Abstract
Kodo (Paspalum scrobiculatum) and little (Panicum sumatrense) millet grains were utilized to minimize their antinutrient content (phytate and tannin) and maximize their antioxidant activity (DPPH) by studying the effect of ultrasonication time, germination time and temperature using central composite rotatable design. Results revealed the optimum conditions for producing ultrasonicated and germinated kodo and little millet flour of the highest antioxidant activity and lowest antinutrient content (phytate and tannin) by using 30 min for ultrasonication, 72 h for germination at 40 °C. Further, a second order model was developed to describe and predict the effect of process variables on antioxidant activity and antinutrient contents. Extended experiments were carried out under the optimized conditions to validate the developed model. The antioxidant activity obtained was 88.46% RSA and 89.06% RSA for kodo and little millet grain flours, respectively whereas antinutrient content for phytate was 0.165 mol/kg and 0.199 mol/kg and for tannin 2.88 mol/kg and 9.51 mol/kg, for kodo and little millet grain flours, respectively. This study provides useful information about the potential utilization of ultrasonicated and germinated kodo and little millet grain flours for the development of functional foods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Millets have been known since ages apparently about 5500 BCE but they gained popularity recently due to their nutritional and health benefits (Sharma et al. 2021). Millets have the power to pullulate even in a scorched land. Millets outperform other cereals like rice and wheat grain in terms of energy, dietary fibre, vitamins (particularly B1 and B2), and minerals (magnesium, phosphorous, and manganese) (Sharma et al. 2015; Liang and Liang 2019). The protein in millets are excellent supplier of all essential amino acids, except for lysine and threonine (Liang and Liang 2019). Kodo and little millets are two types of small millets which are prominent in Madhya Pradesh, India (Chouhan et al. 2019). They are plausibly high in dietary fiber amongst the cereals and also in poly unsaturated fatty acids (PUFA) in terms of fat content (Sharma et al. 2017).
The autogenous plant substances known as antinutrients have the potential to interfere with digestion and/or change the physiological, biochemical, and immune responses in species that use the plants as a source of nutrients. Antinutrients possess various functions depending upon their presence in organisms. In animal nutrition, they show a negative impact by reducing feed potency, inducing growth performance despair, and deteriorating general health (Krogdahl and Bakke 2015). Millets have anti-nutrients that deficit the nutritional property could be eliminated or inactivated through various processing technologies. Anti-nutrients phytate and tannin are found in kodo and little millets, preventing the assimilation of dietary proteins, carbohydrates, and micronutrients (Dey et al. 2022; Fu et al. 2023).
The phenolic compounds present in the millets dominate the antioxidant properties along with dietary fibre primarily found in bran layers (Sharma et al. 2017; Liang and Liang 2019). Many studies stipulate that oxidative stress may cause chronic and degenerative diseases and disorders while antioxidants are believed to play a significant role in lowering oxidative damage. Phenolic acids and flavonoids are the two potent antioxidants found in kodo and little millets. The antioxidants carotenoids and vitamin E finds their place in little millets. The mechanism of action for phenolic acids deals with the capability to make donations of hydrogen atoms to electrons lacking free radicles through hydroxyl groups upon benzene rings, forming a stabilized resonance and less receptive phenoxyl radical as a result. For flavonoids there is a significant antioxidant activity conferred upon the molecule by its many hydroxyl groups. The heterocycle’s activity is increased by a double bond and carbonyl function, which provides an even more robust flavonoid radical by colligation and electron displacement. The carotenoids function as effective antioxidants by squelching free radicals and single oxygen. The prevailing consensus is that the biological actions of tocols are caused by their antioxidant effect, which prevents lipid peroxidation in membranes that are biological. Additionally, the antioxidants like peptides of millets can be enhanced through the process of fermentation and germination (Liang and Liang 2019).
Germination is a process that involves steps like water absorption which helps in rootlet protrusion from the reposed seeds. Germination is among the most basic and successive processes to fix the nutritional constituents by bringing changes in biochemical and sensory elements of cereals by reducing anti-nutritive substances and aggravating the antioxidant levels and free amino acids along with acquirable carbohydrates and other components (Paul et al. 2015; Sharma et al. 2016). The process of germination magnifies the starch and protein digestibility, which proves to be superior to the process of blanching (Dey et. al. 2022). As we go by the definition of germination which starts with the uptake of water, the steeping of grains in water is a fundamental process that can solubilise and thus reduce the concentration of anti-nutrients. Also, steeping helps in inactivation of endoenzymes which starts germination by hydrolysing stored nutrients. The changes in lowering of anti-nutritional elements in cereals and legumes depend on the conditions and period of germination (Bhinder et al., 2021; Fu et al. 2021). Phytase activation upon germination was the reason for the elevation in mineral content. The germinated millets are associated with hydrolysis of phytate by phytase to produce inositol, unbound orthophosphate, and thus help in releasing the micronutrients (Yousaf et al. 2021). Therefore, germination has proved to be an efficient process in lowering anti-nutrients and enhancing the antioxidant activity of millets. Chauhan and Sarita (2018) reported a reduction of antinutrients like oxalic acid, tannin, and phytic acid in millets by germination.
Ultrasonication is a novel non thermal technique which is a boon to the food industry as it keeps a check on the quality and safety of food products along with its application in the association of reduced processing time, reduction in consumption of energy, and maximises the retention of nutrients available in food (Yadav et al. 2021). Ultrasound radiates high frequency sound waves which range in between 20 and 100 kHz having energies higher than 1 W/cm2 to improve the rates of mass transfer, enhancing the efficiency of extraction and functional characteristics of various nutrients (Vanga et al. 2020). A sequence of compression cycles and fractionation is caused by the high energy waves which are transmitted through food media where the molecules get disrupted in the liquid, triggering changes in the food matrices. The principle behind this concept is known as acoustic cavitation by which the energy is transferred to food samples and the process of cavitation causes micro-streaming, which improves heat and mass transfer, and rapid crumpling of cavitation bubbles leading to extreme pressure and temperature fluctuations in the surrounding region. This mechanism causes cell membranes to disintegrate, enzymes to inactivate, micro-channels to develop, and free radicals to generate (Sruthi & Rao, 2021). It has also been found that ultrasonication reduces the concentration of phytates and tannins in sprouted sorghum millet flour while increasing the concentration of phenols, antioxidants, and flavonoids (Hassan et al. 2020).
Although different types of techniques were utilized to reduce the antinutrient content in the millets, the information on the use of ultrasonication and germination on the millets to reduce the antinutrients is very scanty. This research finding provides the use of the kodo and little millets in formulation of the bakery products containing maximum antioxidant activity with low antinutrients. Due to the low antinutrients of kodo and little millets in the bakery products, it will be easy to digest the protein, starch, and minerals. Therefore, the present study deals with the optimization of the processing conditions of kodo and little millets to reduce the antinutrients and increase the antioxidant activity using central composite research design (CCRD) based on various independent variables such as ultrasonication time, germination time, and temperature.
Material and methods
Chemicals
Hydrochloric acid, potassium thiocyanate, ferric chloride, and tannin were purchased from Thermo Fischer, India. 2,2-diphenyl-1-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich (Bangalore, India), Vanillin was purchased from Molychem (Mumbai, India) and all other chemicals used were of analytical grade.
Millet collection and processing
Kodo (Paspalum Scrobiculatum) and little (Panicum Sumatrance) millets were procured from the farmers from Dindori village, Madhya Pradesh, India in February 2021. Both the millets grains were cleaned to remove debris and stored at −20 °C for further analysis.
Experimental design of germination process of kodo and little millets
A Central composite rotatable design (CCRD) of response surface methodology was selected for conducting 19 experimental runs by varying germination time (12–72 h), ultrasonication time (10–30 min), and temperature (20–40 °C). The time and temperature for germination conditions were based on previous and preliminary research (Sharma et al. 2017). Levels of the independent variables (germination time, ultrasonication time, and temperature) along with the experimental plan are shown in Table 1. The second order model was used to describe the effect of processing variables on the antioxidant activity, phytate, and tannin content of treated millet flours. The test of statistical significance was performed on the total error criteria, with a confidence level of 95%. The proportion of germination time, ultrasonication time, and temperature was optimized to find the best germination and ultrasonication conditions of both grains to maximize the antioxidant and minimize the phytate and tannin content. From this study, the functional millet flour was obtained which was rich in antioxidants and low in antinutrients (phytate and tannin content) that could be used as a functional ingredient in the development of nutrient rich food. One-way analysis of variance (ANOVA) generated by software (Design Expert v. 10 (Stat-Ease Inc., Minneapolis, USA) which compare among means with 5% of significance level was used to determine the significant terms for each response. The adequacy of the model was checked by calculating the R2 and the value of R2 near to 1 is most reliable. The regressions of coefficients were then used to make statistical calculations to generate 3-D plots from the regression model. The graphical representations showed the behavior of the responses affected by the independent variables.
Germination and ultrasonication of kodo and little millets
The thickness layer of 4.5–5.5 mm of fifty grams grains of each kodo and little millets were washed and soaked in 250 mL of water into a glass beaker (1000 mL) and kept at different germination times (14 to 72 h) and temperatures (20 to 40 °C) in an incubator chamber (New Brunswick Scientifics, Eppendorf AG, Germany) with relative humidity monitoring (Table 1). After germination both the millets (grains were placed into a glass beaker containing distilled water (1:10 g/mL)) were ultrasonicated (produced under distilled water (4 L)) in an ultrasonic water bath (MRC; DCG-150H, Star Micronic Devices, MRC Israel/heating power-300W/6 L tank capacity/internal tank size (L × H × W) (mm)-300 × 155 × 150) operated at ultrasonic power of 150 W, 40 KHZ with ultrasound intensity of 5.395 W/cm2 for 10–30 min at the same temperature [in ultrasonic water bath temperature was rise due to its heat generation and controlled by switching off the instrument for a short period] (Table 1). Water was changed during the germination process with an equal interval of 8 h and relative humidity of 80–90% within the incubator chamber was maintained by using a tray filled with water (500 mL). The thickness layer of 2–3 mm of both the millets grains were dried to a final moisture content of 10% in an oven (NU-101, Navyug Udyog, India) at 45 °C for 8 h after completion of the treatment and cooled to 25 °C.
Preparation of kodo and little millets flour (raw and treated (germinated and ultrasonicated) grains)
The dried treated (germinated and ultrasonicated) grains of kodo and little millets were ground to flour in a grinder (Sujata, India) and passed through 250 micron screen to obtain uniform particle size. The raw kodo and little millet flour were used as control. All the flours were packed in air tight containers and store at -20 °C for further analysis.
Analysis of dependent variable (Responses)
Analysis of antioxidant activity
Extraction was carried out as per the protocol of (Iftikhar et al. 2020) with minor modifications. Five grams of dried millet flour (250 micron size) that was mixed with 100 mL of absolute ethanol was used and sonicated in an ultrasound water bath (MRC, Israel) operated at 40 kHz at 60 °C for 30 min. The mixture was centrifuged (Sigma 3-18KS, Germany) at 1274 × g at 4 °C for 10 min and filtered with Whatman No. 1 paper. Each filtered extract was stored at -20 °C for further analysis.
Estimation of antioxidant activity of millets
The radical scavenging activity (RSA) of millet extracts by DPPH radical was determined according to Kumar et al. (2020) with minor modifications. An extract aliquot of 0.5 mL of freshly prepared 0.06 mM ethanolic DPPH was added to test tubes with 0.5 mL of millet extracts. The reaction mixture was mixed thoroughly and incubated in the dark for 30 min at room temperature. The absorbance was measured at 517 nm with a multimode microplate reader with a cuvette port (Spectramax M2e system, Molecular Devices, USA) against blank (ethanol). An equal amount of ethanol and DPPH served as control. All determinations were performed in triplicate. The radical scavenging activity was calculated as follows:
where, A0 is absorbance of the control solution, AB is the absorbance of DPPH solution in the presence of extracts, and As is absorbance of the sample extract without DPPH.
Estimation of antinutrient content (phytate and tannin) of millets
Phytate content of millets were determined by method described by (Sharma et al. 2016) with minor modifications. Two gram of ground sample of millets were soaked in 100 mL of 2% HCl for 5 h, filtered the solution with Whatman No. 1 paper. After filtration, 25 mL of filtrate was added to 5 mL of 0.3% potassium thiocyanate solution. Mixture was titrated with a standard solution of FeCL3, having about 0.00195 g of iron per milliliter, till a brownish-yellow colour appeared, which remained for 5 min.
where: T = titer value; M = Molar mass of phytate.
Tannin content of millet extracts was determined as described by Chang et al. (1994) with minor modifications. Two grams of millet flour were added in 0.1% acid (HCl)-methanol solution in a centrifuge tube. Incubate the extracts in a shaker (New Brunswick Scientifics, Eppendorf AG, Germany) at 24 °C for 20 min at 300 rpm. Centrifuged (Sigma 3-18KS, Germany) the extracts at 17,000 × g for 10 min, mixed 1 mL extract supernatant with 5 mL of 2% vanillin-HCl-methanol reagent, and vortexed the mixture for 30 min. The extracts were incubated at room temperature for 20 min and absorbance was measured at 500 nm with a multimode microplate reader with a cuvette port (Spectramax M2e system, Molecular Devices, USA). Tannin was used as a standard with a concentration of 0.1 to 1 mg/mL. All the determinations were performed in triplicates. The result was expressed as Tannic acid equivalent per 100 g (TAE/100 g).
Products optimization
Based on the result, data as obtained for responses after carrying out the runs and optimization, the best fit combinations of independent variables and responses, and the optimized conditions were used to prepare the treated samples of both the millets and subject to analysis to study the effects in detail. The characteristics studied of that of optimized samples of treated millets are detailed in the subsequent section and also compared with raw samples of respective millets.
Statistical analysis
Statistical analysis was conducted using Design-Expert v. 10 (Stat-Ease Inc., Minneapolis, USA). The experimental data were analysed using a second order polynomial equation. Analysis of variance (ANOVA) generated in the software was used for the statistical difference of all polynomial models and their terms. All measurements were carried out in triplicates and the values were reported as mean ± standard deviation.
where, Y: response variable; β0: intercept; β1, β2, β3: linear effects; β11, β22, β33: quadratic effects; β12, β13, β23: interaction effects; and X1, X2, X3: independent factors.
Result and discussion
This study depicted the combined effect of the three independent variables namely germination time (14–72 h), ultrasonication time (10–30 h), and temperature (20–40 °C) on three dependent variables i.e., tannic acid, phytate, and DPPH activity of kodo and little millets.
3.1. Optimization of treated (germination and ultrasonication) condition for production of kodo millet flour high in antioxidant activity and low in anti-nutrients (phytate and tannin content).
The predictive regression equation models for tannic acid, antioxidant activity (DPPH), and phytate content were selected. The tannic acid, antioxidant activity (DPPH), and phytate content of kodo millets flours are varied from 2.59–6.22 mg TAE/100 g extract, 61.69–94.07% RSA and 0.15–0.33 mol/kg, respectively (Table 1). Table 3, summarize the significant coefficients of the second order models for all the responses. A large regression coefficient and a small p-value in a model indicate adequacy and a significant effect on the respective response variables. Analysis of variance showed that tannic acid, antioxidant activity, and phytate content were significantly (p < 0.05) dependent on linear and quadratic terms of germination time (Gt), temperature (T), and ultrasonication time (Ut).
The ANOVA, data for response variables and their level of significance (95%) along with their correlation coefficient showed that the fitted models were suitable showing significant regression, low residual values, no lack of fit with satisfactory determination coefficients (R2) of 0.91, 0.92, and 0.93 for the response of tannin content, antioxidant activity, and phytate content, respectively. These results indicated the suitability of the model to be used in optimizing the conditions of germination for the kodo millet.
Effect of independent variables on tannic acid
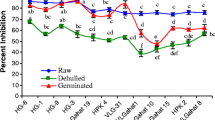
The tannic acid of kodo millet was significantly decreased (p < 0.05) with increasing germination time, ultrasonication time, and temperature (Fig. 1a). The tannic acid of kodo millet decreased from 4.94–2.74 mg TAE/ 100 g. The decrease content of tannin during germination may be due to leaching of the tannin into the water (Shimelis and Rakshit 2007). During germination, it was reported that polyphenol oxidase enzymes activated which are responsible for the degradation of polyphenols (Khandelwal et al. 2010). It has been reported that tannin content was decreased during germination in various cereals, pearl millet, and finger millet (Mbithi-Mwikya et al. 2000; Pushparaj and Urooj 2014). Also, ultrasonicated (76% amplitude, 17.5 min, 40 kHz) treated finger millet showed a 71% reduction in tannin content than the native millet (Dubey and Tripathy 2023). This would be due to the increased amplitude and treatment time during ultrasound treatment facilitated the tannin and hydroxyl radical interaction that converts tannic acid into gallic acid (Silva et al. 2019).
Response surface plot for kodo millet flour (a) Tannin content (TAE), (b) Antioxidant activity (DPPH), (c) Phytate content (In graphs, red dots indicate design points and blue to yellow/red colour indicates the desirability of the process factor combination from least desirable to most desirable, respectively)
Effect of independent variables on antioxidant activity (DPPH)
Antioxidant activity (DPPH) of kodo millet was significantly increased (p < 0.05) with increasing germination time, ultrasonication time, and temperature (Fig. 1b). Antioxidant activity (DPPH) of kodo millet increased from 61.69 to 92.77% RSA after germination (Table 1). The increase in % radical scavenging activity of kodo millets during germination is mainly due to the metabolic changes that take place as endogenous enzymes synthesise the phenolic compounds such as ferulic acid and some enzymes responsible for the degradation of bound phenolics during germination (Sharma et al. 2017). Sharma et al. (2021) reported similar results that the germination (48 h) process increased the DPPH activity from 44.20% to 70.21% in kodo millet (Hyderabad, India). Functional qualities like nutraceutical value and phenolic concentration can be improved by the process of malting. It was reported, the phenolic content in proso millet increased up to 3.5 times during the first five days of germination. The cell wall-degrading enzyme’s activation, which led to the alteration of the seeds’ cell wall and the release of bound phenols as well as an improvement in its antioxidant qualities, may be responsible for the rise in phenolic content (Yousaf et al. 2021).
Effect of independent variables on phytate content
Phytate content of kodo millet was significantly decreased (p < 0.05) with increasing germination time, ultrasonication time and temperature (Fig. 1c). Phytate content of kodo millet decreased from 0.301 to 0.148 mol/kg during germination. It has been depicted that during germination hydrolytic activity of phytase is responsible for the degradation of phytate (Sharma et al. 2017). In the process of germination phytate phosphorous is hydrolysed to inositol monophosphate by the activity of phytase enzyme which contributes in decreasing the phytate content (Sharma et al. 2016). It has been depicted by certain researchers that phytate content was reduced during germination in cereals, millet, soyabean, rice, sorghum, and lima beans (Azeke et al. 2011; Badau et al. 2005; Dave et al. 2008; Suma and Urooj 2014).
The ultrasound treatment helps in the reduction of phytate concentration with increasing soaking time and amplitude. This high amplitude is responsible for generating heat in the millet samples and also increasing the temperature. The content of phytate is decreased as a result of this heat which persuades chemical degradation and decreases the inositol phosphate. The concentration of phytate was found to be at a scale between 185.32 to 486.2 mg/ 100 g in finger millet (Yadav et al. 2021). Similarly, Dubey and Tripathy (2023) reported a 73% reduction in phytate content in ultrasound (76% amplitude, 17.5 min, and 40 kHz) treated finger millet than the native millet (6.89 mg/g). This would be due to the leaching of water-soluble phytate compounds and the higher amplitude of ultrasound treatment facilitated surface disruption. Also, the heat generation during ultrasound treatment degrades the phytate into low molecular weight inositol phosphate (Dubey and Tripathy 2023).
3.2. Optimization of germination conditions for the production of little millet having low anti-nutrients (tannin and phytate content) and high antioxidant activity
The experimental results obtained for each response variable are shown in Table 2. The tannin content, antioxidant activity, and phytate content of germinated little millet flours varied from 9.27–19.62 mg/ 100 g, 61.72–93.92% RSA and 0.199–0.331, respectively. Table 3, summarize the significant coefficients of the second order models for all the responses. A large regression coefficient and a small p-value in a model indicate adequacy and a significant effect on the respective response variables. Analysis of variance showed that tannic acid, antioxidant activity, and phytate content were significantly (p < 0.05) dependent on linear and quadratic terms of germination time (Gt), temperature (T), and ultrasonication time (Ut).
The ANOVA, data for response variables and their level of significance (95%) along with their correlation coefficient showed that the fitted models were suitable showing significant regression, low residual values, no lack of fit with satisfactory determination coefficients (R2) of 0.96, 0.94, and 0.95 for the response of tannin content, antioxidant activity, and phytate content, respectively. These results indicated the suitability of the model to be used in optimizing the conditions of germination for the little millet.
Effect of independent variables on tannin content
Figure 2a–c shows the influence of independent variables on the tannin content. All the independent variables showed a significant effect on the tannin content. The quadratic terms for variables germination time, ultrasonication time, and temperature (Table 2) significantly influence the tannin content of little millet flour (p < 0.05). The coefficient of determination (R2) of the fitted model was 0.96. This value indicates that the model obtained from the results explains 96% of the variance of the observed data (Table 3). A decrease in the tannin content of little millet flour was observed with an increase in germination time and temperature (Fig. 2a). It was reported that during germination tannin content leaches out in water which decrease the level of tannin content in the millets (Chauhan 2018), also tannin formed a hydrophobic-linkages to seed proteins and enzymes (Mbithi-Mwikya et al. 2000). Chauhan (2018) reported decrease in tannin content during germination (870 mg/100 g to 360.5 mg/100 g) in finger millet; similarly, Sharma et al. (2016) reported decreased in the tannin content of barnyard millet from 1.594 mg/100 g to 0.657 mg/ 100 g after germination (soaking time: 11.78 h, germination temperature: 33 °C, germination time: 36.48 h) process. Another reason behind the decreasing quantity of tannins is that upon germination, oxidation of tannins takes place and as a result the structure of the molecule is altered making it impossible for the Folin-Denis reagent to detect its concentrations (Savelkoul et al. 1992).
Response surface plot for little millet flour (a) Tannin content (TAE), (b) Antioxidant activity (DPPH), (c) Phytate content (In graphs, red dots indicate design points and blue to yellow/red colour indicates the desirability of the process factor combination from least desirable to most desirable, respectively)
The effect of ultrasonic treatment on tannin, reduced its concentration with optimum time and temperature combination due to the fact that the higher water concentration present in the sample helps in reduction of total tannin content. OH− and H+ are produced during homolysis of water, which hydrolyses the ester bond in the tannin. The process of ultrasound aids in the transformation of hydrolysable tannic acid in gallic acid. Additionally, it causes the condensed tannin present in millets to seep out, lowering the tannin content (Yadav et al. 2021). The value of total tannin content was found to be between 35.83 and 187.83 mg TAE/ 100 g in finger millet by Yadav et al. (2021).
Effect of independent variables on antioxidant activity (DPPH)
The regression analysis in Table 3 showed that the independent variables had positive effects on tannin content of little millet flour (p < 0.05). It was observed from the response surface plot (Fig. 2b) that the higher germination time leads increase in antioxidant activity which may be due to the biochemical changes of grains during germination which produce secondary plant metabolites and release of bound phenolic and flavonoid compounds (Sharma et al. 2021). Sharma et al. (2021), reported similar results regarding kodo millet that after germination DPPH* radical scavenging activity from 67.34% to 76.34% due to the scavenging of the radicals by hydrogen donation. Zheng et al. (2022) reported that the antioxidant activity (DPPH) in ultrasonic assisted extraction-deep eutectic solvent (UAE-DES) treated foxtail millet bran was found to be higher (13.29 µmol TE/g DW) than native millet bran (7.06 µmol TE/g DW). This would be occurred due to a slow increase of temperature and power during ultrasonication which leads to cavitation and improves the penetration of solvent into the foxtail millet bran particle structures that increase the liberation of intracellular polyphenols into the solvent and increase the antioxidant activity (Zheng et al. 2022).
Effect of independent variables on phytate content
From the Fig. 2c it is shown that germination time, ultrasonication time, and temperature significantly decreased (p < 0.05) the phytate content of little millet. The reduction in phytate may be due to the degradation of phytate and synthesis of phytase enzyme during germination (Dave et al. 2008). Phytase enzyme degrade the phytate into phosphate and myoinositol phosphates (Chauhan 2018). A similar study was reported that phytate content was reduced during germination from 851.4 to 238.5 mg/100 g in finger millet (Chauhan 2018); reduced from 2.91 to 0.282% during 72 h of germination in pearl millet cultivar SOSAT C-88 (Badu et al. 2005). Whereas phytates decreased from 1.344 to 0.997 mol/kg in kodo millets after 13 h of germination. The high phytase activity was the main reason of the decline of phytates (Sharma and Gujral 2020). Grgić et al. (2023) reported the reduction in phytic acid (PA) content in oat bran (17% reduction) and barley bran (39% reduction) after ultrasound assisted treatment (with different specific energies 87 kJ/kg, 217.5 kJ/kg, and 348 kJ/kg) than the native oat bran (PA: 17.35 mg/g dw) and barley bran (PA: 11.53 mg/g dw). This would be observed due to increase in the phytase activity in ultrasound assisted treated oat bran and barley bran (Grgić et al. 2023).
Optimized conditions of germination of kodo and little millets
Optimization of germination conditions of kodo and little millets was carried out to obtain desired criteria for each response (dependent variables-tannin content, antioxidant activity, and phytate content). Numerical multi-response optimization of RSM was applied to determine the optimum combination of germination time, ultrasonication time, and temperature for the maximize the antioxidants while minimizing the anti-nutrients (tannin and phytate content) (Table 4). The independent variables such as germination time kept at maximum level, whereas ultrasonication time and temperature were kept in range. The responses like antioxidant activity were maximized, whereas anti-nutrients (tannin and phytate content) were minimized based on the desirable characteristics of the kodo and little millets.
Kodo millet
A numerical multi-response optimization technique of RSM (CCRD) was applied to determine the optimum combination of germination time, ultrasonication time, and temperature for the production of germinated kodo millet flour with low anti-nutrients (tannin and phytate content) and high antioxidant activity. Second-order polynomial models obtained in this study were utilized for each response to determine the specified optimum germination condition. The optimization was applied for selected ranges of germination time, ultrasonication time, and temperature as 14–72 h, 10–30 min, and 20–40 °C, respectively. By applying the desirability function method, the desired solution was obtained for the optimum covering criteria with a value of 0.900. The solutions were obtained for the optimum combination of process variables (germination time = 72 h, ultrasonication time = 30 min, and temperature = 40 °C). At this moment, the predicted values of antioxidant activity (DPPH) and anti-nutrients (tannin and phytate content) were 89.60% RSA, 2.80 mg TAE/ 100 g, and 0.166 mol/kg, respectively. It was observed from the Table 4 that the predicted and experimental values showed the suitability of the model for optimizing the germination process for kodo millet.
Little millet
A numerical multi-response optimization technique of RSM (CCRD) was applied to determine the optimum combination of germination time, ultrasonication time, and temperature for the production of germinated little millet flour with low anti-nutrients (tannin and phytate content) and high antioxidant activity. Second-order polynomial models obtained in this study were utilized for each response to determine the specified optimum germination condition. The optimization was applied for selected ranges of germination time, ultrasonication time, and temperature as 14–72 h, 10–30 min, and 20–40 °C, respectively. By applying the desirability function method, the desired solution was obtained for the optimum covering criteria with a value of 0.939. The solutions were obtained for the optimum combination of process variables (germination time = 72 h, ultrasonication time = 30 min, and temperature = 40 °C). At this moment, the predicted values of antioxidant activity (DPPH) and anti-nutrients (tannin and phytate content) were 89.06% RSA, 9.51 mg TAE/ 100 g, and 0.199 mol/kg, respectively. It was observed from Table 4 that the predicted and experimental values showed the suitability of the model for optimizing the germination process for kodo millet.
Conclusion
The present study indicated that the minimum antinutrients content of phytate (0.165 mol/kg and 0.199 mol/kg) and tannin (2.88 mol/kg and 9.51 mol/kg) and highest antioxidant activity (88.46% RSA and 89.06% RSA) was obtained from the optimized conditions of ultrasonication time (30 min), germination time (72 h) and temperature (40 °C) of kodo and little millets, respectively. Predicted values were verified experimentally, confirming the general agreement with the model generated from the analysis. The present research concluded that ultrasonicated and germinated kodo and little millet grains could be used as an ingredient to develop a functional food that will be high in antioxidant content and rich in nutrient contents.
Data availability
Not applicable.
Code availability
Not applicable.
References
Azeke MA, Egielewa SJ, Eigbogbo MU, Ihimire IG (2011) Effect of germination on the phytase activity, phytate and total phosphorus contents of rice (Oryza sativa), maize (Zea mays), millet (Panicum miliaceum), sorghum (Sorghum bicolor) and wheat (Triticum aestivum). J Food Sci Technol 48(6):724–729. https://doi.org/10.1007/s13197-010-0186-y
Badau MH, Nkama I, Jideani IA (2005) Phytic acid content and hydrochloric acid extractability of minerals in pearl millet as affected by germination time and cultivar. Food Chem 92(3):425–435. https://doi.org/10.1016/j.foodchem.2004.08.006
Chang MJ, Collins JL, Bailey JW, Coffey DL (1994) Cowpeas tannins related to cultivar, maturity, dehulling and heating. J Food Sci 59(5):1034–1036. https://doi.org/10.1111/j.1365-2621.1994.tb08183.x
Chauhan ES (2018) Effects of processing (germination and popping) on the nutritional and anti-nutritional properties of finger millet (Eleusine coracana). Curr Res Nutr Food Sci 6(2):566–572. https://doi.org/10.12944/CRNFSJ.6.2.30
Dave S, Yadav BK, Tarafdar JC (2008) Phytate phosphorus and mineral changes during soaking, boiling and germination of legumes and pearl millet. J Food Sci Technol 45(4):344
Dubey A, Tripathy PP (2023) Ultrasound-mediated hydration of finger millet: effects on antinutrients, techno-functional and bioactive properties, with evaluation of Ann-PSO and Rsm optimization methods. Techno-functional and bioactive properties, with evaluation of Ann-PSO and rsm optimization methods.
Fu Y, Zhang F, Liu Z, Zhao Q, Xue Y, Shen Q (2021) Improvement of diabetes-induced metabolic syndrome by millet prolamin is associated with changes in serum metabolomics. Food Biosci 44:101434. https://doi.org/10.1016/j.fbio.2021.101434
Fu Y, Chen B, Liu Z, Wang H, Zhang F, Zhao Q, Zhu Y, Xue Y, Shen Q (2023) Effects of different foxtail millet addition amounts on the cognitive ability of mice. Food Biosci 51:102286. https://doi.org/10.1016/j.fbio.2022.102286
Grgić T, Pavišić Z, Maltar-Strmečki N, Voučko B, ČukeljMustač N, Ćurić D, Le-Bail A, Novotni D (2023) Ultrasound-assisted modification of enzymatic and antioxidant activities, functional and rheological properties of oat and barley bran. Food Bioproc Tech 28:1–14
Hassan S, Imran M, Ahmad MH, Khan MI, Xu C, Khan MK, Muhammad N (2020) Phytochemical characterization of ultrasound-processed sorghum sprouts for the use in functional foods. Int J Food Properties 23(1):853–863
Iftikhar M, Zhang H, Iftikhar A, Raza A, Begum N, Tahamina A, Syed H, Khan M, Wang J (2020) Study on optimization of ultrasonic assisted extraction of phenolic compounds from rye bran. LWT-Food Sci Technol 134:110243. https://doi.org/10.1016/j.lwt.2020.110243
Khandelwal S, Udipi SA, Ghugre P (2010) Polyphenols and tannins in Indian pulses: Effect of soaking, germination and pressure cooking. Food Res Int 43(2):526–530. https://doi.org/10.1016/j.foodres.2009.09.036
Krogdahl Å, Bakke AM (2015) Antinutrients. Dietary Nutr Addit Fish Health 29:211–235
Kumar Y, Singhal S, Tarafdar A, Pharande A, Ganesan M, Badgujar PC (2020) Ultrasound assisted extraction of selected edible macroalgae: effect on antioxidant activity and quantitative assessment of polyphenols by liquid chromatography with tandem mass spectrometry (LC-MS/MS). Algal Res 52:102114. https://doi.org/10.1016/j.algal.2020.102114
Liang S, Liang K (2019) Millet grain as a candidate antioxidant food resource: a review. Int J Food Prop 22(1):1652–1661
Mbithi-Mwikya S, Van Camp J, Yiru Y, Huyghebaert A (2000) Nutrient and antinutrient changes in finger millet (Eleusine coracan) during sprouting. LWT-Food Sci Technol 33(1):9–14. https://doi.org/10.1006/fstl.1999.0605
Pushparaj FS, Urooj A (2014) Antioxidant activity in two pearl millet (Pennisetum typhoideum) cultivars as influenced by processing. Antioxidants 3(1):55–66. https://doi.org/10.3390/antiox3010055
Savelkoul FHMG, Van der Poel AFB, Tamminga S (1992) The presence and inactivation of trypsin inhibitors, tannins, lectins and amylase inhibitors in legume seeds during germination. A Review Plant Foods Hum Nutr 42(1):71–85. https://doi.org/10.1007/BF02196074
Sharma B, Gujral HS (2020) Modifying the dough mixing behavior, protein & starch digestibility and antinutritional profile of minor millets by sprouting. Int J Biol Macromol 153:962–970. https://doi.org/10.1016/j.ijbiomac.2019.10.225
Sharma S, Saxena DC, Riar CS (2016) Analysing the effect of germination on phenolics, dietary fibres, minerals and γ-amino butyric acid contents of barnyard millet (Echinochloa frumentaceae). Food Biosci 13:60–68. https://doi.org/10.1016/j.fbio.2015.12.007
Sharma S, Saxena DC, Riar CS (2017) Using combined optimization, GC–MS and analytical technique to analyze the germination effect on phenolics, dietary fibers, minerals and GABA contents of Kodo millet (Paspalum scrobiculatum). Food Chem 233:20–28. https://doi.org/10.1016/j.foodchem.2017.04.099
Sharma S, Jan R, Riar CS (2021) Analyzing the effect of germination on the pasting, rheological, morphological and in-vitro antioxidant characteristics of kodo millet flour and extracts. Food Chem 361:130073. https://doi.org/10.1016/j.foodchem.2021.130073
Shimelis EA, Rakshit SK (2007) Effect of processing on antinutrients and in vitro protein digestibility of kidney bean (Phaseolus vulgaris L.) varieties grown in East Africa. Food Chem 103(1):161–172. https://doi.org/10.1016/j.foodchem.2006.08.005
Silva DAROD, Jorge LMDM, Jorge RMM (2019) Estudo e modelagem da cinética de hidratação de grãos de sorgo. Rev Cienc Agron 50(1):44–53
Suma P, Urooj A (2014) Influence of germination on bioaccessible iron and calcium in pearl millet (Pennisetum typhoideum). J Food Sci Technol 51(5):976–981. https://doi.org/10.1007/s13197-011-0585-8
Yadav S, Mishra S, Pradhan RC (2021) Ultrasound-assisted hydration of finger millet (Eleusine Coracana) and its effects on starch isolates and antinutrients. Ultrason Sonochem 73:105542. https://doi.org/10.1016/j.ultsonch.2021.105542
Yousaf L, Hou D, Liaqat H, Shen Q (2021) Millet: a review of its nutritional and functional changes during processing. Food Res Int 142:110197. https://doi.org/10.1016/j.foodres.2021.110197
Zheng B, Yuan Y, Xiang J, Jin W, Johnson JB, Li Z, Luo D (2022) Green extraction of phenolic compounds from foxtail millet bran by ultrasonic-assisted deep eutectic solvent extraction: optimization, comparison and bioactivities. Lwt-Food Sci Technol 154:112740
Acknowledgements
First author is grateful to the Vice Chancellor, Late Prof. Manjeet Aggarwal and Dr. Komal Chauhan, National Institute of Food Technology Entrepreneurship and Management (NIFTEM-K) for providing necessary facilities to carry out the research work.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SD conceived, carried out the experiments and wrote the manuscript; AS supervised the work, revised, and edited the manuscript; YK revised and edited the manuscript; TM revised the experiments; AT revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author confirm that they have no conflicts of interest with respect to the work described in this manuscript.
Ethical approval
Not applicable.
Consent to participate
All the authors declare there in no conflict of interest in publishing this manuscript.
Consent for publication
All the authors provide the consent to participate.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dey, S., Saxena, A., Kumar, Y. et al. Optimizing the effect of ultrasonication and germination on antinutrients and antioxidants of kodo (Paspalum scrobiculatum) and little (Panicum sumatrense) millets. J Food Sci Technol 60, 2990–3001 (2023). https://doi.org/10.1007/s13197-023-05837-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-023-05837-6