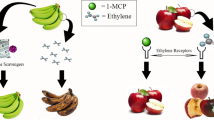
Abstract
Fruit ripening is an unfolding of a series of genetically-programmed modifications and tend to be highly orchestrated irrevocable phenomenon mediated by ethylene. Phytohormone ethylene also leads to over-ripening, senescence, loss of texture, microbial attack, reduced post-harvest life and other associated problems during storage and transportation of fruits. Its harmful impacts on fresh fruits, vegetables, and ornamentals result in substantial product losses even up to 80%. Curbing of this inevitable menace is therefore need of the hour. Accrual of ethylene in packaging system should fundamentally be ducked to extend the shelf-life and uphold an adequate superiority of perishables in visual and organoleptic terms. The current review discusses about properties, factors affecting and impact of ethylene, intimidation of its impact at gene vis-à-vis activity level using gene-modification/inhibition techniques, chemical/physical in conjunction with other suitable approaches. It also entails the most commercially cultivated approaches worldwide viz. KMnO4-based oxidation together with adsorption-based scrubbing of ethylene in thorough details. Future ethylene removal strategies should focus on systematic evaluation of KMnO4-based scavenging, exploring the mechanism of adsorption, adsorbent(s) behavior in the presence of other gases and their partial pressures, volatiles, temperature, relative humidity, development of hydrophobic adsorbents to turn-up under high RH, regeneration of adsorbent by desorption, improvement in photocatalytic oxidation etc. and further improvements thereof.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The process of fruit ripening is an unfolding of a series of genetically programmed modifications in terms of its biochemical, physiological, textural, sensorial and organoleptic attributes and tend to be a highly orchestrated irrevocable phenomenon. Phytohormone ethylene originated during beginning of ripening of fruits spearheads genes for manufacturing various ripening enzymes (Kumar et al. 2019). The origin of ethylene can be biological or non-biological environmentally. Higher plant tissues, few algae, certain mosses and numerous bacterial species constitute ethylene biologically (Vermeiren et al. 2003); conversely, major non-biological contributors of ethylene include fossil fuels’ incomplete incineration, fluorescent lights, burning of agricultural wastes, emissions from automobiles, smoke, and seepage of industrial polyethylene production plants etc. The estimated annual total global (atmospheric) ethylene emission is to the tune of 18 − 45 × 106 tons, of which 74 and 26% arise due to natural and manmade sources, respectively. The demolition of ethylene takes place primarily in the troposphere of earth’s atmosphere where it reacts with •OH radicals (89%) and O3 (8%). The estimated atmospheric lifespan of ethylene oscillates somewhere amid 2–4 days (Keller et al. 2013; Sawada and Totsuka 1986).
Ethylene is a natural ripening phyto-hormone necessarily required for natural ripening of fruits, though each fruit has its own dose requirements depending upon fruit type, variety, stage of ripening, as well as ripening behaviour (climacteric/non-climacteric). Fresh produce releases different concentrations of ethylene, and susceptibility to ethylene exposure also varies for various perishables. The gaseous hormone can alter the physico-chemical stability of commodities at the concentration 10–100 nL L−1 (Aghdam et al. 2019; Sadeghi et al. 2021). On the other hand, it leads to over-ripening, senescence, loss of texture, microbial attack, less post-harvest life and other associated problems during storage and transportation of fruits. It is no exaggeration to say that ethylene is one of the most notorious contributors to post-harvest losses. Ethylene, chemically a simple molecule of the alkene type has multifarious effects on growth, development and storage life of many fruits, vegetables, and ornamental crops in a dose dependent manner (Saltveit 1999). It exerts its effect as physiological ripening agent at enormously low concentrations ranging from ppm (μL L−1) to ppb (nL L−1) under controlled conditions. It has been observed that even insignificant quantities of ethylene all through shipping and storage can lead to quicker deterioration of fresh fruits, vegetables, and ornamentals. The ethylene can harm perishables resulting in significant product losses oscillating between 10 and 80% (Kader 2003; Keller et al. 2013). The generation of ethylene during ripening and storage leads to rapid senescence, decay, loss of firmness and subsequent diminished shelf-life as ethylene acts as an elicitor of ripening-mediated variations. The evident symptoms of short shelf-life include decay, weight loss, deterioration of appearance, textural and nutritional quality attributes (Ulloa 2007). These changes are arbitrated by cell wall degrading enzymes (cellulases, pectinases), pathogenesis related (PR) proteins responsible for tissue softening and decay, color as well as flavor/aroma related changes. Inhibiting ethylene action can have fabulous benefits commercially during the storage of ethylene sensitive perishable commodities. For example, 1% wastage in a refrigerated cargo ship having a load of 8000 tons amounts to 80 tons loss of perishable produce, which is a mammoth amount (Keller et al. 2013). Thus, in order to control the post-harvest losses, decay and senescence, the management of ethylene concentration remains to be of utmost importance. The present review discusses various ways researched throughout the world to curb the menace of ethylene thus maintaining the freshness of perishables and minimizing post-harvest losses while storage and transportation with specific reference to oxidative and absorptive means.
Climacteric versus non-climacteric fruits
Fruits are generally classified into climacteric or non-climacteric types depending upon the pattern of respiration, and responsiveness to externally supplemented ethylene. The major differences between both can be enumerated as given in Table 1.
Effects and impact of ethylene
Inhibiting the action of ethylene or removing it from perishables’ surroundings can have incredible advantages in terms of commerce for the storage of ethylene sensitive perishables. Accumulation of ethylene in the packaging system should fundamentally be avoided/stopped in order to protract the shelf-life and uphold an acceptable quality of fruits and vegetables with regard to visual and organoleptic characteristics (Chaves and Mello-Farias 2006; Sadeghi et al. 2021). Modern technologies for foods like active packaging these days are engineered to confiscate unwanted elements (including ethylene) from the headspace of packaging via absorption, adsorption or scavenging. The said target is fulfilled by incorporating a sachet/blanket/filter etc. with commodity or a physical or chemical absorbent/adsorbent (agent) is placed directly in the packaging itself (Gaikwad et al. 2018; Sadeghi et al. 2021). The containment of ethylene for maintaining the freshness and lowering post-harvest losses of perishables is impetus and need of the hour. Many leading companies in the world offer newer ways to curb the menace of ethylene and save the produce from losses. According to the extensive review of Keller et al. (2013), the work related to ethylene curbing can be categorized in to two major ways: plant level actions for inhibiting ethylene production as well as action (genetic as well as chemical approaches), whereas another way concerns environment level actions (avoidance, inhibition, and removal). The inhibition includes controlled atmosphere (CA), modified atmosphere (MA), modified atmospheric storage (MAP) and hypobaric storage whereas the removal strategies comprise of ventilation, destructive oxidation and adsorption. The oxidation can be chemical mediated (KMnO4, UV-C, ozone, photocatalysis etc.) and by biological means (use of biofilters, microorganisms etc.) (Keller et al. 2013). Further, various researchers have signposted that ethylene has capability to infuse through physical materials like cardboard boxes, wooden packaging, and concrete walls thus, additionally complicate the curbing process. Wills et al. (2000) have reported that at obviously higher concentrations, ethylene can undergo diffusion with ease from one storage room to another. Potassium permanganate-based scrubbing of ethylene is practiced worldwide by leading companies of the world. In order to enhance the scavenging capacity, KMnO4 is usually embedded in various inert support materials such as minerals or nanoparticles in the form of permeable sachets (Sadeghi et al. 2021). Other approaches also have their own pros and cons.
In addition to ripening, ethylene is also involved in regulating various other vital processes during plant growth and development. These include fruit/vegetable ripening, seed germination, cell elongation, defence against pathogens, flowering, dormancy, senescence, geotropism and response to external stress factors (Gaikwad and Lee 2017; Gaikwad et al. 2020; Zhu et al. 2019). Apart from its role of fruit ripening (which makes the perishables an elixir) and as a base of ‘n’ number of branches of science viz. Physiology, Food Science, Biochemistry, Aroma and Flavor Science etc., ethylene has been considered problematic while post-harvest management of horticultural foodstuffs (fruits, flowers and vegetables). When ethylene gets attached to its receptor, it accelerates a series of reactions for ripening, texture softening and organoleptic changes at the level of synthesis as well as activity at enzyme level (cellulase, pectinase, ethylene synthesis etc.). It is involved in accelerating chlorophyll degradation and inducing yellowing of green tissues viz. leafy green vegetables (spinach), immature cucumbers and broccoli, flavour changes, conversion of starch to sugar, loss of acidity and textural changes (Zagory 1995). Table 2 is enumerating the specific effects of ethylene exposure on post-harvest shelf-life, disorders and marketable quality of fresh fruits and vegetables. Ethylene also boosts the chances of pathogenesis as a consequence of stimulating physiological ripening and senescence (Vermeiren et al. 2003).
Various ethylene control approaches
Historically in 1864, leakage of an illuminating gas (containing ethylene) in greenhouse resulted in premature senescence and defoliation of plants and trees growing near the gas lines. Ethylene (C2H4) is a small colourless gaseous molecule having molecular weight 28.05 g/mol (density 1.178 kg/m3 at 15 °C) and is lighter than CO2 (44 g/mol) and O2 (32 g/mol). Ethylene’s double bond makes it amenable to be modified or tarnished in various ways and offers a myriad of opportunities for profit-making methodologists to remove/curb ethylene levels (Chowdhury et al. 2017). Table 3 represents various approaches researched for curbing the menace of ethylene either at the level of synthesis or during storage and transportation. One of the main things to be kept in mind that once ripening of a fruit has progressed towards its climacteric stage, reducing the external ethylene concentration cannot reverse ripening. However, during the initial stage of fruit ripening, when internally very low ethylene concentration is present, either inhibiting the production and/or removal of ethylene can slow down the ripening of climacteric perishables to a larger extent (Saltveit 1999).
Factors affecting ethylene production and its response/action during storage and transport of perishables
Various studies indicated that although ethylene may exhibit little toxicity yet pose no potential risk to human health. Interestingly, ethylene was used as an anaesthetic for many years before the advent of modern anaesthetics, but replaced eventually due to its high explosion risk as ethylene in air oscillating between 2.75 and 28.6% at 0.1 MPa and 20 °C may explode (Keller et al. 2013). The minimum and maximum odour threshold levels are 299 ppm and 4600 ppm, respectively. Ethylene molecules are perceived by its receptor present in cell membranes of plants. The ethylene-receptor binding unlocks the receptor, which in turn results in a myriad of chemical reactions at cell and tissue level leading to genetic as well as colour, texture and other related changes which further enhance the production of ethylene (Keller et al. 2013). The latter authors reported that perishables are loaded usually ∼85% by volume, of the container capacity. Depending upon the perishable goods stored, the air circulation rates vary accordingly.
-
a.
Temperature: Horticultural commodity has its own recommended temperature. At low temperature, there is substantial decrease in fruit metabolism leading to significantly sluggish response to ethylene, while the perishable commodities’ deterioration increases two to threefold for every 5 °C upsurge in temperature. The recommended temperature and relative humidity (RH) for short term storage of fruit and vegetables are (provided the concentration of ethylene should remain < 1 ppm in storage locations) as follow: cole crops, most of the leafy vegetables (0–2 °C; 90–98%), fruits and berries (temperate type) (0–2 °C; 85–95%), citrus, other subtropical fruits and various vegetables resembling fruits (7–10 °C; 85–95%), general root-type vegetables, melons, winter squash and most tropical fruits (13–18 °C; 85–95%) (Keller et al. 2013).
-
b.
Relative humidity (RH): The relative humidity can significantly affect water loss, uniformity of fruit ripening, progress of decay and physiological disorders. While storing fruits, the most suitable RH arrays between 85 and 95%, although in case of vegetables, the corresponding range fluctuates between 90 and 100% (Kader and Rolle 2004).
-
c.
Ethylene: At sea, remote sites and rural areas, ethylene concentration ranges to < 1 − 5 ppb while in urban and indoor areas, it may reach up to the level of 50 ppb. In sealed cold store, controlled atmospheric storage and mixed cargoes, the ethylene may be very high, which could result in deterioration of perishable commodities. Ethylene is able to pervade through cardboard boxes, wooden packaging, and concrete walls and to diffuse one storage compartment to another (Keller et al. 2013; Wills et al. 2000). The C2H4 concentrations greater than 0.1 μL L−1 affect fresh produce storage life firmly and C2H4 concentration between 0.1 and 0.5 μL L−1 is considered as threshold level to induce ripening in fruits like banana, melon, avocado and pear etc. (Blanke 2014).
-
d.
Oxygen: Low oxygen (than 21%) leads to less respiration and ethylene production specifically in modified and controlled atmospheric storage as well as in modified atmospheric packaging (Keller et al. 2013).
-
e.
Carbon dioxide: High CO2 accumulated due to respiration may decrease ethylene production.
The concentration of C2H4 in the product’s vicinity is undesirable and remains as a factor of utmost importance to be controlled as it often leads to more rapid post-harvest deterioration of fresh produce. This happens especially while their storage and transport and generally leads to significant losses to perishables. Importantly, refrigeration (low temperature) and humidity slow down the decay, but are unable to arrest ethylene generation completely. They don't halt the production of harmful ethylene gas completely. The climacteric fruits are the major sources of C2H4 and capable of altering horticultural produce environments (Pathak et al. 2017). Cleavage, chemical modification, absorption and adsorption of ethylene molecule are employed in manufacturing the ethylene scavengers (Alves et al. 2022). Ethylene scavenging can be done by chemical or physical methods (absorption, adsorption and/or other oxidation mechanisms) from the surrounding environment to maintain good keeping quality of fresh fruits and vegetables for comparatively longer periods (Chopra et al. 2017; Yildirim et al. 2018). Chemical molecules like electron deficient dienes and trienes like benzene and pyridine (Alves et al. 2022) and resveratrol (Li et al. 2022) can also be explored for ethylene scavenging. As reported in the review of Álvarez-Hernández et al. (2018), in terms of value, advanced packaging captures nearly 5% share of the total packaging market worldwide, out of which 35% (nearly 1/3rd) owes to active packaging. However, C2H4 scavengers epitomize 3% of total market share of gas elimination packaging technologies (Gaikwad and Lee 2017; Wyrwa and Barska 2017).
KMnO4-based oxidation and adsorption-based ethylene elimination
In further section, detailed discussion of KMnO4-based oxidation and adsorption-based methods has been elaborated as the former is most commercially cultivated while the latter is one of the technologies of choice for research in the area of ethylene elimination.
KMnO4-based oxidation
Ethylene scavengers effectively eradicate ethylene generated by packed fresh produce via absorbing/scavenging it, thus containing postharvest fatalities (Gaikwad and Ko 2015). By far the most successful and commercially viable scavenger of ethylene from horticultural products, used worldwide is KMnO4-based oxidation on activated alumina support, though other adsorbents also find place either as support material or as ethylene adsorbent itself (Álvarez-Hernández et al. 2018; Gaikwad and Lee 2017; Keller et al. 2013). The commercially accessible scavengers can scavenge ethylene to the tune of 3 to 6.5 L/kg (Scully and Horsham 2007). Table 4 enlists various manufacturers involved in KMnO4-based oxidation system providers, their trade names, support materials and other accessible information as per the available literature to have a thorough glimpse of scavenging-based ethylene removal methods.
KMnO4-based oxidation process is a sort of destructive approach allowing irreversible as well as continuous ethylene removal (Keller et al. 2013). One more thing should be kept in mind while dealing with ethylene scavenging mechanisms that natural convection and diffusion are the only driving forces involved in gas movement. Therefore, for enhanced oxidation of ethylene by KMnO4, the latter is generally reinforced onto solid carrier materials which are inert, have minute particle size with a large surface area such as celite, activated alumina, vermiculite, silica gel, activated carbon, limestone, clay, zeolite, perlite, pumice, brick, or glass etc. (Shaabani et al. 2005; Spricigo et al. 2017). These inert materials adsorb/absorb ethylene and provide huge surface area for latter’s smooth interaction with KMnO4 (Álvarez-Hernández et al. 2018; Gaikwad et al. 2020; Wills and Warton 2004). The concentration of KMnO4 may vary from 2.5 to 12% as reported by various researchers. Typically, the average concentration of KMnO4 remains about 4–6%. The scavenging capacity largely depends upon surface area of material and KMnO4 concentration (Gaikwad et al. 2020; Zagory 1995). The oxidation of ethylene with potassium permanganate gets accomplished in a two-step process. On reaction, ethylene (C2H4) gets converted to acetaldehyde (CH3CHO) via oxidation, which is then gets oxidised to acetic acid (CH3COOH). Acetic acid can further be transformed to harmless entities like carbon dioxide and water in an oxidative reaction. The entire process can be represented as:
KMnO4 being powerful oxidant, oxidises C2H4 to harmless CO2 and H2O in a cheap and easy way. KMnO4-based scavengers can be used in an array of ways viz. in active packaging, storage, transportation and domestic refrigerators (Gaikwadet al. 2020; Keller et al. 2013). These scrubbers are available in the form you name e.g., sachets, bags, tube filters, blankets, labels or films etc., though sachets are the most widely used form due to their suitability for individual packaging (Janjarasskul and Suppakul 2018) and ease of application (Álvarez-Hernández et al. 2018). However, KMnO4 cannot be placed in direct contact with foodstuffs owing to its toxic nature and insufficient enduring efficacy under high moisture conditions which remains a pre-requisite during storage of most of the fresh fruits and vegetables, KMnO4 cannot be used in direct contact with foodstuffs (Gaikwad et al. 2019; Wyrwa and Barska 2017; Yildirim et al. 2018). Post oxidation, potassium permanganate changes its color from purple to brown due to consequent reduction of MnO4 to MnO2. Studies conducted by various researchers have revealed the effectiveness of these sachets in removing ethylene from packages of bananas, diced onions, apples, mango and tomato etc. (Vermeiren et al. 2003). Warsiki (2018) prepared chitosan and KMnO4-based active packaging and used it for tomato ripening inhibition. The fruits packed with KMnO4-based active packaging possessed high hardness compared to control at ambient storage, however, the tomato stored at refrigerated storage had lower hardness value when compared with respective control. Spricigo et al. (2017) studied the effect of particle size (viz. micro- versus nanoparticles) as support material; KMnO4 content (2.5, 5 and 10%) and RH (45, 60, 75 and 90%) on KMnO4-based oxidation. KMnO4-based ethylene scrubbers (0.3 g sample used for each experiment) supported onto SiO2 and Al2O3 (at 25 °C) oxidized 7.48 ml/L C2H4 after 1 h of exposure. Ethylene removal rates showed an upsurge with decreased particle size and increased KMnO4 concentration, regardless of the support material used. The KMnO4-based scavenging system can be utilized in conjunction with a controlled or modified atmosphere or in active packaging to confiscate the concentration of C2H4 accrued within a closed environment (Keller et al. 2013). Bhattacharjee and Dhua (2017) observed comparatively higher shelf-life of pointed gourd fruits stored at 29–33 °C with 68–73% RH (in polypropylene bags) with celite as support compared to silica gel for KMnO4 as ethylene scrubber (4–8 g scrubber kg−1 fruit). The baby bananas were stored (@18 °C; 70–80% RH) each with 17 g of KMnO4 (kg−1 fruit) supported on montmorillonite, vermiculite, kaolinite and zeolite as diverse support materials (García et al. 2012). Vermiculite was found to be the best while kaolinite occupied the bottom place (Álvarez-Hernández et al. 2018). In another experiment, polyolefin elastomer having nanoparticles of nanosilica and nanoclay impregnated with KMnO4 showed increased ethylene absorption at higher concentrations due to higher KMnO4 concentration. The nanoparticles were able to extend the shelf life of bananas up to 15 days at ambient conditions (Ebrahimi et al. 2021). The KMnO4-loaded sepiolite mediated ethylene scavenging was utilized in conjunction with encapsulated thymol for inhibiting Botrytis cinerea in cherry tomatoes (Álvarez-Hernández et al. 2021). Ni et al. (2021) utilized the 1-MCP and molecular sieves loaded with potassium permanganate as ethylene scavenger for preserving Agaricus bisporus. The potassium permanganate loaded halloysite nanotubes (HNTs) onto low density polyethylene had higher ethylene scavenging effect and thus delays the changes associated with ethylene (Joung et al. 2021). Ahmad et al. (2023) found that combination of 1-MCP and hypobaric storage was effective in improving the post-harvest storage life of Shughri pears.
In most technologies dealing with oxidation,for effective elimination of ethylene, the air within the storage premise (room/transport vehicle) should essentially be circulated past the scrubbing material (Keller et al. 2013). The capability of KMnO4 to lessen ethylene concentration from the atmosphere surrounding horticultural commodity (apple) was proved for the first time by Forsyth et al. (1967). Afterwards, many studies have been done but no scientific studies were performed regarding the potassium permanganate concentration and the effect of ethylene concentration (Keller et al. 2013). Blanpied et al. (1985) showed that for a 200 tonnes Empire apple store, the elimination effectiveness of KMnO4 beads declined to 25% after twelve days of continuous use and thus needed regular replacements. However, they postulated that this decrease was mainly owed to the moisture which hindered competitively ethylene scavenging. Keller et al. (2013) postulated a dire 50% diminution of KMnO4 efficiency when the relative humidity augmented from 70 to 90%. According to an exhaustive study of Wills and Warton (2004), if a commodity yielding 1 μl kg−1 h−1 ethylene is held at 20 °C with 90% RH, nearly 6 g absorbent per kg of commodity should be required to plummet the ethylene concentration by 90%, on the other hand, with a commodity generating 10 μl kg−1 h−1 ethylene under similar conditions, the amount of absorbent required should be 60 g of absorbent per kg of stored commodity. This implies that the potassium permanganate-based scrubbing appears feasible for perishables producing little ethylene. Coming to these simulations, for a 20 kg of packed commodity producing ethylene @10 μl/kg/h, the amount of KMnO4 compulsory would be near about 1.2 kg, which will concomitantly release 0.8 kg of KOH as residual directly within the solid body (Keller et al. 2013).
Adsorption based ethylene removal
Ethylene adsorption is prompted by van der Waals forces among the adsorbent and the adsorbate molecules. Adsorption (surface phenomenon) of any molecule depends on quite a few parameters: adsorbent concentration, temperature, gas composition, and RH. Adsorption of ethylene can be accomplished on activated charcoal, crystalline aluminosilicates, bentonite, aluminium oxide, Fuller’s earth, brick dust, silica gel, clay materials (cristobalite and zeolite) etc. However, activated carbon, zeolite, carbon fibre and silica gel come under the category of standard physical adsorbents (Álvarez-Hernández et al. 2018). In addition, certain sorbent chemicals, like propylene glycol, hexylene glycol, squalene, phenylmethyl silicone, polyethylene and polystyrene, adsorb ethylene and they can be reused by regenerating and after purging (Vermeiren et al. 2003; Zagory 1995). The solid adsorbents when offered with certain alkaline treatments viz. making to react under a gas stream or with certain agents, can develop the selectivity to a specific adsorbate (Gaikwad et al. 2019). Various adsorbent materials used as such or as support for enhancing the scavenging capacity of KMnO4 have been elaborated in Table 5.
Although there are various KMnO4 based commercially viable ventures in the field of ethylene removal, yet certain adsorbent-based ventures also do exist. Table 6 reviews various manufacturers involved in adsorbent-based ethylene removal system providers, their trade names, principal adsorbents and other accessible information as per the available literature. Also, the adsorbent materials have found place in some patents in the field of ethylene removal. These include Orega bags (US patent) consisting of pumice-tuff, zeolite, activated carbon etc. and synthetic resin film sheet (Nissho and Co., Japan; US patent) with crushed coral and calcium carbonate (Vermeiren et al. 2003).
KMnO4 (oxidation) and adsorption-based ethylene scavenging
The capability to perform under the atmosphere of high humidity is a must for an effective adsorbent of ethylene in order to simulate the conditions during storage and transportation of perishables. However, as reviewed extensively by Keller et al. (2013), published results on adsorption of ethylene by many adsorption/oxidation-based strategies on horticultural perishables stored under various environmental conditions like low ethylene and temperature, high relative humidity along with the presence of other volatile entities as well as ethylene adsorption isotherm and the adsorption capacity of the adsorbent are rare. Abe and Watada (1991) reported less ethylene accumulation and consequent reduction of softening in kiwifruit and banana and diminished loss of chlorophyll in spinach leaves, when palladium chloride catalyst infused charcoal was used as an ethylene absorbent at 20 °C; although, it did not give promising results in case of broccoli. Marzano-Barreda et al. (2021) used biodegradable active packaging containing synthetic zeolite Watercel ZF as ethylene scavenger for packing fresh broccoli florets. The adsorbent-based ethylene removal is different from potassium permanganate-based oxidation and the differences between KMnO4 and adsorption-based ethylene scavenging systems has been compiled and presented in Table 7.
Activated carbon/charcoal
Activated carbon/charcoal (AC) is a sort of porous carbon, non-crystallinein nature and produced by pyrolytic treatment of carbonous materials (Ben-Mansour et al. 2016; Sneddon et al. 2014). Various forms of AC include granular, powdered or fibre form, though due to comparatively easy regeneration and versatile nature, the granular form is the most preferred. For preparation of AC, the carbon containing material is first carbonized at high temperature followed by activation. The activation step of carbon is performed to generate more pores vis-à-vis to change their pore volume, form and size etc. The latter step can be attained by physical as well as chemical methods (Álvarez-Hernández et al. 2018; Yang 2003). Activated carbon offers advantages owing to its hydrophobic behaviour, more surface area, being lightweight and low production cost (Ben-Mansour et al. 2016; Gaikwad et al. 2020). Most commercial grades of AC typically own a diameter ranging between 10 and 25 Å for pore volume along with surface area oscillating between 300 and 4000 m2 g−1, though the latter can reach as much as 5000 m2 g−1 for some ACs (Martínez-Romero et al. 2007; Yang 2003). Bailén et al (2006) stored Beef’ tomato (8 °C; 90% RH) in polypropylene bags (20 μm thickness) under modified atmosphere packaging. The packaging was loaded with sachets comprising granular AC (5 g) with surface area of 226 m2 g−1. The granular AC deferred the quality changes in colour, physiological weight, and overall firmness of tomato while storage and was able to reduce significantly C2H4 levels up to 2 weeks inside packages. The AC can be united or impregnated with other ethylene adsorbing/scavenging compounds like KMnO4 to further enhance its usefulness. Mukti et al. (2018) used mangosteen rind powder (a waste with 180–355 µm size) for porous carbon preparation. The rind powder was carbonized at 575 °C for 3.5 h followed by pyrolysis/activation upto 850 °C and kept for 15 min under flowing nitrogen and steam. The highest surface area obtained was 1080 m2 g−1 thus falling in the category of mesoporous carbon with the ethylene adsorption capacity of 40.12 cm3 g−1. In another experiment, rice husk was carbonized at 300 °C for 3 h to make it silica free followed by its activation with activating agents (viz. NaOH, ZnCl2, and KOH) separately in 1:1 ratio at 900 ◦C under nitrogen flow. The KOH activated samples had high specific surface area (2342 m2 g−1) and large pore volume (2.94 cm3 g−1) (Shrestha et al. 2019). Liu et al. (2006) reported that activated carbon has approx. 11–78 mmol/kg ethylene absorption capacity which can quench 64–1000 ppm ethylene at 30 °C, while palladium catalyst with activated carbon has corresponding ethylene absorption capacity of 7–71 mmol/kg and has similar ethylene quenching capacity at 30 °C.
Conclusion
Fruit ripening encompasses a progressive series of physiological, biochemical, sensorial, textural and organoleptic amendments. Ethylene responsible for fruit ripening is invariably produced in climacteric and non-climacteric plants from methionine. It is required for natural ripening of fruits and also leads to over-ripening, senescence, loss of texture, microbial attack, less post-harvest life and other associated problems during storage and transportation of fruits. Therefore, ethylene accumulation in packaging system should be avoided to lengthen the shelf-life of perishable commodities. KMnO4-based oxidation is the most commercially cultivated approach worldwide. However, adsorption-based scrubbing is also a vital phenomenon which can be tapped alone or in combination with earlier existing processes for ethylene removal. Future research should focus on systematic evaluation of KMnO4-based scavenging, exploring the mechanism of adsorption, adsorbent(s) behavior in the presence of other gases and their partial pressures, volatile organic compounds, temperature, relative humidity, development of hydrophobic adsorbents to turn-up under high RH conditions, improvement in adsorption by π-complexation, regeneration of adsorbent by desorption (temperature or pressure swing), and improvement in photocatalytic oxidation etc. Heat input and air flow patterns also need to be considered while designing ethylene removal strategy for a large-scale storage. Banking upon novel approaches including combination of one or more strategy which is economical as well as amenable to scale-up may revolutionize the perishables’ shelf-life and in turn global economy.
Availability of data and material
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
References
Abe K, Watada AE (1991) Ethylene absorbent to maintain quality of lightly processed fruits and vegetables. J Food Sci 56(6):1589–1592
Aghdam MS, Luo Z, Jannatizadeh A, Sheikh-Assadi M, Sharafi Y, Farmani B, Fard JR, Razavi F (2019) Employing exogenous melatonin applying confers chilling tolerance in tomato fruits by upregulating ZAT2/6/12 giving rise to promoting endogenous polyamines, proline, and nitric oxide accumulation by triggering arginine pathway activity. Food Chem 275:549–556
AgraCo Technologies International (2014) Ethylene filters for large cold rooms in conjunction with electric blowers. AgraCo Technologies International, LLC, PE, USA. https://www.agraconew.com/products/ethylene-filters. Accessed on October 16, 2020
Ahmad A, Hashmi MS, Durrani Y, Khan NA, Khan MR, Siddiqi MZ, Riaz A, Alam M, Rahman WU (2022) Synergy of 1-MCP and hypobaric treatments prevent fermented flavour and improve consumers’ acceptability of ‘Shughri’ pear. J Food Sci Technol 60(1):200–210
Álvarez-Hernández MH, Artés-Hernández F, Ávalos-Belmontes F, Castillo-Campohermoso MA, Contreras-Esquivel JC, Ventura-Sobrevilla JM, Martínez-Hernández GB (2018) Current scenario of adsorbent materials used in ethylene scavenging systems to extend fruit and vegetable postharvest life. Food Bioproc Tech 11(3):511–525
Álvarez-Hernández MH, Martínez-Hernández GB, Avalos-Belmontes F, Castillo-Campohermoso MA, Contreras-Esquivel JC, Artés-Hernández F (2019) Potassium permanganate-based ethylene scavengers for fresh horticultural produce as an active packaging. Food Engg Rev 11(3):159–183
Álvarez-Hernández MH, Martínez-Hernández GB, Castillejo N, Martínez JA, Artés-Hernández F (2021) Development of an antifungal active packaging containing thymol and an ethylene scavenger. Validation during storage of cherry tomatoes. Food Packag Shelf Life 29:100734
Alves J, Gaspar PD, Lima TM, Silva PD (2023) What is the role of active packaging in the future of food sustainability? A systematic review. J Sci Food Agric 103(3):1004–1020
Bailén G, Guillén F, Castillo S, Serrano M, Valero D, Martínez-Romero D (2006) Use of activated carbon inside modified atmosphere packages to maintain tomato fruit quality during cold storage. J Agric Food Chem 54(6):2229–2235
Beaudry RM (1999) Effect of O2 and CO2 partial pressure on selected phenomena affecting fruit and vegetable quality. Postharvest Biol Technol 15(3):293–303
Befresh Technology (2018) Products Befresh. Befresh Technology, Spain. http://www.befreshtech.com/en/products. Accessed on October 16, 2020
Ben-Mansour R, Habib MA, Bamidele OE, Basha M, Qasem NA, Peedikakkal A, Laoui T, Ali MJ (2016) Carbon capture by physical adsorption: materials, experimental investigations and numerical modeling and simulations–a review. Appl Energy 161:225–255
Bhattacharjee D, Dhua R (2017) Ethylene absorbents improve the shelf life of pointed gourd (Trichosanthes dioica Roxb.) fruits. Int J Pure Appl Biosci 5(1):64–71
Bioconservación. BiOn, Barcelona, Spain: https://www.bioconservacion.com/. Accessed on November 26, 2020
Biopac Postharvest at work. Ethylene filters and sachets. Biopak, West Burleigh, Australia. https://www.biopac.com.au/ethylene-control/. Accessed on October 16, 2020
BioXTEND Co. (2020) BioX®. BioXTEND, Fort Myers, Florida, USA. https://bioxtend.com/. Accessed on October 16, 2020
Blanke MM (2014) Reducing ethylene levels along the food supply chain: a key to reducing food waste? J Sci Food Agric 94(12):2357–2361
Blanpied GD, Bartsch JA, Turk JR (1985) A commercial development programme for low ethylene-controlled atmosphere storage of apples. In: Roberts JA, Tucker JA (eds) Ethylene and plant development. Butterworths, London, pp 343–404
Bry-Air (Asia) Pvt. Ltd. Technical specification of BRYSORBTM chemical media. BryAir pdf, Gurugram, India. https://www.bryair.com. Accessed on May 23, 2020
Chamara D, Illeperuma K, Galappatty T, Sarananda KH (2000) Modified atmosphere packaging of ‘Kolikuttu’ bananas at low temperature. J Hortic Sci Biotech 75(1):92–96
Chaves AL, Mello-Farias PC (2006) Ethylene and fruit ripening: from illumination gas to the control of gene expression, more than a century of discoveries. Genet Mol Biol 29:508–515
Chopra S, Dhumal S, Abeli P, Beaudry R, Almenar E (2017) Metal-organic frameworks have utility in adsorption and release of ethylene and 1-methylcyclopropene in fresh produce packaging. Postharvest Biol Technol 130:48–55
Chowdhury P, Gogoi M, Borchetia S, Bandyopadhyay T (2017) Nanotechnology applications and intellectual property rights in agriculture. Environ Chem Lett 15(3):413–419
Circul-Aire Inc. (2006) MULTI-MIX® chemical media: Gas phase filtration: Industrial and commercial applications. Circul-Aire Inc., Georgia, USA. www.circul-aire.com. Accessed on May 23, 2020
Coloma A, Rodríguez FJ, Bruna JE, Guarda A, Galotto MJ (2014) Development of an active film with natural zeolite as ethylene scavenger. J Chilean Chem Soc 59(2):2409–2414
DeltaTrak Inc. White papers. Extending shelf Life: Ethylene absorption packaging strategies for produce transport. DeltaTrak Inc., California, USA. https://www.deltatrak.com. Accessed on May 27, 2020
Dennis Green Ltd. Green's Extra Life Produce Preserver. Dennis Green Ltd., USA. https://www.amazon.ca/Dennis-Green-Ltd-Produce-Preserver/dp/B0006GSLDQ. Accessed on May 27, 2020
Desiccare Inc. Ethylene eliminator pack. Desiccare Inc., Nevada, USA. https://www.desiccare.com/ethylene-absorber-1. Accessed on November 07, 2020
Dry Pak Industries Inc. Shelf life extension products. Dry Pak, Encino, CA, USA. www.drypak.com/ethyleneAbsorbers.html. Accessed on November 07, 2020
Ebrahimi A, Khajavi MZ, Mortazavian AM, Asilian-Mahabadi H, Rafiee S, Farhoodi M, Ahmadi S (2021) Preparation of novel nano–based films impregnated by potassium permanganate as ethylene scavengers: an optimization study. Polym Test 93:106934
Ethylene Control Inc. (2020) Sachets. Ethylene Control Inc., Selma, California, USA. https://ethylenecontrol.com/sachets. Accessed on October 30, 2020
Evert-Fresh (2017) Green Bags. Evert-Fresh, Texas, USA. https://www.evertfresh.com/. Accessed on November 07, 2020
Forsyth FR, Eaves CA, Lockhart CL (1967) Controlling ethylene levels in the atmosphere of small containers of apples. Can J Plant Sci 47(6):717–719
Gaikwad KK, Ko S (2015) Overview on in polymer-nano clay composite paper coating for packaging application. J Material Sci Eng 4(1):151
Gaikwad KK, Lee YS (2017) Current scenario of gas scavenging systems used in active packaging-A review. Korean J Packag Sci Technol 23(2):109–117
Gaikwad KK, Singh S, Lee YS (2018) High adsorption of ethylene by alkali-treated halloysite nanotubes for food-packaging applications. Environ Chem Lett 16(3):1055–1062
Gaikwad KK, Singh S, Negi YS (2020) Ethylene scavengers for active packaging of fresh food produce. Environ Chem Lett 18(2):269–284
García JC, Balaguera-López HE, Herrera AO (2012) Conservación del fruto de banano bocadillo (Musa AA Simmonds) con la aplicación de permanganato de potasio (KMnO4). Rev Colomb Cienc Hortíc 6(2):161–171
Greenkeeper Iberia. GK3-GK4-Greenkeeper. Greenkeeper Iberia, Toledo, Madrid, Spain. https://greenkeeperiberia.es/en/gk3-y-gk4/. Accessed on October 30, 2020
Grofit Plastics. Biofresh bags: Liners for cartons. Grofit Plastics, M.P. Eilot, Israel. www.grofitplastics.com. Accessed on November 07, 2020
Isolcell Spa (2018) Purethyl absorbers. Isolcell Spa, Laives, Italy. https://storage.isolcell.com/wp-content/uploads/2018/08/PURETHYL.pdf. Accessed on October 16, 2020
It’s Fresh Ltd. The it’s fresh! technology. It’s Fresh Ltd., London, UK. https://itsfresh.com/technology/. Accessed on December 25, 2020
Jal PK, Patel S, Mishra BK (2004) Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta 62(5):1005–1028
Janjarasskul T, Suppakul P (2018) Active and intelligent packaging: the indication of quality and safety. Crit Rev Food Sci Nutr 58(5):808–831
Joung J, Boonsiriwit A, Kim M, Lee YS (2021) Application of ethylene scavenging nanocomposite film prepared by loading potassium permanganate-impregnated halloysite nanotubes into low-density polyethylene as active packaging material for fresh produce. LWT 145:111309
Kader AA (2003) A perspective on postharvest horticulture (1978–2003). HortScience 38(5):1004–1008
Kader AA (2004) Controlled atmosphere storage. In: Gross KC, Wang CY, Saltveit ME (eds) The commercial storage of fruits, vegetables and florist and nursery stocks. USDA, Washington DC, pp 1–4
Kader AA, Rolle RS (2004) Post-harvest treatments designed to manipulate the environment around produce in order to enhance quality. In: Rolle RS (ed) The role of post-harvest management in assuring the quality and safety of horticultural produce, FAO Agricultural Services Bulletin 152. Italy, Rome, pp 35–41
Kaur N, Kishore D (2012) Montmorillonite: An efficient, heterogeneous and green catalyst for organic synthesis. J Chem Pharm Res 4(2):991–1015
Keep It Fresh. Profile. Keep It Fresh, California, USA. https://kif-usa.com/profile/. Accessed on October 15, 2020
Keep-Cool (2019) Ethylene absorbing filters. Keep-Cool, Moline de Segura, Spain. https://keep-cool.es/en/how-it-works/ethylene-absorbing-filters/. Accessed on November 07, 2020
KeepFresh Technologies. Product profile. Malaga, WA, Australia. https://keepfresh.com.au/product-profile/. Accessed on October 30, 2020
Keller N, Ducamp MN, Robert D, Keller V (2013) Ethylene removal and fresh product storage: A challenge at the frontiers of chemistry: Toward an approach by photocatalytic oxidation. Chem Rev 113(7):5029–5070
Knee M (1995) Copper reverses silver inhibition of flower senescence in Petunia hybrida. Postharvest Biol Technol 6(1–2):121–128
Kumar S, Kumar R, Pal A, Chopra DS (2019) Enzymes. In: Yahia EM, Carrillo-Lopez (eds) Postharvest physiology and biochemistry of fruits and vegetables, Woodhead Publishing, Elsevier, UK, pp 335–358
Li Y, Yu FX, Wang W, Jiang L, Cao S, Fan T (2022) Resveratrol improves postharvest quality of tomato fruits by enhancing the antioxidant defense system and inhibiting ethylene biosynthesis. J Food Sci Technol 59(11):4313–4321
Liu ZX, Park JN, Abdi SH, Park SK, Park YK, Lee CW (2006) Nano-sized carbon hollow spheres for abatement of ethylene. Top Catal 39(3):221–226
Mallakpour S, Khadem E (2015) Recent development in the synthesis of polymer nanocomposites based on nano-alumina. Prog Poly Sci 51:74–93
Martínez-Romero D, Bailén G, Serrano M, Guillén F, Valverde JM, Zapata P, Castillo S, Valero D (2007) Tools to maintain postharvest fruit and vegetable quality through the inhibition of ethylene action: a review. Crit Rev Food Sci Nutr 47(6):543–560
Marzano-Barreda LA, Yamashita F, Bilck AP (2021) Effect of biodegradable active packaging with zeolites on fresh broccoli florets. J Food Sci Technol 58:197–204
Miatech Inc (2020a) Erisfilter: How it works. Miatech Inc., Clackamas, Oregon, USA. https://eris-filter.com/how-it-works/. Accessed on October 30, 2020
Molecular Products Limited (2013) SofnofilTM: SofnofilTM is a general order absorbent for use in the air purification industry. Molecular products, Technical datasheet, Version 4, July 22, 2013, MCL, EK, Essex, UK. www.molecularproducts.com. Accessed on May 23, 2020
Mukti NI, Prasetyo I, Mindaryani A (2018) Preparation of porous carbon as ethylene adsorbent by pyrolysis of extraction waste Mangosteen rinds. MATEC Web of Conferences. EDP Sciences 154:1–5
Ni X, Yu J, Shao P, Yu J, Chen H, Gao H (2021) Preservation of Agaricus bisporus freshness with using innovative ethylene manipulating active packaging paper. Food Chem 345:128757
Ozeano Urdina SL (2013). Product datasheet OZEANO "SACHET S5". Ozeano, May 01, 2013, Bizkaia, Spain. http://www.ozeano.net. Accessed on May 23, 2020
Patdhanagul N, Rangsriwatananon K, Siriwong K, Hengrasmee S (2012) Combined modification of zeolite NaY by phenyl trimethyl ammonium bromide and potassium for ethylene gas adsorption. Microporous Mesoporous Mater 153:30–34
Pathak N, Caleb OJ, Geyer M, Herppich WB, Rauh C, Mahajan PV (2017) Photocatalytic and photochemical oxidation of ethylene: Potential for storage of fresh produce—A review. Food Bioprocess Technol 10(6):982–1001
Pathak S, Sriramulu S, Thandavan SP, Jothimani G, Banerjee A, Marotta F (2018) Enhancement of Shelf Life of the Climacteric Fruits= A Review on Application of CRISPRi Technology. Trends Tech Sci Res 1(2):23–29
Peakfresh products. Superior packaging to extend the life of your fruit, vegetables and flowers. Peakfresh, Australia. www.peakfresh.com. Accessed on May 27, 2020
Prodew Inc. Ethylene control: Extend shelf life. Prodew Inc. Marietta, Georgia, USA. https://www.prodew.com/flyers/eth_flyer_web.pdf. Accessed on October 16, 2020
Purafil Inc (2015) Product bulletin for purafil chemisorbent media. Purafil Inc., ProdBltn–CHM-03, Doraville, Georgia, USA. www.purafil.com. Accessed on May 23, 2020
Ranjeet S, Giri SK (2014) Shelf-life study of guava (Psidium guajava L.) under active packaging: an experiment with potassium permanganate salt as ethylene absorbent. Arch Lebensmittelhyg 65(2):32–39
Retarder SRL (2020) Retarder SRL: FRUIT LOGISTICA – Exhibitor. Retarder, C44327, Verzuolo, Italy. https://www.virtualmarket.fruitlogistica.de/en/Retarder-SRL,c44327. Accessed on October 30, 2020
Rodrıguez FI, Esch JJ, Hall AE, Binder BM, Schaller GE, Bleecker AB (1999) A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 283(5404):996–998
Sadeghi K, Lee Y, Seo J (2021) Ethylene scavenging systems in packaging of fresh produce: a review. Food Rev Int 37(2):155–176
Saltveit ME (1999) Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol Technol 15(3):279–292
Saltveit ME (2003) Is it possible to find an optimal controlled atmosphere? Postharvest Biol Technol 27(1):3–13
Santosa E, Widodo WD (2010) The use of clay as potassium permanganate carrier to delay the ripening of Raja Bulu Banana. J Hort Indonesia 1(2):88–95
Sawada S, Totsuka T (1986) Natural and anthropogenic sources and fate of atmospheric ethylene. Atmos Environ 20:821–831
Schaller GE, Binder BM (2017) Inhibitors of ethylene biosynthesis and signaling. In: Binder BM, Schaller GE (eds) Ethylene Signaling: methods and protocols, methods in molecular biology, Humana Press, New York, 1573, pp 223–235
Scully AD, Horsham MA (2007) Active packaging for fruits and vegetables. CRC Press, Boca Raton, Florida, pp 57–73
Sensitech Inc (2020b) Ryan® Ethylene absorption filters and sachets. Sensitech Inc., p1399017, Beverly, MA, USA. https://www.virtualmarket.asiafruitlogistica.com/en /Ryan®-Ethylene-Absorption Filters-and-Sachets. Accessed on Accessed November 27, 2020
Shaabani A, Tavasoli-Rad F, Lee DG (2005) Potassium permanganate oxidation of organic compounds. Synth Commun 35(4):571–580
Shrestha LK, Thapa M, Shrestha RG, Maji S, Pradhananga RR, Ariga K (2019) Rice husk-derived high surface area nanoporous carbon materials with excellent iodine and methylene blue adsorption properties. J Carbon Res 5(1):10
Sneddon G, Greenaway A, Yiu HH (2014) The potential applications of nanoporous materials for the adsorption, separation, and catalytic conversion of carbon dioxide. Adv Energy Mater 4(10):1301873
Spricigo PC, Foschini MM, Ribeiro C, Corrêa DS, Ferreira MD (2017) Nanoscaled platforms based on SiO2 and Al2O3 impregnated with potassium permanganate use color changes to indicate ethylene removal. Food Bioprocess Technol 10(9):1622–1630
Tas CE, Hendessi S, Baysal M, Unal S, Cebeci FC, Menceloglu YZ, Unal H (2017) Halloysite nanotubes/polyethylene nanocomposites for active food packaging materials with ethylene scavenging and gas barrier properties. Food Bioprocess Technol 10(4):789–798
Ulloa JA (2007) Frutas auto estabilizadas en el envase por la tecnología de obstáculos, 1st edn. Universidad Autónoma de Nayarit, México
Vermeiren L, Devlieghere F, van Beest M, de Kruijf N, Debevere J (1999) Developments in the active packaging of foods. Trends Food Sci Technol 10(3):77–86
Vermeiren L, Heirlings L, Devlieghere F, Debevere J (2003) Oxygen, ethylene and other scavengers. In: Ahvenainen (eds) Novel food packaging techniques, Woodhead Publishing Limited and CRC Press LLC, pp 22–49
Warsiki E (2018) Application of chitosan as biomaterial for active packaging of ethylene absorber. In: IOP Conference series: earth and environmental science, vol 141, issue 1. IOP Publishing, p 012036
Warton MA, Wills RB, Ku VV (2000) Ethylene levels associated with fruit and vegetables during marketing. Aust J Exp Agric 40(3):465–470
Watkins CB (2000) Responses of horticultural commodities to high carbon dioxide as related to modified atmosphere packaging. HortTechnology 10(3):501–506
Wills RB, Warton MA (2004) Efficacy of potassium permanganate impregnated into alumina beads to reduce atmospheric ethylene. J Am Soc Hortic Sci 129(3):433–438
Wills RBH, Warton MA, Ku VVV (2000) Ethylene levels associated with fruit and vegetables during marketing. Aus J Exp Agric 40(3):465–470
Wyrwa J, Barska A (2017) Innovations in the food packaging market: active packaging. Eur Food Res Technol 243(10):1681–1692
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interface Sci 209:172–184
Yahia EM, Carrillo-Lopez A (2019) Postharvest physiology and biochemistry of fruits and vegetables. Woodhead Publishing, UK
Yang RT (2003) Adsorbents: fundamentals and applications. John Wiley and Sons, New Jersey
Yildirim S, Röcker B, Pettersen MK, Nilsen-Nygaard J, Ayhan Z, Rutkaite R, Radusin T, Suminska P, Marcos B, Coma V (2018) Active packaging applications for food. Compr Rev Food Sci 17(1):165–199
Zagory D (1995) Ethylene removal packaging. In: Rooney ML (ed) Active food packaging. Blackie Academic and Professional, London, UK, pp 38–54
Zhu Z, Zhang Y, Zhang Y, Shang Y, Zhang X, Wen Y (2019) Preparation of PAN@ TiO2 nanofibers for fruit packaging materials with efficient photocatalytic degradation of ethylene. Mater 12(6):896
Acknowledgements
The authors are thankful to Indian Council of Agricultural Research for providing facilities to write this review.
Funding
Indian Council of Agricultural Research.
Author information
Authors and Affiliations
Contributions
SK: Corresponding author, Conceptualization, literature collection, Writing original draft; Writing—review and editing. RK: Conceptualization, Writing original draft, literature collection, editing. BRB: Formal analysis, editing. Prerna Nath:Formal analysis, literature collection. RKS: Project administration, Conceptualization, Supervision. SM: Review, editing. AP: Review, editing. RS: Literature collection, review. AK: Review, editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, S., Kumar, R., Bibwe, B.R. et al. Postharvest handling of ethylene with oxidative and absorptive means. J Food Sci Technol 61, 813–832 (2024). https://doi.org/10.1007/s13197-023-05777-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-023-05777-1