Oren‐Shamir 2009). Anthocyanins could provide photoprotection against photooxidative damage at high temperature (Shao et al. 2007) and under drought (Danae et al. 2003; Van den Berg and Perkins 2007). In our experiments, cytokinin addition alone or in combination with mycorrhizal inoculation improves plant survival through an increase of the anthocyanin levels. Although no longer find a synergism caused by the combination of cytokinin and AMF, the same protective effect was found with cytokinin, AMF and both. Chalker-Scott (1999) found an increase in anthocyanins levels in cowpea seedlings and resurrection plants. Baslam and Goicoechea (2012) also found that levels of anthocyanins were increased in mycorrhizal lettuce plants. Thus arbuscular mycorrhizal symbiosis can stimulate synthesis of these plant secondary metabolites (Gianinazzi et al. 2010). Foliar anthocyanin is accumulated in young leaves, expanding foliage and also in the old one before fall in deciduous species as response to diverse stresses (nutrient deficiency, ultraviolet radiation exposure, herbivores or pathogenic fungal infection) (Close and Beadle 2003). Also, anthocyanins protect the photosynthetic apparatus during leaf senescence (Hoch et al. 2001).
Exogenous addition of cytokinins relieved salt stress condition and chlorophyll content of wheat seedlings (Mumtaz et al. 1997). We found that under water deficit conditions, stress with respect to chlorophyll a and a + b was only relieved in pomegranate plants that were mycorrhiza and not given cytokinin addition. Other authors have observed an increase of chlorophyll contents in mycorrhizal plants (Carpio et al. 2005; Wu and Ren-Xue 2006; Latef and Chaoxing 2011; Rahmaty and Khara 2011; Ruiz-Lozano et al. 2012). Recently, Baslam and Goicoechea (2012) observed that total chlorophyll was enhanced in mycorrhizal lettuce plants regardless of the water regime applied, suggesting that mycorrhizae protect by the improving the photosynthetic rate. Such increases in chlorophyll content in leaves of plants associated with AMF inoculation could be related to a greater uptake of nutrients (e.g.,N, Mg, Fe, Z and Cu) essential for chlorophyll biosynthesis (Marschner and Dell 1994; Eftekhari et al. 2010; Khattab et al. 2011c). In our experiments only mycorrhizal inoculation without cytokinin addition increased the chlorophyll content over that observed in control plants at 23 % SFC. Interestingly, cytokinin addition regardless of mycorrhizal inoculation resulted in an intermediate value for total chlorophyll. The Chlorophyll a/b ratio gives a clue about senescence as chlorophyll a is degraded faster than chlorophyll b (Hughes et al. 2005). In our experiments on pomegranate we found a significative decrease in the chlorophyll a/b ratio in mycorrhizal plants without cytokinin addition at 23 % SFC. However, the proportion of chlorophyll b was significantly higher indicating that plants were protected by the mycorrhizal association. Rivero et al. (2010) showed that an increase in endogenous cytokinin activates transcription genes that code for chlorophyll biosynthesis. The combination of exogenous cytokinin and mycorrhizal inoculation should increase endogenous levels of cytokinins and lead to a synergistic effect on chlorophyll synthesis content. As this was not observed in our study, it is possible that in pomegranate there is a balance between biosynthesis and degradation of cytokinins.
A higher protein content was evident in mycorrhizal pomegranate plants, particularly in the shoots. Najafi et al. (2012) also found that inoculation of winter barley plants with R. irregularis improved the protein content and endogenous levels of cytokinins. In our experiments increases in protein content and biomass were evident in plants provided with both mycorrhizal inoculation and exogenous cytokinin addition.
In general, enzyme activities decreased in both shoots and roots of treated pomegranate plants. Das et al. (2012) argue that anthocyanin biosynthesis is related to the electron transport chain. Anthocyanins detoxify H2O2, O2 −, ONOO−, and possibly OH− and 1O2 (Yamasaki 1997; Neill et al. 2002; Hughes et al. 2005; Van Den Berg and Perkins 2007; Ying and Tai 2012). In our study, anthocyanins could be the main compounds involved in detoxifying toxic molecules generated by a reduction in water availability, particularly in pomegranate plants given exogenous cytokinin addition treatment. More studies including those on plant nutrient status following inoculation with different AM fungal strains should be carried out and adjustment of cytokinin levels should be done to throw light upon the regulatory networks involved.
References
Achard P, Cheng H, De Grawe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311:91–94
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126
Ainley WM, McNeil KJ, Hill JW, Lingle WL, Simpson RB, Brenner ML, Nagao RT, Key JL (1993) Regulatable endogenous production of cytokinins up to toxic levels in transgenic plants and plant tissue. Plant Mol Biol 22:13–23
Arndt DS, Baringer MO, Johnson MR (2010) State of the climate in 2009. Bull Am Meteorol Soc 91(7):1–224
Aseri GK, Jain N, Panwar J, Rao AV, Meghwal PR (2008) Biofertilizers improve plant growth, fruit yield, nutrition, metabolism and rhizosphere enzyme activities of Pomegranate (Punica granatum L.) in Indian Thar Desert. Sci Hortic 117:130–135
Augé RM (2001) Water relations, drought and vesicular arbuscular symbiosis. Mycorrhiza 11:3–42
Azcón-Bieto J, Talón M (2000) Citoquininas. In: Fundamentos de Fisiología Vegetal. McGraw-Hill SA Interamericana de España Editorial, España, pp 343–360
Baas R, Kuiper D (1989) Effects of vesicular-arbuscular mycorrhizal infection and phosphate on Plantago major ssp. pleiosperma in relation to internal cytokinin concentrations. Physiol Plant 76:211–215
Barker SJ, Tagu D (2000) The roles of auxins and cytokinins in mycorrhizal symbiosis. J Plant Growth Regul 19(2):144–154
Baslam M, Goicoechea N (2012) Water deficit improved the capacity of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of antioxidant compounds in lettuce leaves. Mycorrhiza 22:347–359
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72:248–254
Bray EA, Bailey-Serres J, Weretilnyk E (2000) Responses to Abiotic Stresses. In: Buchanan B, Gruissem R, Jones R (eds) Biochemistry and molecular biology of plants. American society of plant physiologists, pp 1158–1203
Carpio LA, Davies FT Jr, Arnold MA (2005) Arbuscular mycorrhizal fungi, organic and inorganic controlled-release fertilizers: effect on growth and leachate of container-grown bush morning glory (Ipomoea carnea ssp. fistulosa) under high production temperatures. Am Soc Horticult Sci 130(1):131–139
Castaneda‐Ovando A, Pacheco‐Hernandez MD, Paez‐Hernandez ME, Rodriguez JA, Galan‐Vidal CA (2009) Chemical studies of anthocyanins: a review. Food Chem 113:859–871
Capannesi C, Palchetti I, Mascini M, Parenti A (2000) Electrochemical sensor and biosensor for polyphenols detection in olive oils. Food Chem 74(4):553–562
Cecotto JA, Taiariol DR, Cáceres S (2007) Colección de frutos tropicales de la EEA INTA Bella Vista. Technical Serie N 21 1–17, ISSN 1515–9299
Chalker-Scott L (1999) Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70(1):1–9
Clewer AG, Scarisbrick DH (2001) Factorial experiments. In: Wiley J, Ltd S (eds) Practical statistics and experimental design for plant and crop science. The Atrium, Southern Gate, Chicheste, West Sussex England, pp 159–181
Close DC, Beadle CL (2003) The ecophysiology of foliar anthocyanin. Bot Rev 69(2):149–161
Danae E, Pratt R, Stephen D (2003) Reddening and regreening: The role of anthocyanins in water stressed leaves of a sclerophyllous shrub. ESA Annu Meet
Das PK, Shin DH, Choi SB, Yoo SD, Choi G, Park YI (2012) Cytokinins enhance sugar-induced anthocyanin biosynthesis in Arabidopsis. Mol Cells 34(1):93–101
Eftekhari M, Alizadeh M, Mashayekhi K, Asghari H, Kamkar B (2010) Integration of arbuscular mycorrhizal fungi to grape vine (Vitis vinifera L.) in nursery stage. J Adv Lab Res Biol 1(1):102–111, ISSN 0976–7614
Fuleki T, Francis FJ (1968) Determination of total anthocyanin and degradation index for cranberry juice. J Food Sci 33(1):78–83
Gianinazzi S, Gollotte A, Binet MN, van Tuinen D, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20:519–530
Giovanetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Gogorcena Y, Iturbe-Ormaetxe I, Escuredo PR, Becana M (1995) Antioxidant defense against activated oxygen in pea nodules subjected to water stress. Plant Physiol 108:753–759
Goicoechea N, Antolin MC, Sánchez-Díaz M (1997) Gas exchange is related to the hormone balance in mycorrhizal or nitrogen-fixing alfalfa subjected to drought. Physiol Plant 100:989–997
Goicoechea N, Antolin MC, Strnad M, Sánchez-Díaz M (1996) Root cytokines, acid phosphates and nodule activity in drought-stressed mycorrhizal or nitrogen fixing alfalfa plants. J Exp Bot 47:683–686
Goicoechea N, Dolezal K, Antolin MC, Strnad M, Sánchez-Díaz M (1995) Influence of mycorrhizae and Rhizobium on cytokinin content in drought-stressed alfalfa. J Exp Bot 46:1543–1549
Hare PD, Cress WA, Van Staden J (1997) The involvement of cytokinins in plant responses to environmental stress. Plant Growth Regul 23:79–103
Hewitt EJ (1952) Sand and water culture methods in the study of plant nutrition. Tech Com Agric Bur 22
Hoch WA, Zelding EL, McCown BH (2001) Physiological significance of anthocyanins during autumnal leaf senescence. Tree Physiol 21:1–8
Hughes NM, Neufeld HS, Burkey KO (2005) Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytol 168:575–587
Inskeep WP, Bloom PR (1985) Extinction coefficients of chlorophyll a and b in N, N-dimethylformamide and 80 % acetone. Plant Physiol 77:483–485
Javid MG, Sorooshzadeh A, Sanavy SAMM, Allahdadi I, Moradi F (2011) Effects of the exogenous application of auxin and cytokinin on carbohydrate accumulation in grains of rice under salt stress. Plant Growth Regul 65(2):305–313
Khattab MM, Shaban AE, El-Shrief AH, El-Deen Mohamed AS (2011a) Growth and productivity of pomegranate trees under different irrigation levels. II: fruit quality. J Hort Sci Ornamen Plants 3(3):259–264
Khattab MM, Shaban AE, El-Shrief AH, El-Deen Mohamed AS (2011b) Growth and productivity of pomegranate trees under different irrigation levels. I: vegetative growth and fruiting. J Hort Sci Ornamen Plants 3(2):194–198
Khattab MM, Shaban AE, El-Shrief AH, El-Deen Mohamed AS (2011c) Growth and productivity of pomegranate trees under different irrigation levels. III: leaf pigments, proline and mineral content. J Hort Sci Ornamen Plants 3(3):265–269
Knight P, Coker CH, Anderson JM, Murchison DS, Watson CE (2005) Mist interval and K-IBA concentration influence rooting of orange and mountain azalea. Native Plants 6:111–117
Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R (2003) Analysis and biological activities of anthocyanins. Phytochem 64:923–933
Krüger M, Krüger C, Walker C, Stockinger H, Schüßler A (2012) Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol 193:970–984
Ksouri R, Megdiche W, Debez A, Falleh H, Grignon C, Abdelly C (2007) Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritime. Plant Physiol Biochem 45:244–249
Kung’U JB, Lasco RD, De La Cruz IU, De La Cruz RE, Husain T (2008) Effect of vesicular arbuscular mycorrhiza (VAM) fungi inoculation on coppicing ability and drought resistance of Senna spectabilis. Pak J Bot 40(5):2217–2224
Latef AAHA, Chaoxing H (2011) Arbuscular mycorrhizal influence on growth, photosynthetic pigments, osmotic adjustment and oxidative stress in tomato plants subjected to low temperature stress. Acta Physiol Plant 33:1217–1225
Liu X, Huang B (2002) Cytokinin effects on creeping bentgrass response to heat stress: II. Leaf senescence and antioxidant metabolism. Crop Sci 42:466–472
Liu X, Huang B, Banowetz G (2002) Cytokinin effects on creeping bentgrass responses to heat stress: I. Shoot and root growth. Crop Sci 42:457–465
Marschner H, Dell B (1994) Nutrient uptake in mycorrhizal symbiosis. Plant Soil 159(1):89–102
Martínez GJ (2011) Uso de plantas medicinales en el tratamiento de afecciones transmitidas por el agua en una comunidad Toba (QOM) del impenetrable (Chaco, Argentina): una perspectiva etnoecológica y sanitaria. Bonplandia 20(2):329–352, ISSN: 0524–0476
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Mittler R, Vanderauwera S, Gollery M, van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9(10):490–498
Mumtaz S, Naqvi SSM, Shereen A, Khan MA (1997) Salinity stress and the senescence process in wheat (Triticum aestivum L.). Pak J Bot 29:299–303
Nacif de Abreu I, Mazzafera P (2005) Effects of water and temperature stress on the content of active constituents of Hypericum brasilienne Choisy. Plant Physiol Biochem 43:241–248
Najafi A, Reza Arkadami M, Rejali F, Sajedi N (2012) Response of winter barely to co-inoculation with Azotobacter and mycorrhiza fungi influenced by plant growth promoting rhizobacteria. Ann Biol Res 3(8):4002–4006
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5:388–395
Oren‐Shamir M (2009) Does anthocyanin degradation plays a significant role in determining pigment concentration in plants? Plant Sci 177:310–316
Patumi M, D’Andria R, Marsilio V, Fontanazza G, Morelli G, Lanza B (2002) Olive and olive oil quality after intensive monocone olive growing (Olea europaea L., cv Kalamata) in different irrigation regimes. Food Chem 77:27–34
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55(1):158–161, IN16-IN18
Pospísilová J, Synková H, Rulcová J (2000) Cytokinins and water stress. Biol Plant 43(3):321–328
Rahmaty R, Khara J (2011) Effects of vesicular arbuscular mycorrhiza Glomus intraradices on photosynthetic pigments, antioxidant enzymes, lipid peroxidation, and chromium accumulation in maize plants treated with chromium. Turk J Biol 35:51–58
Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S et al (2007) Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci U S A 104:19631–19636
Rivero RM, Gimeno J, Deynze AV, Walia H, Blumwald E (2010) Enhanced cytokinin synthesis in tobacco plants expressing PSARK: IPT prevents the degradation of photosynthetic protein complexes during drought. Plant Cell Physiol 54(11):1929–1941
Ruiz-Lozano JM, Azcón R, Palma JM (1996) Superoxide dismutase activity in arbuscular mycorrhizal Latuca sativa plants subjected to drought stress. New Phytol 134(2):327–333
Ruiz-Lozano JM, Porcel R, Azcón C, Aroca R (2012) Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. J Exp Bot 63(11):4033–4044
Safir GR, Boyer S, Gerdemann JW (1972) Nutrient status and mycorrhizal enhancement of water transport in soybean. Plant Physiol 49:700–703
Shao L, Shu Z, Sun S, Peng C, Wang X, Lin Z (2007) Antioxidation of anthocyanins in photosynthesis under high temperature stress. J Integrat Plant Biol 49(9):1341–1351
Singh LP, Singh Gill S, Tuteja N (2011) Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal Behav 6(2):175–191
Sreenivasulu N, Grimm B, Wobus U, Weschke W (2000) Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica). Physiol Plant 109:435–442
Stahl PD, Frost SM, Williams SE, Schuman GE (1998) Arbuscular Mycorrhizae and water stress tolerance of wyoming big sagebrush seedlings. Soil Sci Soc Am J 62(5):1309–1313
Taiz L, Zeiger E (1998) Cytokinins, In: plant physiology. Sinauer Associates Inc Publishers, Sunderland Massachusetts, pp 621–650
Vadassery J, Ritter C, Venus Y, Camehl I, Varma A, Shahollari B, Novák O, Strnad M, Ludwig-Muller J, Oetmuller R (2008) The role of auxins and cytokinins in the mutualistic interactions between Arabidopsis and Piriformospora indica. Mol Plant-Microbe Interact 21:1371–1383
Van Den Berg AK, Perkins TD (2007) Contribution of anthocyanins to the antioxidant capacity of juvenile and senescing sugar maple (Acer saccharum) leaves. Funct Plant Biol 34(8):714–719
Van Staden J, Cook E, Noodén LD (1988) Cytokinins and senescence. In: Noodén LD, Leopold AC (eds) Senescence and aging in plants. Academic, San Diego, pp 281–328
Vomácka L, Pospísilová J (2003) Rehydration of sugar beet plants after water stress: effect of cytokinins. Biol Plant 46(1):57–62
Wang J, Letham DS, Cornish E, Wei K, Hocart CH, Michael M, Stevenson KR (1997) Studies of cytokinin action and metabolism using tobacco plants expressing either the ipt or the GUS gene controlled by a chalcone synthase promoter. II. Ipt and GUS gene expression, cytokinin levels and metabolism. Aust J Plant Physiol 24:673–683
Wolters H, Jurgens G (2009) Survival of the flexible: hormonal growth control and adaptation in plant development. Nat Genet 10:305–317
Wu QS, Ren-Xue X (2006) Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J Plant Physiol 163:417–425
Wu QS, Zou YN (2009) Mycorrhiza has a direct effect on reactive oxygen metabolism of drought-stressed citrus. Plant Soil Environ 55(10):436–442
Yamasaki H (1997) A function in colour. Trends Plant Sci 2(1):7–8
Ying YS, Tai XB (2012) Photoprotective mechanisms of leaf anthocyanins: research progress. NCBI PubMed – indexed for MEDLINE
Zhu XC, Song FB, Liu SQ (2011) Arbuscular mycorrhiza impacts on drought stress of maize plants by lipid peroxidation, proline content and activity of antioxidant system. J Food Agric Environ 9(2):583–587
Acknowledgments
The authors would like to acknowledge to Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Ministerio de Ciencia y Tecnología (MINCyT) and Universidad de Buenos Aires (UBA) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bompadre, M.J., Fernández Bidondo, L., Silvani, V.A. et al. Combined effects of arbuscular mycorrhizal fungi and exogenous cytokinins on pomegranate (Punica granatum) under two contrasting water availability conditions. Symbiosis 65, 55–63 (2015). https://doi.org/10.1007/s13199-015-0318-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-015-0318-2