Abstract
Protein hydrolysates (P-HS) from edible bird’s nest co-product prepared without and with ultrasound (US) pretreatment in combination with heating before hydrolysis using alcalase at different concentrations were characterized. US treatment of co-product in water at 60% amplitude for 20 min, followed by heating at 95 °C for 3 h was done before enzymatic hydrolysis. The degree of hydrolysis (DH), yield and sialic acid (SL) content of P-HS samples were not different (p > 0.05) when 1 or 2% alcalase was used for hydrolysis. The highest protein content and lightness (L*) were observed in P-HS prepared from co-product subjected to US treatment (60% amplitude for 20 min) using 1% alcalase for hydrolysis. When antioxidant activities of dried P-HS were determined, P-HS from co-product subjected to US treatment had higher DPPH and ABTS radical scavenging activities, ferrous reducing antioxidant power and oxygen radical absorbance capacity, compared to those prepared from non-US treated co-product. P-HS with higher DH contained greater amount of small peptides having MW lower than 1,883 Da. The P-HS produced under optimum condition had major essential amino acids (EA-A) including leucine, threonine, lysine and valine of 4.28, 3.53, 3.30 and 3.08%, respectively. Therefore, P-HS from co-product could serve as both nutrients and functional ingredients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Edible bird’s nest (B-nest) is produced from sticky saliva of swiftlets, which can be glued with cave wall (cave nest) or man-made house (house nest) (Ling et al., 2020). B-nest consists of protein as a major constituent (58.6–66.9%) followed by carbohydrate, ash and lipid (Quek et al., 2018). Nowadays B-nest is still in high demand in the world market owing to its health benefit. Southeast Asia, especially Thailand, can supply 200 tonnes of B-nest annually, in which B-nest are collected from both natural sources and B-nest houses. This business generates around US$ 280 million per year (Department of International Trade Promotion, 2020). According to Chantakun and Benjakul (2020), B-nest contains 85–97% saliva and 3–15% feather. During the cleaning process, co-products consisting of broken B-nest, feather, eggshell, etc. are obtained. The amount is approximately 30% of whole B-nest. Price of the co-product is in the range of 500–1,000 Thai baht per kilogram. Gan et al., (2020) documented that co-product can be hydrolyzed to produce protein hydrolysates with higher bioactivities, compared to that of the original B-nest.
Protein hydrolysates (P-HS) can be another choice for increasing the value of co-product (Ling et al., 2020). The hydrolysis process is conducted under mild conditions, in which racemization reaction can be minimized (Idowu et al., 2019). Enzymes generally cleave peptide bonds of proteins at specific sites. Time and temperature for hydrolysis, degree of hydrolysis (DH), pH and type of enzyme used, etc. are reported as key factors governing hydrolysis efficacy of proteins (Klompong et al., 2007). Alcalase has been used for B-nest P-HS production because of its non-specific nature in cleavage of peptide bonds (Babji et al., 2018). However, DH is the vital factor influencing the functional properties and antioxidant capacity of P-HS (Ng et al., 2020). In recent years, high-intensity ultrasound-assisted process (US) has gained interest in augmenting enzymatic hydrolysis, accelerating the release of peptides and producing bioactive peptides (Yang et al., 2020). Zhang et al., (2015) reported that US pretreatment induced unfolding of proteins and also remarkably improved enzymatic hydrolysis of wheat gluten. In addition, US treatment under optimal conditions potentially escalates the extraction efficiency of collagens from fish skin without affecting their physiochemical properties (Petcharat et al., 2021). Nevertheless, the harsh condition, especially energy-divergent type US pretreatment, could promote Maillard reaction or flavor development of resulting P-HS (Yang et al., 2020). The co-product could be better exploited via production of P-HS having antioxidant activity with the aid of appropriate pretreatment. Thus, this work aimed to investigate the effect of US pretreatment in combination with the heating process on recovery of the remaining broken B-nest from co-product before enzymatic hydrolysis using alcalase at different levels and to characterize and determine antioxidant activity of resulting P-HS.
Materials and methods
Chemicals
Alcalase from Bacillus licheniformis was obtained from Novozymes (Bagsvaerd, Denmark). 2-diphenyl‐1picrylhydrazyl (DPPH), 2,4,6‐tritrobenzenesulphonic acid (TNBS), 2,2‐azinobis (3ethylbenzothiaziline‐6‐sulphonic acid), diammonium salt (ABTS), 6‐hydroxy‐ 2,5,7,8‐tetramethylchroman‐2carboxylic and 2,4,6‐tripyridyltriazin (TPTZ) were procured from Sigma Chemical Co. (St. Louis, MO, USA). All chemicals were of analytical grade.
Collection of B-nest co-product
Co-product from house B-nest was procured from My Great (Thailand) Co., Ltd., Songkhla, Thailand. The sample (20 g) was added to distilled water (1 L) and stirred continuously at room temperature (25–27 °C) for 60 min to remove the small or soluble residues or contaminants. The mixture was drained for 5 min on the screen. Subsequently, the washed sample was dried at 60 ± 2ºC for 6 h, in which the final moisture content was below 15%. Dried co-product was blended using a blender (Panasonic, Model MX-898 N, Berkshire, UK) and sieved using a shaker (AS 200, Retsch, Germany) equipped with the screen having the holes of 18 mesh to remove the feathers, which retained unblended. The co-product powder with a uniform size was obtained. The powder sample was kept in the airtight nylon plastic container at 4ºC until use.
Preparation of protein solution from co-product
Dried co-product powder containing small feathers and eggshells (2 g) was added with distilled water (100 mL). The ultrasonic processor model Vibra-Cell (Sonics & Material, Inc., Newtown, CT, USA) and a flat tip probe (diameter 13 mm) were used for ultrasonication (US). US was operated at 750 W with a single frequency of 20 kHz in a pulse mode (5 s acting and 5 s resting time). Various levels of amplitude (20%, 40% and 60%) and different total operation times (20 and 40 min) were used. During US, the temperature of the mixture was maintained at 25–30 °C using an iced water. The samples without US treatment (non-US) and US treated samples were subsequently heated in a water bath with continuous stirring at 95 °C for 3 h. the mixtures were cooled down to 25 °C and centrifuged for 10 min at 9000×g (25 °C) using a centrifuge (Allegra 25-R, Beckman Instruments Inc., Palo Alto, CA, USA). The supernatant was determined for protein and α-amino group (A-AG) contents (Chantakun and Benjakul 2020). The resulting solution yielding the highest protein and α-amino group contents were selected for enzyme hydrolysis.
Production of co-product hydrolysate
The protein solution prepared using US at 60% amplitude for 20 min or “US-60”, which rendered high A-AG and protein contents and had a short operation time was selected for preparation of co-product hydrolysate. Protein solution without US treatment (non-US) was also used for hydrolysate preparation. Firstly, the protein solution (100 mL) was transferred into a beaker and placed in a water bath (Model W350, Memmert, Schwabach, Germany) at 60 °C. The mixture was stirred gradually using an overhead stirrer (IKA®-Werke GmbH & CO.KG, Stanfen, Germany) at 5,000 rpm. Thereafter, the solution was adjusted with either 0.1.M HCl or 0.1 M NaOH to obtain pH of 8.0. Thereafter, the mixture was added with alcalase at 1 and 2% w/w (based on protein content). Hydrolysis was monitored as a function of time (0–210 min) with continuous stirring. At designated times (0, 10, 20, 30, 45, 60, 75, 90, 120, 150, 180 and 210 min), the mixtures (1 mL) were collected and enzymes were inactivated by immersing the solution in hot water (90 °C) for 20 min. P-HS obtained at all the designated times were determined for DH as tailored by Idowu et al. (2019).
Characterization of protein hydrolysates from co-product
All P-HS samples from co-product without and with US treatment, followed by heating before hydrolysis using 1% or 2% alcalase at the selected hydrolysis time, showing the highest DH, were selected. The samples were centrifuged at 9,000×g for 15 min. The supernatants were lyophilized with the aid of a freeze-dryer (CoolSafe 55, ScanLaf A/S, Lynge, Denmark). P-HS powder samples were then analyzed.
Yield

The yield of P-HS was calculated as follows:
Proximate compositions
Protein, fat, ash and carbohydrate contents were determined using AOAC analytical methods (AOAC, 2002). The values were expressed as g/100 g (dry weight basis).
Sialic acid (SL) content
The high-performance liquid chromatography (HPLC, Agilent technologies 1200 series, Santa Clara, CA, USA) equipped with C18 column (Water Sunfire, 150 × 4.6 mm id., 5 μm) was used to determine SL content as detailed by Quek et al. (2018). Firstly, three milligrams of sample were added with 1 mL of sodium bisulfate solution (0.5 M) and heated in a water bath at 80 °C for 30 min. The solution was further mixed with 1 mL of 20 mg/mL Ο-phenylenediamine dihydrochloride solution and heated at 80 °C for 40 min for pre-column derivatization. SL quantification was done using N-acetylneuraminic acid (0–1,000 mg/L) as standard. SL content was reported as mg/100 g (dry weight basis).
Color
The color of samples was measured using a colorimeter (Color Flex, Hunter Lab Inc., Reston, VA, USA). L*, a*, and b*-value were recorded (Chantakun and Benjakul, 2020).
Antioxidative activity assays
P-HS sample (0.1 g) was dissolved in 0.9 mL of distilled water. The sample was filtered using a membrane filter (0.45 μm). The resulting filtrates were determined for antioxidative activities. DPPH and ABTS radical scavenging activities were determined as detailed by Binsan et al. (2008). FRAP was examined as tailored by Benzie and Strain (1996). Oxygen radical absorbance capacity (ORAC) was also determined (Chotphruethipong et al., 2019). The activities were reported as µmol Trolox equivalents (TE)/g dry samples.
Molecular weight distribution
Molecular weight (MW) distribution of P-HS samples from co-product was determined using a 2.5 × 50 cm Sephadex G-25 gel filtration column (GE Healthcare Bio‐Science AB, Uppsala, Sweden) (Chotphruethipong et al., 2019). The MW standards used consisted of insulin chain B (3,495.89 Da), vitamin B12 (1,355.4 Da), glycinetyrosine (238.25 Da), and tyrosine (181.2 Da). Distilled water was used as an elution solution with a flow rate of 0.5 mL/min and the fractions (3.0 mL) were collected. The absorbance at 220 and 280 nm was monitored. MW of fractions was calculated (Chotphruethipong et al., 2019).
Amino acid analysis
The amino acid analyzer (JLC-500/V AminoTacTM; JEOL Inc., Peabody, MA, USA) was used to determine the amino acid composition of the selected P-HS (Petcharat et al., 2021). The amino acid composition was expressed as g/ 100 g sample.
Statistical analysis
A completely randomized design (CRD) was implemented for entire studies. All the experiments and analyses were done in triplicates. One-way analysis of variance (ANOVA) was used and Duncan’s multiple range test was carried out to analyze the significant differences among samples at a level of (p < 0.05) using SPSS version 11.0 (SPSS Inc., Chicago, IL, USA).
Results and discussion
Effect of pretreatment on the characteristic of protein solution
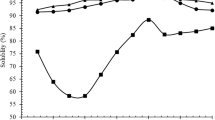
Protein and α-amino group (A-AG) contents of the obtained solutions from co-product after pretreatment using ultrasound-assisted process (US) combined with heating are shown in Fig. 1. All solutions of co-product subjected to US at various amplitudes and times, followed by heating had protein content ranging from 3.10 to 4.11 mg/mL (Fig. 1 A). The samples treated with US at 60% amplitude for 20, 40 min or at 40% amplitude for 40 min, followed by heating showed higher protein content (p < 0.05), compared to other samples. The control sample (non-US) and samples subjected to US at 20% amplitude for 20 or 40 min possessed the lowest protein content (p < 0.05). Wang et al., (2020) found that the assistance of US resulted in higher extraction efficacy of pea protein and extraction time was shortened. US plausibly caused the changes in the secondary and tertiary structure of the protein, associated with partial unfolding. In addition, all the samples treated with US combined with heating had higher A-AG content than the control (p < 0.05). When an amplitude of 60% was used for pretreatment, the highest A-AG content was attained for both US treatment times (20 and 40 min) (p < 0.05). Aguilar-Acosta et al., (2020) found that US application could induce the structural damage of proteins. The cavitation effect of US might induce the cleavage of peptide bonds, as witnessed by the increased A-AG content. However, the lowest A-AG content was found in the control (without US) (p < 0.05). The combined effect of US with heating had a marked impact on protein and A-AG contents of co-product. US might help remove B-nest from co-product, while it also augmented the hydrolysis of protein in B-nest, leading to increased liberation of peptides. Thus, the optimum condition of US was 60% amplitude for 20 min, followed by heating at 95 °C for 3 h. This process was selected for hydrolysis study.
Degree of hydrolysis (DH) and yield (%) of protein hydrolysate (P-HS)
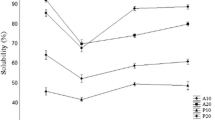
The DH of P-HS has been used to determine the degree of cleavage of peptides relative to the total number of peptide bonds in a protein. The co-product was hydrolyzed by alcalase at 1 or 2% for an optimum time (45 min) (data not shown). The US-treated samples yielded P-HS with higher DH (36.06 and 37.45%, respectively) than those produced from non-US samples (26.18 and 33.75%, respectively) (Table 1). However, both alcalase concentrations had no significant effect on DH of P-HS produced from US pretreated samples (p > 0.05). Generally, the US could augment the extraction efficiency. High shear gradient generated via micro-bubble collapse loosens protein structure (Petcharat et al., 2021). This result indicates that US (60% amplitude) treatment released higher proteins, which were further hydrolyzed by alcalase. Also unfolded proteins could be hydrolyzed by alcalase more effectively. Simultaneously, P-HS from co-product subjected to US (60% amplitude) and hydrolyzed using either 1 or 2% alcalase showed higher yield (p < 0.05) (26.52 and 26.75%, respectively) than those of P-HS produced from the non-US sample (15.50 and 17.90%, respectively) (Table 1). It was proposed that 1% alcalase was sufficient for hydrolysis of proteins in co-product, particularly when US was used for pretreatment. According to Petcharat et al. (2021), an optimum condition of US (80% amplitude for 10 min) was able to increase the yield of collagen from clown featherback (Chitala ornata) skin, (p < 0.05), compared to the control (typical extraction process). In the present study, hydrolysis of co-product pretreated with US (60% amplitude for 20 min) with subsequent heating (95 °C for 3 h) using 1% alcalase resulted in P-HS with the highest DH and coincidentally highest yield. Also, DH of P-HS using 2% alcalase was not different from that of P-HS using 1% alcalase (p > 0.05). This result was in line with Bhaskar et al. (2008) who found that DH of hydrolysate from visceral waste proteins of Catla (Catla catla) remained constant when alcalase concentration was above 1%. This was plausibly due to the limited available substrate for hydrolysis by enzymes added (Bhaskar et al., 2008). As a result, the increased level of enzyme did not markedly augment DH of P-HS.
Proximate composition
Proximate compositions of P-HS samples prepared from co-product under different conditions are shown in Table 1. Protein was the main component of all P-HS samples, constituting 58.98 to 64.35%. Comparing between P-HS from US treated sample using 1 and 2% alcalase concentration, the former had higher (p < 0.05) protein content (64.35%), whereas the latter had a protein content of 61.89%. This indicated that 1% alcalase showed effectiveness in hydrolysis toward protein in co-product. The use of 2% alcalase might induce autolysis of enzyme, leading to the lower amount of active enzyme for hydrolysis as witnessed by lower yield. Normally, the concentration of protease in free form may determine its stability. Higher concentration of protease can induce its autolysis (Tacias-Pascacio et al., 2020). No difference in protein content was found for P-HS from non-US treated sample as affected by alcalase concentration (p > 0.05). Ng et al. (2020) reported that 1% alcalase was used to produce P-HS from two types of B-nest (clean and co-product B-nest). The resulting P-HS had an improved protein efficiency ratio, indicating higher protein quality than raw B-nest samples. In general, an unsuitable enzyme/substrate ratio could lower the activity, leading to a decreased product generated (Idowu et al., 2019). In addition, all P-HS samples had no difference in fat content (p > 0.05), which was in the range of 0.89–0.92%. Among animal products, B-nest has the lowest fat content (0.01 to 0.16%), compared to other animal products (Chantakun and Benjakul 2020; Quek et al., 2018).
Ash content of P-HS ranged from 4.63 to 5.25%. The result was in line with that of Ma and Liu (2012) who reported that ash contents of B-nest hydrolysates were in the range of 2.1–7.3%. The pH adjustment using NaOH and HCl before enzymatic hydrolysis more likely resulted in the formation of salt as indicated by ash content (Benjakul and Morrissey 1997). This was mainly associated with the salt formed. US pretreatment in combination with heating and enzyme hydrolysis using 1% alcalase could increase the protein content of P-HS from co-product.
For carbohydrates, P-HS produced from US treated co-product showed a lower content than those from the control (non-US). The lowest carbohydrate content (29.59%) was found in P-HS produced from US-treated sample in combination with heating with the aid of 1% alcalase. It was presumed that alcalase at 1% could selectively hydrolyze proteins associated with carbohydrates, mainly glycoproteins. As a result, higher protein content was found in this sample. B-nest consists of glycoprotein, in which proteins are associated with some sugars or carbohydrates (Quek et al., 2018).
Sialic acid (SL) content
Table 1 shows the SL content in all P-HS samples from co-product with different pretreatment process and alcalase levels used. SL has several basic forms involving 2-keto-3-deoxynonulosonic acid (Kdn), N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc). Among those forms, N-acetylneuraminic acid was the dominant SL found in B-nest (Schauer, 2009). P-HS samples from US-treated co-product and hydrolyzed by alcalase at both concentrations (1 and 2%) showed no difference in SL content (p > 0.05) (18.79 and 18.01 mg/g sample, respectively). For P-HS from the control (non-US), SL contents ranged from 13.23 to 13.89 mg/g sample, which was lower than those found in P-HS derived from US-treated co-product, regardless of alcalase level used. For the same preparation process of co-product, alcalase at different concentrations did not affect SL content. Haghani et al. (2017) reported that SL content of P-HS from house B-nest from Gua Madai and Teluk Intan of Malaysia ranged from 3.2 to 6.7 mg/g sample. Normally, the SL content in raw B-nest was documented to be about 8.3–13.6% by Quek et al. (2018) and 1–6% by Thavamanithevi et al. (2014). The lower SL content was noticeable in P-HS than the raw B-nest sample. Destruction of SL induced by heat or enzymatic hydrolysis might occur. Thus, the chemical composition of P-HS from co-product varied, as influenced by pretreatment and hydrolysis process.
Color
L*-(lightness), a*-(redness/greenness) and b*-(yellowness/blueness) values of P-HS samples from co-product with different pretreatments and levels of alcalase used are shown in Table 1. The samples showed different L*, a* and b*-values (p < 0.05). The higher L*-values of P-HS sample pretreated with US using 1% alcalase were noticeable, compared to other samples (p < 0.05). The lowest L*-value was attained for P-HS produced from non-US sample hydrolyzed by 1% alcalase (p < 0.05). Nevertheless, the highest a* and b*-values were found for P-HS samples from non-US samples using 1% alcalase (p < 0.05). No differences in a* and b*-values between P-HS samples prepared from US-treated co-product using both alcalase concentrations were found (p > 0.05). Ling et al. (2020) documented that the enzymatic hydrolysis increased the yellowness (b*) of hydrolysate product, compared to raw B-nest. In general, color is an essential quality attribute of food products (Idowu et al., 2019). When comparing the yellowness (b*-value) between P-HS prepared from non-US and US-treated co-product, P-HS produced with the aid of US showed lower a* and b*- values than that produced from non-US co-product. US with cavitation effect might destroy the pigments present in B-nest, leading to the lower yellowness or redness. This coincided with the increased whiteness. The color of co-product P-HS was therefore varied, depending on US pretreatment and enzymatic hydrolysis conditions.
Antioxidative activities of dried co-product protein hydrolysate (P-HS)
DPPH radical scavenging activity
Figure 2 A shows DPPH radical scavenging activity of P-HS from co-product produced without and with US treatment and hydrolyzed by alcalase at 1 and 2%. Different P-HS showed differences (p < 0.05) in DPPH radical scavenging activity. P-HS from US-treated co-product and hydrolyzed using 2% alcalase showed the highest (p < 0.05) DPPH radical scavenging activity (95.32 µmol TE/L sample), followed by P-HS prepared using 1% alcalase with US treatment (88.99 µmol TE/L sample). The lowest DPPH radical scavenging activity was found in P-HS from non-US co-product, using both alcalase concentrations (p < 0.05). This assay has been used as an indicator of donating hydrogen to radicals to form a stable diamagnetic molecule (Chantakun et al., 2020). When US treatment (60% amplitude) was implemented, the P-HS samples had higher activity than those from non-US co-product (p < 0.05). The result indicated that US pretreatment combined with heating, followed by hydrolysis using alcalase had different peptides with various DPPH radical scavenging activity. Idowu et al. (2019) reported that P-HS from mince salmon frame prepared using papain or alcalase had different DPPH radical scavenging activity (247.38–293.12 µmol TE/g sample), depending on the forms of frame and enzyme used for hydrolysis. In addition, Wen et al. (2020) found that DPPH radical scavenging activity of arrowhead (Sagittaria sagittifolia L.) hydrolysates prepared with the aid of ultrasonication was higher than other samples without ultrasonication. US could make the particle size much smaller, thus favoring hydrolysis by the enzyme. As a result, peptides with DPPH radical scavenging activity were increased.
DPPH and ABTS radical scavenging activities and ferric reducing antioxidant power (FRAP) of P-HS from co-product prepared without and with US pretreatment, followed by heating before hydrolysis using alcalase at different levels
Bars represent the standard deviation (n = 3). Different lowercase letters on the bars denote significant different (p < 0.05)
ABTS radical scavenging activity
P-HS from co-product without and with US treatment combined with heating showed the difference (p < 0.05) in ABTS radical scavenging activity, as seen in Fig. 2B. Similarly, the P-HS produced by US-treated co-product using alcalase at both levels showed higher ABTS radical scavenging activity than P-HS from non-US co-product. No differences in ABTS radical scavenging activity were observed between P-HS from US-treated co-product (p > 0.05). However, P-HS from non-US co-product hydrolyzed by 2% alcalase exhibited higher ABTS radical scavenging activity than those using 1% alcalase (p < 0.05). The former might contain peptides with higher ABTS radical scavenging activity at higher amounts. Normally, ABTS radical scavenging activity can reflect the hydrogen donating and chain-breaking capacity of the compound. This assay has been used to test both hydrophilic and lipophilic compounds (Binsan et al., 2008). Stefanovic´ et al. (2014) found that egg white P-HS obtained from the US pretreatment process having peptides with MW between 1 and 10 kDa had the stronger ABTS radical scavenging activity, compared with that without US treatment. The lower ABTS radical scavenging activity of P-HS from non-US co-product was likely due to the lower amount of peptides with ABTS radical scavenging activity. Those peptides generated during US treatment and enzymatic hydrolysis were more likely involved in ABTS radical scavenging activity.
Ferrous reducing antioxidant power (FRAP)
FRAP of P-HS samples produced from co-product using alcalase at two concentrations as affected by US pretreatment is shown in Fig. 2 C. The co-product pretreated with US combined with heating and subjected to enzymatic hydrolysis process by 1% alcalase showed the highest FRAP (p < 0.05). No difference in FRAP was observed in other samples (p > 0.05). Klompong et al. (2007) documented that the ability of antioxidants in providing an electron to free radicals, which reduce ferric ions to ferrous ions (Fe3+ to Fe 2+), was related with FRAP. The results revealed that P-HS from co-product produced with the aid of US treatment in combination with heating process before hydrolysis using 1% alcalase had highest FRAP.
Oxygen radical absorbance capacity (ORAC)
ORAC of all P-HS samples from co-product is shown in Fig. 3. The protective effect of all the P-HS based on net integrated area of the fluorescence decay curve (AUC) is shown in Fig. 3. The highest inhibition was observed in P-HS sample prepared from US treated co-product using 1% alcalase, whereas the fluorescence decay was most pronounced in those from non-US co-product. Overall, the highest ORAC value was found in P-HS from co-product treated with US in combination with heating before hydrolysis (Fig. 3b). Similar ORAC values were found between P-HS when 1 and 2% alcalase were used (p > 0.05), regardless of pretreatment. Basically, ORAC assay has been used to examine the radical chain-breaking ability of antioxidants by measuring the inhibition of peroxyl radical (Chotphruethipong et al., 2019). Ikram et al. (2020) found that the highland barley brewer’s spent grain protein hydrolysates using US pretreatment before enzymatic hydrolysis showed a higher ORAC value than that produced from non-US samples. Peptides with hydrogen atom donating ability most likely had the augmented ORAC. The result suggested that 1% alcalase could be used for enzymatic hydrolysis of co-product to produce P-HS with high ORAC.
Relative fluorescence intensity (A) and oxygen radical absorbance capacity (ORAC) (B) of P-HS from co-product prepared without and with US pretreatment, followed by heating before hydrolysis using alcalase at different levels
Bars represent the standard deviation (n = 3). Different lowercase letters on the bars denote significant different (p < 0.05)
Molecular weight distribution
The different elution profiles of P-HS from co-product without and with US pretreatment in conjunction with heating before hydrolysis by alcalase at different levels using Sephadex G-25 gel filtration chromatography are illustrated in Fig. 4. A220 and A280 were used to monitor peptide bond and protein, respectively. P-HS from non-US co-product using 1 and 2% alcalase had two major peaks of both A220 and A280 (11,860 and 10,175 Da), while peptides with different molecular weights (MW) ranging from 4,726 to 11,860 Da were also found. However, the peak height was slightly different, indicating the varying number of peptides generated in both samples when alcalase at different concentrations was used. No difference in minor peaks of A220 and A280 (4,730 Da) were found in both P-HS from non-US co-product. Both P-HS samples from US treated co-product showed peptides with smaller MW (750, 1,880 and 8,730), compared with those found in P-HS from non-US co-product both. This result indicated that US pretreatment could help reduce the size of peptides in P-HS from co-product. In addition, the increase in DH (Table 1) of P-HS was in line with low MW peptides in P-HS. Petcharat et al. (2021) documented that US treatment could induce fragmentation of peptides via cavitation effect. Karnjanapratum and Benjakul (2015) found that varying MW profiles revealed the differences in DH, which was governed by the hydrolysis process and the enzyme used. Thus, US treatment and alcalase concentration used mainly affected the MW distribution of peptides in P-HS from co-product.
Elution profile by Sephadex G-25 size exclusion chromatography of P-HS from co-product prepared without and with US pretreatment, followed by heating before hydrolysis using alcalase at different levels. Absorbance at 280 nm (close circle), 220 nm (open circle). A; non-US pretreatment using 1% alcalase, B; non-US pretreatment using 2% alcalase, C; US treatment using 1% alcalase, D; US treatment using 2% alcalase
Amino acid composition
Table 2 shows the amino acid compositions of P-HS from co-product (US treatment at 60% amplitude in combination with heating using 1% alcalase). Essential amino acids (EA-A) are important amino acids in protein in terms of nutrition value and also have an effect on the functional properties of food proteins (Quek et al., 2018). P-HS had leucine, threonine, lysine and valine as major EA-A. In addition, non-essential amino acids (NEA-A), which could be synthesized by the human body such as proline, aspartic acid, serine and glutamic acid were also found in P-HS. However, no bitterness was detected in the P-HS (data not shown). The result was in agreement with Ng et al. (2020) who documented that P-HS from co-product had valine, threonine, leucine as major EA-A and aspartic acid, serine, proline as NEA-A. However, the major EA-A of P-HS from co-product was higher in concentration than other high protein foods such as chicken egg yolk, quail egg yolk or quail egg white (Chua et al., 2015). Thus, P-HS from co-product could be another alternative source of EA-A, in which the daily requirement can be met (Quek et al., 2018).
Conclusions
P-HS from co-product could be prepared with the aid of US pretreatment (60% amplitude) combined with heating before enzymatic hydrolysis (1% alcalase). P-HS had whiter color and high yield. It showed high radical scavenging activity and reducing power. The MW of peptides in P-HS became smaller in size when US treatment was implemented before hydrolysis. Therefore, US pretreatment along with heating could be an alternative technique for co-product preparation before enzymatic hydrolysis. Overall, P-HS with antioxidative property could be used as a functional ingredient or nutraceutical for health benefit.
Availability of data and material
Research data are not shared.
Code Availability
Not Applicable.
References
AOAC (2002) Official method of analysis of AOAC International, 17th edn. Association of Official Analytical Chemists, Washington DC
Aguilar-Acosta LA, Serna-Saldivar SO, Rodríguez-Rodríguez J, Escalante-Aburto A, Chuck-Hernández C (2020) Effect of ultrasound application on protein yield and fate of alkaloids during lupin alkaline extraction process. Biomolecules 10:292. https://doi.org/10.3390/biom10020292
Babji A, Ibrahim ESK, Daud N, Nadia N, Akbar H, Ghassem M, Najafian L, Salma M (2018) Assessment on bioactive components of hydrolysed edible bird nest. Int Food Res J 25:1936–1941. ISSN19854668
Benzie I, Strain J (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1:70–76. https://doi.org/10.1006/abio.1996.0292
Bhaskar N, Benila T, Radha C, Lalitha RG (2008) Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Bioresour Technol 99:335–343. https://doi.org/10.1016/j.biortech.2006
Binsan W, Benjakul S, Visessanguan W, Roytrakul S, Tanaka M, Kishimura H (2008) Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei). Food Chem 106:185–193. https://doi.org/10.1016/j.foodchem.2007.05.065
Chantakun K, Benjakul S (2020) Effect of pretreatments and retort process on characteristics and sensory quality of edible bird’s nest beverage. Int J Food Sci Technol 55:2863–2871. https://doi.org/10.1111/ijfs.14542
Chantakun K, Nuthong P, Benjakul S (2020) Influence of different alginate pretreatments on characteristics of edible bird’s nest flakes and their sterilized beverage. LWT-Food Sci Technol 131:109695. https://doi.org/10.1016/j.lwt.2020.109695
Chotphruethipong L, Aluko RE, Benjakul S (2019) Hydrolyzed collagen from porcine lipase-defatted seabass skin: Antioxidant, fibroblast cell proliferation, and collagen production activities. J Food Biochem 43:e12825. https://onlinelibrary.wiley.com/doi/10.1111/jfbc.12825
Chua P, Chan S, Bloodworth B, Li S, Leong L (2015) Identification of edible bird’s nest with amino acid and monosaccharide analysis. J Agric Food Chem 63:279–289. https://doi.org/10.1021/jf503157n
Department of International Trade Promotion (DITP) (2019) Bird’s nest in Thailand. https://ditp.go.th/contents_attach/150637/150637.pdf. (Accessed 10.05.20.)
Gan JY, Chang LS, Mat Nasir NA, Babji AS, Lim SJ (2020) Evaluation of physicochemical properties, amino acid profile and bioactivities of edible Bird’s nest hydrolysate as affected by drying methods. LWT-Food Sci Technol 131:109777. https://doi.org/10.1016/j.lwt.2020.109777
Haghani A, Mehrbod P, Safi N, Kadir FAA, Omar AR, Ideris A (2017) Edible bird’s nest modulate intracellular molecular pathways of influenza A virus infected cells. BMC Complement Altern Med 17:1–13. https://doi.org/10.1186/s12906-016-1498-x
Idowu AT, Benjakul S, Sinthusamran S, Sookchoo P, Kishimura H (2019) Protein hydrolysate from salmon frames: Production, characteristics and antioxidative activity. J Food Biochem 43:e12734. https://doi.org/10.1111/jfbc.12734
Ikram S, Zhang H, Ahmed M, Wang J (2020) Ultrasonic pretreatment improved the antioxidant potential of enzymatic protein hydrolysates from highland barley brewer’s spent grain (BSG). J Food Sci 85:1045–1059. https://doi.org/10.1111/1750-3841.15063
Karnjanapratum S, Benjakul S (2015) Characteristics and antioxidative activity of gelatin hydrolysates from unicorn leatherjacket skin as affected by autolysis‐assisted process. J Food Process Preserv 39:915–926. https://doi.org/10.1111/jfpp.12304
Klompong V, Benjakul S, Kantachote D, Shahidi F (2007) Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem 102:1317–1327. https://doi.org/10.1016/j.foodchem.2006.07.016
Ling JWA, Chang LS, Babji AS, Lim SJ (2020) Recovery of value-added glycopeptides from edible bird’s nest (EBN) co-products: Enzymatic hydrolysis, physicochemical characteristics and bioactivity. J Sci Food Agr 100:4714–4722. https://doi.org/10.1002/jsfa.10530
Ma F, Liu D (2012) Sketch of the edible bird’s nest and its important bioactivities. Food Res Int 48:559–567. https://doi.org/10.1016/j.foodres.2012.06.001
Ng SR, Mohd Noor HS, Ramachandran R, Tan HY, Ch′ng SE, Chang LS, Babji AS, Lim SJ (2020) Recovery of glycopeptides by enzymatic hydrolysis of edible bird’s nest: The physicochemical characteristics and protein profile. J Food Meas Charact 14:2635–2645. https://doi.org/10.1007/s11694-020-00510-4
Petcharat T, Benjakul S, Karnjanapratum S, Nalinanon S (2021) Ultrasound-assisted extraction of collagen from clown featherback (Chitala ornata) skin: yield and molecular characteristics. J Sci Food Agric 101:648–658. https://onlinelibrary.wileyhttps://doi.org/10.1002/jsfa.10677
Quek MC, Chin NL, Yusof YA, Law CL, Tan SW (2018) Characterization of edible bird’s nest of different production, species and geographical origins using nutritional composition, physicochemical properties and antioxidant activities. Food Res Int 109:35–43. https://doi.org/10.1016/j.foodres.2018.03.078
Schauer R (2009) Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol 19:507–514. https://doi.org/10.1016/j.sbi.2009.06.003
Tacias-Pascacio VG, Morellon-Sterling R, Siar EH, Tavano O, Berenguer-Murcia Á, Fernandez-Lafuente R (2020) Use of alcalase in the production of bioactive peptides: A review. Int J Biol Macromol 165:2143–2196. https://doi.org/10.1016/j.ijbiomac.2020.10.060
Thavamanithevi S, Sarifah R, Lim CG, Theanmalar M, Aidawati MS, Devi MD, Saleha AA (2014) Characterization and standardization of edible bird’s nest (EBN)-determination of sialic acid. Proceedings of the Edible Bird Nest Industry Conference. November 2014 Putrajaya, pp 25–26
Wang F, Zhang Y, Xu L, Ma H (2020) An efficient ultrasound-assisted extraction method of pea protein and its effect on protein functional properties and biological activities. LWT-Food Sci Technol 127:109348. https://doi.org/10.1016/j.lwt.2020.109348
Wen C, Zhang J, Zhou J, Cai M, Duan Y, Zhang H, Ma H (2020) Antioxidant activity of arrowhead protein hydrolysates produced by a novel multi-frequency S-type ultrasound-assisted enzymolysis. Nat Prod Res 34:3000–3003. https://doi.org/10.1080/14786419.2019.1601192
Yang X, Li Y, Li S, Ren X, Olayemi Oladejo A, Lu F, Ma H (2020) Effects and mechanism of ultrasound pretreatment of protein on the Maillard reaction of protein-hydrolysate from grass carp (Ctenopharyngodon idella). Ultrason Sonochem 64:104964. https://doi.org/10.1016/j.ultsonch.2020.104964
Zhang Y, Ma H, Wang B, Qu W, Li Y, He R, Wali A (2015) Effects of Ultrasound Pretreatment on the Enzymolysis and Structural Characterization of Wheat Gluten. Food Biophys 10:385–395. https://doi.org/10.1007/s11483-015-9393-4
Funding
The authors would like to express their sincere thanks to Prince of Songkla University for the financial support under Prachayacharn program (Grant No. AGR6402088N).
Author information
Authors and Affiliations
Contributions
Kasidate Chantakun: Investigation, data analysis, methodology, validation. writing-original draft. Soottawat Benjakul: Conceptualization, funding acquisition and editing of manuscript.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
The authors declare no conflict of interest.
Ethics approval
The experiment was reviewed and approved by the ethical committee (ethical number FIRIn 2562/023) of Prince of Songkla University, Hat Yai, Thailand.
Consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chantakun, K., Benjakul, S. Effect of ultrasound-assisted pretreatment in combination with heating on characteristics and antioxidant activities of protein hydrolysate from edible bird’s nest co-product. J Food Sci Technol 59, 3908–3917 (2022). https://doi.org/10.1007/s13197-022-05420-5
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-022-05420-5