Abstract
The present study concludes the impact of storage on changes in physico-chemical characteristics of fermented whey cereal (pearl millet and moth bean) beverage. The beverage was prepared by fermented whey (standardised to 4% fat and 18% total solids) supplemented with germinated pearl millet and moth bean slurry & using NCDC-167 as starter culture for 6–8 h at 37 °C. The developed beverage was then stored at 5 °C for 4 weeks. The samples were analysed for physico-chemical characteristics (pH, titrable acidity, viscosity, tyrosine, FFA, wheying off), sensory qualities changes and microbial quality changes (standard plate count, lactic acid bacteria count, coli form counts) at 3 days’ interval for 4 weeks at 4 °C. Control was prepared standardised whey (4% fat and 18% total solids) while treatments were prepared using standardised milk. During storage: acidity, tyrosine values, free fatty acid values and wheying off increased in all the samples The upsurge was on higher side in non nisin treated and non thermised samples compared to control formulation. However, all the samples remained sensorily acceptable upto 12th day of storage. Basic and thermised as well as nisin treated fermented whey cereal products epitomize innovative dairy products with desired functional characteristics with decent shelf life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Now a day, there is rising demand by the consumers for functional/nutraceutical products which triggers the amalgamation of probiotic in milk products. Due to advancement in technologies, change in communal outlooks, the consumer’s awareness about fermented milk products acquiring drive, (Stanton et al. 2011). Fermented dairy foods constitutes a part of the human regime globally. Fermented dairy products like buttermilk boost the healthy microflora in the intestine. Whey is treasured with minerals like readily absorbable minerals (calcium and phosphorus) and vitamins like vitamins B and vitamin A. It also comprises superior protein, vitamins A, C, E, carotenoids, enzymatic antioxidants including glutathione peroxidase etc. (Kruni´c and Rakin 2022). It is more bioavailable which means its amino acids are more absorbed by the intestinal cells to make them available for further utilisation to make the proteins required. Fermented milk or whey based beverages also makes the maximum nutrients bioavailable. (Krunic, Rakin, Bulatovic, and Zaric 2018). Whey and whey based drinks also provide some beneficial effects like antimicrobial, immune-stimulating etc. (Khan et al. 2015). Whey is easy to use for preparation of beverages because of its liquid state, which act as good solvent (Chavan et al. 2015).
In many Asian and African nations, millets contribute majorily in the diet of underprivileged segments as a part of their staple diet and traditional recopies. Millets are considered to be beneficial for health specially for cancer, cardiac disease, tumour as well as for gut health (Gupta et al. 2012). Milk and cereal based composites are enriched with superior nutrients (Callaghan et al. 2019). There is improvement in nutritional value resulting from fermentation of the milk e.g., increasing the bioavailability of certain minerals such as Ca, Mg, Zn, Cu and Fe; decreasing the anti-nutritional factors; enhancing the release of bioactive peptides; increasing the levels of free amino acids and B group vitamins; and reducing the lactose content. (Rahmawati and Suntornsuk 2016). So, keeping this fact in mind, functional properties of milk proteins need to be utilised. Therefore, to utilise the underutilised highly nutritious whey and millet grains, the research was designed to prepare tasty and heathy fermented dairy products based on traditional cereals by blending whey, skim milk powder and millet grains (pearl millet).
Materials and methods
Raw materials
Std. Milk and cream (50% fat) was procured from LPT, LUVAS. Bacteriocin nisin and pectin was obtained from Himedia, Mumbai. The starter culture NCDC-167 (Lactococcus lactis ssp. lactis, Lactococcus lactis ssp. cremoris and Lactococcus lactis ssp. lactis biovar. diactetylactis in 1:1:1 ratio) was purchased from ICAR- NDRI, Karnal. SMP (Nova brand), pearl millet, moth bean, cumin, black pepper and salt, packaging materials i.e. glass bottles of 200 ml capacity were purchased from the local market. Paneer whey, standardized milk (4.5% fat & 8.5% SNF) and cream was acquired from Livestock Products Technology Department, LUVAS, Hisar.
Preparation of whey cereal beverage
Whey preparation
Whey was strained using muslin cloth was neutralized by sodium bicarbonate (@0.5gm/100 ml of whey) to pH 6.8 ± 0.2. Filtered and neutralized whey was standardised to 4% fat and 18% total solids level with the help of cream and skim milk powder and stored under refrigeration till further use.
Formulation of whey cereal based beverage (Lassi) with Thermization and Nisin
As shown in Fig. 1, The products (by using 4% fat, whey-germinated pearl millet and moth bean slurry and inoculation with NCDC-167) were prepared as shown in flow diagram 1 and subjected to three treatments: thermization (T) (65 °C-5 min), nisin (N) addition (400 RU/ml), and nisin + thermization (N + T), control was kept non thermized and non nisin treated. Four treatments viz. Control, C + T, C + N and C + N + T were prepared for whey pearl millet beverage (Lassi) and four treatments for whey moth bean beverage (Lassi). Glass bottles of 200 ml capacity were used for packaging after sterilizing the glass bottles in autoclave (121 °C for 15 min). All the treatments with packaging were kept at 5 ± 1 °C up to 4 weeks and the analysis was done at 3 days’ interval. Samples were analysed for physic-chemical parameters like pH, titrable acidity and viscosity, wheying off, tyrosine and free fatty acid.
Physicochemical analysis
pH
pH meter of Elico India Ltd. was used to determine the pH of fermented beverage with calibrations by standard buffers of pH 4.0, 7.0 and 9.1. After calibrating, electrode was dipped into samples and reading was recorded.
Titrable acidity
For estimating the acidity of fermented beverage (lassi), simple titration method was used in the presence of an indicator (phenolphthalein) as per AOAC (2007). For calculation, following formula was used:
% lactic acid = 0.09 × volume of NaOH × Normality of NaOH used × 100/Weight of Sample.
Viscosity
For determination of viscosity Rheology International Viscometer was used. For this, spindle was fixed (ASTM Disk Spindle-6 at room temperature) and then viscosity of homogenously mixed samples was recorded at 100 rpm, stated in centipoise.
Wheying off
For measuring the wheying off, 10 ml of sample was taken in a clean 15 ml graduated capped tube and then this tube was kept under refrigeration (5 ± 1 °C) for 24 h without disturbing. Then the separated amount of whey recorded as % wheying off.
Tyrosine
Tyrosine was estimated using Strange et al. (1977) method. Tyrosine extract was prepared by mixing 5 ml sample in 20 ml, 10% tyrosine and filtering it through Whatman 1 filter paper. Using 2.5 ml of this tyrosine and to this extract, 2.5 ml of distilled water was added. To this 3 ml of Folin’s reagent followed by 5 ml, 1N NaOH. Homogenous mixing of mixture was done and kept at 37 °C for 15 min. The readings were taken at 660 nm using Toshniwal Visible Spectrophotometer TVS 25. The tyrosine value was reported as mg of tyrosine per 100 g sample. The standard curve was prepared by taking different volumes of 0.0 ml, 0.5 ml, 1.0 ml, 1.5 ml and 2.0 ml of tyrosine solution (20 mg/100 ml).
Free fatty acids (FFA)
Free fatty acids (FFA) was determined using method of Deeth and Fitzgerald 1976. To three ml of sample (W), 5 mL of distilled water was added. Ten mL of extraction mixture (isopropanol, petroleum ether and 4 N sulphuric acids in the proportion of 40:10:1) was added along with six mL of petroleum ether. Then test tubes containing this mixture were kept at 40 °C for 10 min. The contents were vigorously shaken for 20 s. The two layers were formed in 5–10 min and a 5 ml aliquot from the upper layer was taken out in a 50 mL conical flask. Then the titration was performed using 0.02 N methanolic potassium hydroxide in the presence of 1% methanolic phenolphthalein indicator (six drops), One blank was also run. Following formula was used for calculating FFA: FFA = 1000VN/VW [μg/ml of fat] (normality of methanolic potassium hydroxide).
Sensory evaluation
For sensory evaluation, a semi trained panel consisting of faculty members and post graduate students of 25–55 age groups with 55–80 kg body weight with preference for dairy products, using a 9-point Hedonic scale as per method of Stone et al. 1974, assessed the sensory qualities viz. colour and appearance, flavour, consistency and overall acceptability as below. All the treatments with suitable codes were presented to the sensory panel for sensory evaluation. In between the samples the mouth was rinsed using water.
Evaluation card for taste panel
Name: Date/Time:
Product:
Parameters under 9-point hedonic scale | Score |
|---|---|
Liked extremely | 9 |
Liked very much | 8 |
Liked moderately | 7 |
Liked slightly | 6 |
Neither liked nor disliked | 5 |
Disliked slightly | 4 |
Disliked moderately | 3 |
Disliked very much | 2 |
Disliked extremely | 1 |
Assay for microbiological quality
Procedures of American Public Health Association (APHA 1992) were used for finding Standard plate count (SPC), lactic acid bacteria (LAB) count, coliforms count and yeast and mold (YMC) counts of the samples.
Procedure of sampling and serial dilutions
A pre-sterilized inoculation chamber of laminar flow (RH-58–03, Rescholar equipment’s, Ambala) was used to keep the samples. Sample (10 ml) was mixed in ratio of 1:9 to sterile 0.1% peptone water in pre-sterilized conical flask. The sample was thoroughly mixed to make further dilutions using sterile pipettes. Aseptic measures were taken during whole time.
Standard plate count (SPC)
Plate count agar (M091; Hi-Media Laboratories Pvt. Ltd., Mumbai) was prepared as per instructions and autoclaved. Pour plate technique was used. Incubation was done for 48–72 h at 35 °C in an inverted position. Colonies between 30 and 300 were counted and represented in log cfu/g.
Lactic acid bacteria (LAB)
Man Rogosa and Sharp (MRS) agar was prepared as per instructions and autoclaved. Pour plating method was used. Incubation was done for 48–72 h at 35 °C in an inverted position. Colonies between 30 and 300 were counted and represented in log cfu/g.
Coli forms counts
Violet Red Bile Glucose Agar was used for coliform count. For creation of anaerobic environment, an extra layer of agar was added. Incubation was done for 48–72 h at 35 °C in an inverted position. Red purple Colonies with about 0.5 mm diameter surrounded by a zone of precipitated bile between 30 and 300 were counted and represented in log cfu/g.
Yeast and mold counts
Potato dextrose agar was used. Acid was added after heating. Incubation was done for 5 days at 25 °C. Pour plate method was used incubated at 25 °C. Colonies with black, white, red and greenish black colour were counted and represented as log cfu/g.
Statistical analysis
The experiments were performed thrice for accuracy of results with duplicate samples while three observations were used for microbial analysis and sensory evaluation. Analysis was done statistically on ‘SPSS-16.0’ (SPSS Inc., Chicago II, USA) software package as per standard methods (Snedecor and Cochran 1994). Duncan’s Multiple Range Test was used for evaluation of significant treatments at 5% level (P ≤ 0.05) before ANOVA.
Results and discussion
Physicochemical analysis
Figure 2a and b shows the pH and titrable acidity at zero day and during the storage period of all samples of whey pearl millet and whey moth bean beverage respectfully. pH on zero day were around 4.33 in whey pearl millet and 4.82 in whey moth bean beverage without differences among formulations. There was significant difference in control as well as in treatments with storage; pH of all the samples decreased significantly at each day of investigation with advancement of storage. Such decreasing drift was also reported by Shaghaghi et al., (2013) in pH values of symbiotic yoghurts during storage. Behare and Prajapati (2007) reported a steady decline in pH and observed decline of higher rate in T1 (control) i.e. 4.34 and T2 (55 °C/5 min) i.e. 4.45 on 21 and 28 days than T3 (60 °C/5 min.) i.e. 4.66 and T4 (65 °C/5 min.) i.e. 4.72 on 35th day, however statistically non-significant. However, Ramana and Tiwari (1999) reported that there was no noticeable change in pH of lassi on heating. In nisin treated samples decrease in pH was slower in comparison to the non nisin treated samples.
Titrable acidity had shown increasing trend in all the treatments with storage. Increase was significantly higher in control as compared to treatments (T1, T2 and T3). On 21st day of storage T2 sample had significantly low acidity among all the samples. On zero days T3 had significantly lower acidity in comparison to other samples. Titrable acidity of the samples followed the trend such as C > T2 > T1 > T3 and till control got spoiled. The rise in acidity of nisin treated sample (T2) was lesser in comparison to non nisin treated treatments. A similar upsurge in the titrable acidity of probiotic lassi prepared using L. acidophilus and S. thermophillus was reported by Patidar and Prajapati (1998). Results are in accordance with observations of Behare and Prajapati 2007 on cultured buttermilk, where pH of all treatments decreased significantly (P ≤ 0.05) with storage. Prajapati et al. (1992) attributed slow development of acidity to the biochemical action of culture microorganisms and contaminants in heat treated shrikhand during storage. Pawar et al. (2010) observed that acidity score increased up to 3rd day and after that decrease was observed. Sarkar (2016) observed that nisin addition lowered down the acid development in yoghurt in comparison to non nisin samples due to inhibitory action of nisin in yoghurt cultures.
As shown in Tables 1 and 2, on zero days, viscosity values of C, T1, T2 and T3 of whey pearl millet beverage were 35.60, 35.50, 36.42, 36.00 and of whey moth bean beverage were 36.60, 36.10, 36.56 and 36.40 of whey pearl millet beverage which increased on 3rd day (which may be due to gel like structure formation due to interaction between pectin and water molecules (Oliveira et al. 2001) and thereafter a decrease was noticed to 31.00, 30.00, 31.20, 30.20 and of whey moth bean beverage to 30.00, 28.00, 30.90 and 28.90 respectively on 21st days of storage. The decrease in viscosity may be attributed to starch degradation due to microbial action during storage (Modha and Pal 2011). Similar results Similar trend was observed when respective treatments were compared on subsequent days of storage. Ramana and Tiwari (1999) described that increase in temperature of heating led to decrease in viscosity of homogenized samples, showing maximum decrease in samples heated to 70 °C for 5 min. This indicated a loose dispersion of hardened curd particles in serum phase of heat treated lassi. Similar result was reported by Hussain and Pal (2012) in microgard treated samples of sorghum malt based fermented milk beverage.
It is evident from results that non thermized (control & T2) samples had significantly lower tyrosine values in comparison to thermized (T1 & T3) samples. When non thermal treated samples were compared results indicated that the nisin treatment had shown remarkable effect on increase in tyrosine. Hussain and Pal (2012) also observed similar results in all sprouted sorghum lassi samples i.e. increase in tyrosine values on storage. Increase was more in control compared to others. This may be because of more microbial degradation of protein in control due to more microbial growth in control as compared to other samples.
A significant (P ≤ 0.05) increase was observed in FFA in all treatment on storage. Increase in free fatty acid is a result of progressive lipolysis. Nisin treated and thermised samples had lower values than the non nisin treated and non thermised respectively and the during storage same trend was observed. Similar results were observed by Hussain and Pal (2012) for all thermized samples all the time. Behare and Prajapati (2007) also reported a steady increase in FFA but at higher rate in T1 and T2 and at lower rate in T3 and T4. Patel (1984) also reported slower rate of FFA production in thermized yoghurt.
There was no wheying off up to 3rd day of storage in all the samples of whey-germinated pearl millet and moth bean based lassi stored at refrigeration storage. A significant (P ≤ 0.05) increase in wheying off was observed on each day of study irrespective of treatment of lassi samples. Similar observations were made by Modha and Pal (2011) up to 3rd day of storage in the rabadi like fermented milk beverage thereafter increase was seen. Lysis of starch may be responsible for this. (Cronk et al. 1977).
Sensory analysis
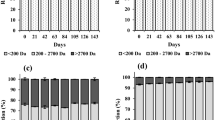
Figure 3a and b shows the sensory parameters which depicts that, in colour and appearance of freshly prepared whey-germinated pearl millet and moth bean lassi samples judges scored control at par with treatments. Thermized samples obtained significantly lower scores for colour & appearance throughout the storage study However, significantly higher value for colour and appearance was noticed for T2 sample in comparison to all other samples on 15th day of storage. Pawar et al. 2010 prepared Lassi by using different levels of nisin i.e. 100 IU/mI–400 IU/mI reported that nisin treated thermized samples scored significantly lowest score in comparison to rest of the samples on storage.
The consistency of whey-germinated pearl millet and moth bean lassi was judged equal in all the fresh lassi sample. On the basis of judges rating of consistency of control, T1 and T3 were still acceptable on the basis of consistency but rejected due to other sensory parameters results and not offered for further sensory evaluation whereas T2 sample remained accepted up to 21st day of storage. Heating in cultured products may lead to dehydration of proteins which results in whey separation. (Rasic and Kurmann 1978). Coarse mouth feel, whey separation and thin consistency were also observed by Ramaan and Tiwari (1999) for heat treated lassi (> 60 °C).
In freshly prepared whey-germinated pearl millet and moth bean lassi sample’s flavor was judged as liked moderately and no significant difference between control and treatments was noticed. On 15th day of storage judges rated T2 sample as liked moderately and other samples included control were rated as neither liked nor disliked on 9-point hedonic scale and therefore were rejected while T2 sample remained acceptable till 21st day. On heating, breakdown of preservative compounds may occur which may lead to decline in flavour in acidic environment, the same was prevented in nisin treated sample. Modha and Pal (2011) reported a decrease in flavour scores of rabadi like fermented beverage from 7.3 to 6.9 on 7th day of storage. Upsurge in acidity on storage may results decrease in flavour scores. As per judges’ overall acceptability of T2 remained acceptable till 21st day of storage whereas other samples (control, T1, & T3) were rejected on 15th day of storage and not offered for further sensory evaluation. Similar results were reported by Hussain and Pal (2012) for thermized samples than non thermized samples in sorghum malt milk composite beverage. Decrease in sensory scores of lassi in heat treated were also observed by Raman and Tiwari (1999).
Viable bacterial count
Figure 4a and b shows microbial count of lassi samples of whey pearl millet beverage and of whey moth bean beverage respectively, it was noticed that T1 and T3 (thermized samples) had significantly lower standard plate count (SPC) in comparison to control and T2 (non thermized samples) in both type of beverage, low counts in these samples may be due to thermization treatment given to these samples to extend the shelf-life. Similar trend was evident during the entire storage period i.e. 21st day. On refrigerated storage, low temperature and organic acids affect the the viability of the starter culture (Talwalkar and Kailasapathy 2004). This led to the decrease in standard plate count also. However, the nisin treated sample had second highest count on zero day, 9th day on ward it was found that the nisin treatment constantly maintained a significantly (P ≤ 0.05) low SPC in whey- germinated pearl millet lassi and the same trend was there till the product spoilage i.e. 21st day. The nisin treated thermized sample (T3) also resulted in lower SPC and the a significant (P ≤ 0.05) difference was observed as coompared to the other samples. This may be due to synergistic effect of nisin and thermization. This may be due to the fatal effect of thermization leading to the destruction of microorganisms. Behare and Prajapati (2007) also reported that as the thermization temperature increased, the rate of destruction of organisms also increased. A similar trend was evident till the product spoilage i.e. 21st day. Nisin can lead to termination of all biosynthetic process because of depolarization of cytoplasmic membrane (Kramer et al. 2004).
Fresh lassi samples when analysed for LAB it was noticed that T2 of both type of whey cereal beverage had significantly high count in comparison to all other samples followed by control. T1 and T3 (thermized samples) had significantly lower count, low counts in these samples may be due to thermization treatment given to these samples to extend the shelf-life. Similar trend was evident during the entire storage period i.e. 21st day. Acidity and hydrogen peroxide affect the sustainability of L. acidophilus (Dave and Shah 1997b). An increasing trend in acidity of the all samples was observed throughout the whole study. Survival of probiotic strains is markedly affected by low temperature and organic acids (Talwalkar and Kailasapathy 2004).
No coli forms were detected throughout the study. Other workers also reported similar results (Sarkar 2016; Sharma et al. 2016). It is apparent from the results that thermized samples (T1 & T3) has comparatively lower count than non thermized samples (control & T2). Nisin had no significant (P ≤ 0.05) effect on of yeast and mould counts during the study. Hussain et al. (2012) also noticed similar results in all the samples.
On 3rd day of storage, an increase was observed in viscosity of all samples of lassi and then slowly decreased on storage. On zero days, viscosity of all samples did not differ significantly to each other and follow the same trend on advancement of storage. Similar trend was observed when respective treatments were compared on subsequent days of storage. Ramana and Tiwari (1999) reported that viscosity of homogenized samples decreased with the increase in temperature. This indicated a loose dispersion of hardened curd particles in serum phase of heat treated lassi. Similar result was reported by Hussain and Pal (2012) in in microgard treated samples of sorghum malt based fermented milk beverage. Firstly, there was increase in viscosity of all the samples from day 0 to 3rd day and later gradually it decreased till the end of storage. Hussain and Pal (2012) also observed similar results. This increase may be contributed to gel structure formed by pectin and water molecules (Oliveira et al. 2001) and later on decrease may to enzymatic degradation of starch by microbes (Modha and Pal 2011). Breakdown of starch results into smaller molecules which cannot hold water (Cronk et al. 1977) which may result in decrease in viscosity.
Conclusion
-
Physico-chemical physiognomies of fermented whey based pearl millet beverage revealed that nisin and thermisation can be used as a tool for extension of shelf life of fermented whey beverage based on traditional cereals (pearl millet and moth bean) with storage in glass bottles at 5 ± 1 °C.
-
Nisin treated and thermised samples were shelf stable upto 21st day of storage while control and non nisin treated as well non thermsied were safe to consume up to 12 days
-
Fermented whey beverages based on traditional cereals (pearl millet and moth bean (Lassi) can be prepared standardized whey by inoculation with NCDC-167 culture.
-
Hence, the developed beverages based on whey-cereals (pearl millet and moth bean) can be commercialized and marketed with lower cost.
Data availability
Data will be provided, if required. Data supporting the results reported in the article can be found including, where applicable, hyperlinks to publicly archived datasets analysed or generated during the study.
Code availability
Not applicable.
References
APHA (1992) Compendium of methods for the microbiological examination of foods. In: Vandergant C, Splittstoesser DF, American Public Health Association, Washington, D.C, Adwan L, (eds) Fermented dairy drinks under pressure (online). Euromonitor international archive. Available from: http://www.euromonitor.com/article.asp?id=1371
AOAC (2007) Official methods of analysis, 18th edn. Association of Official Analytical Chemists, Washington, D.C.
Behare PV, Prajapati JB (2007) Thermization as a method for enhancing the shelf life of cultured buttermilk. Indian J Dairy Sci 60(2):86–93
O’Callaghan YC, Shevade AV, Guinee TP, O’Connor TP, O’Brien NM (2019) Comparison of the nutritional composition of experimental fermented milk:wheat bulgur blends and commercially available kishk and tarhana products. Food Chem 278:110–118
Chavan RS, Shraddha RC, Kumar A, Nalawade T (2015) Whey based beverage: its functionality, formulations, health benefits and applications. J Food Proc Technol 6(10):495
Cronk TC, Steinkraus KH, Hackler LR, Mattick LR (1977) Indonesian tape ketan fermentation. Appl Environ Microbiol 33:1067–1073
Dave RI, Shah NP (1997) Viability of yogurt and probiotic bacteria in yogurt made from commercial starter culture. Int Dairy J 7:31–41
Deeth HC, FitzGerald CH (1976) Lipolysis in dairy products: a review. Aust J Dairy Technol 31(2):53–64
Gupta N, Srivastava AK, Pandey VN (2012) Biodiversity and nutraceutical quality of some Indian millets. Proc Natl Acad Sci India Sect B Biol Sci 82:265–273. https://doi.org/10.1007/s40011-012-0035-z
Hussain SA, Gargh FC, Pal D (2012) Effect of different preservative treatments on the shelf life of sorghum malt based fermented milk beverage. J Food Sci Technol. https://doi.org/10.1007/s13197-012-0657-4
Khan S, Amin N, Ansari Z, Majumder DR (2015) WHEY: waste to health and wealth. Int J Curr Microbiol Appl Sci 2:245–325
Kramer NE, Smid EJ, Kok J, de Kruijff B, Kuipers OP (2004) Resistance of gram positive bacteria to nisin is not determined by lipid ΙΙ levels. FEMS Microbiol Lett. 239:157–161
Kruni´c TZ, Rakin MB (2022) Enriching alginate matrix used for probiotic encapsulation with whey protein concentrate or its trypsin-derived hydrolysate: impact on antioxidant capacity and stability of fermented whey-based beverages. Food Chem 370:130931
Krunic T, Rakin M, Bulatovic M, Zaric D (2018) Chapter 9—the contribution of bioactive peptides of whey to quality of food products. In food processing for increased quality and consumption; grumezescu. In: Holban AM (ed) Handbook of food bioengineering. Academic Press, Cambridge, pp 251–285
Modha H, Pal D (2011) Optimisation of rabadi like fermented milk beverage using pearl millet. J Food Sci Technol 48(2):190–196
Oliveira JD, Silva DA, de Paula RCM, Feitosa JPA, Paula HCB (2001) Composition and effect of salt on rheological and gelation properties of enterolobium contortisiliquum gum exudate. Int J Biol Macromol 29:35–44. https://doi.org/10.1016/S0141-8130(01)00151-9
Patel MT (1984) Evaluation of the effects of methods of lactose hydrolysis and post- incubation thermization in relation to manufacture and quality of stirred and frozen yoghurts. Gujrat Agricultural University, Anand campus, Gujrat
Patidar SK, Prajapati JB (1998) Standardization and evaluation of lassi prepared using Lactobacillus acidophilus and Streptococcus thermophilus. J Food Sci Technol 35(5):428–431
Pawar BK, Choudhari DM, Kamble DK (2010) Effect of nisin on shelf life of lassi. Asian J Dairy Food Res 29(2):79–85
Prajapati PS, Gupta GR, Patel AA (1992) Studies on the preparation of Shrikhand by using selected starter. Indian J Dairy Sci 1992:1–5
Rahmawati SI, Suntornsuk W (2016) Effects of fermentation and storage on bioactive activities in milks and yoghurts. Procedia Chem 18:53–62. https://doi.org/10.1016/j.proche.2016.01.010
Ramana BLV, Tiwari BD (1999) Effect of processing variables on sensory and physico-chemical qualities of heat treated lassi. Indian J Dairy Sci 52(5):272–278
Rasic JL, Kurmann JA (1978) Yoghurt: Scientific grounds, technology, manufacture and preparations. Technical Dairy Publishing House, Copenhagen, p 466
Sarkar S (2016) Effect of nisin on technological and microbiological characteristics of stirred yoghurt. J Microbiol Microbial Technol 1(1):01–06
Shaghaghi M, Pourahmad R, Mahdavi Adeli HR (2013) Synbiotic yogurt production by using prebiotic compounds and probiotic lactobacilli. Int Res J Appl Basic Sci 5:839–846
Sharma S, Sreeja V, Prajapati BJ (2016) Development of symbiotic lassi containing honey: studies on probiotic viability, product characteristics and shelf life. Indian J Dairy Sci 69(2):148–153
Snedecor GW, Cochran WG (1994) Statistical methods, 9th edn. Iowa State University Press, Ames, Iowa
Stanton C, Gardiner G, Meehan H, Collins K, Fitzgerald G, Lynch PB, Ross RP (2011) Market potential for probiotics. Am J Clin Nutr 73(2):476S-483S
Stone H, Sidel J, Oliver S, Woosley A, Singleton RC (1974) Sensory evaluation by quantitative descriptive analysis. Food Technol 28:24–34
Strange ED, Benedict RC, Smith JL, Swift CE (1977) Evaluation of rapid test for monitoring alteration in meat quality during storage. J Food Prot 40:843–847
Talwalkar A, Kailasapathy K (2004) A review of oxygen toxicity in probiotic yoghurts: influence on the survival of probiotic bacteria and protective techniques. Compr Rev Food Sci Food Saf 3:117–124
Acknowledgements
Authors are highly thankful to Vice-Chancellor, LUVAS for providing needful facilities in Department of Livestock Products Technology. Authors are also thankful to Department of Science and Technology, India for financial assistance throughout the study.
Funding
Authors are thankful to, LUVAS for providing needful facilities in Department of Livestock Products Technology. Authors are also thankful to Department of Science and Technology, India for financial assistance.
Author information
Authors and Affiliations
Contributions
MR conceived, carried out the experiments and wrote the MS, while RSD supervised the work and SB and GJ corrected the manuscript and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics approval
Not required.
Consent to participate
I hereby submit my consent for publication of the manuscript to JFST.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rani, M., Dabur, R.S., Bishnoi, S. et al. Influence of storage on physico-chemical physiognomies of fermented whey cereal (pearl millet and moth bean) beverage. J Food Sci Technol 61, 117–128 (2024). https://doi.org/10.1007/s13197-023-05825-w
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-023-05825-w