Abstract
The objective of this study was to investigate the protease family caspases in skeletal muscle and their potential contribution to postmortem proteolysis and meat tenderization. Nine yaks were slaughtered, and samples of Longissimus dorsal were injected with AC-DEVD-CHO at a ratio of 1:1 (w/v) and then stored at 4 °C for 2, 6, 12, 24, 72, and 120 h. Results indicate that the morphological changes of the muscle fibers are significantly obstructed, which is not conducive to the subsequent degradation of proteins. After inhibiting the activity of Caspase- 3, the activity of Caspase-8 and 9 and the energy metabolism was affected. In the case of without inhibition of caspase, the pH value decreased and then increased. The meat color and the water retention are better, the muscle fiber skeleton protein degradation is remarkable, the tenderness is improved. Furthermore, yak meat tenderness was improved by apoptotic pathway during aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apoptosis is a programmed cell death that depends on an organism’s energy. A number of complex biochemical reactions are involved in meat tenderness that develops during muscle aging, and these reactions are influenced by various factors (Ouali et al. 2006). Apoptosis is the first step in the conversion of muscles to meat, and its subsequent steps induce internal changes (Chen et al. 2011). The postmortem tenderization of muscle is a complex process that improves meat quality and similar maturation occur because postmortem muscle tissues disrupt the main communication with the external environment and similar maturation occur because postmortem muscle tissues disrupt the main communication with the external environment (Cao et al. 2010). Current studies have also investigated apoptotic mechanisms to explain the maturation of meat after slaughter. The involvement of the postmortem degradation of myofibrillar proteins in meat tenderness has also been described. The conversion of muscles into meat is a sequence of changes and events, but underlying pathways have been poorly characterized. Koohmaraie and other researchers found that the degradation of cytoskeletal proteins is the main factor influencing beef tenderness during postmortem aging (Herrera-Mendez et al. 2006). Lee and Kauffman indicated that biochemical and morphological features reveal postmortem skeletal muscle cells in early death in the form of apoptosis (Wolf et al. 2017; Koohmaraie and Geesink 2006). The above scholars explained the effect of apoptosis on tenderness from protein degradation or other aspects. They also provided a theoretical basis for further investigations on cell apoptosis in enzyme maturation (Chen et al. 2011). Recent studies have attempted to emphasize the molecular mechanisms of apoptosis and the influence of postmortem biochemical factors on meat quality (Cao et al. 2010; Herrera-Mendez et al. 2006). However, additional evidence is necessary to evaluate the apoptotic activation and its contribution to internal environment changing during muscle postmortem aging.
Underwood showed that caspases are closely related to meat tenderness (Dimri et al. 1995). Postmortem tenderization consists of a series of complex biochemical activities in muscle cells. The activities of the caspase family in animal skeletal muscle cells during maturation are dependent on physiological conditions. Caspase activities also affect cell deformation, protein degradation, and quality changes. At present, caspase-3, caspase-8, and caspase-9 are involved in the maturation and other relevant processes of yak meat after slaughter. The effect of these enzymes on the quality of yak meat should be further investigated.
Chen demonstrated that maturation during postmortem glycolysis decreases the pH of the intracellular environment of meat and consequently deforms muscle cell (Kauffman et al. 2003). Biochemical mechanisms that stimulate these changes are dependent on the pH of postmortem muscles. Despite several findings on apoptosis, the involvement of pH and energy metabolism in cell death mechanisms and in caspase-3, caspase-8, and caspase-9 activation in different cellular systems remains poorly understood.
Considering that caspases play a pivotal role in the apoptotic death of muscle fibers. To further investigate the effect of apoptosis in postmortem regulation, we investigated the effects of injection of Ac-DEVD-CHO, a specific caspase-3 inhibitor in postmortem yak longissimus dorsi (LD) muscle. In muscle fibers exposed to AC-DEVD-CHO, the scientists already found an increase in myofiber cleavage, suggesting that caspase-3-like proteases were activated. Although the inhibition of caspase-3 enzyme activities with AC-DEVD-CHO attenuated the death of myofibers for up to 12 h, blocking caspase activities with peptide inhibitors did not provide any substantial long-term protection. AC-DEVD-CHO treatment blocks myofiber degradation and cell death for an extended period and over a broad range of AC-DEVD-CHO concentrations. These findings prompted us to examine whether an inhibitor of caspase-3-like proteases likely blocks cell death by caspase-3, caspase-8, and caspase-9. Our study aimed to investigate the correlation of caspase-3, caspase-8, and caspase-9 with the tenderness and internal environment changing of yak meat and to explore the theoretical basis for yak meat apoptosis.
Materials and methods
Sample preparation
The M. longissimus lumborum (LL, the anterior 12th rib to the last lumbar vertebrae) were randomly extracted from an abattoir (Yushu Tibetan Autonomous Prefecture (4500 m), Qinghai Province, China. Nine yak bulls (weight 241–280 kg) for each group were of the similar age and two groups have similar feeding and carcasses conditions. The experiment was conducted in accordance with the guidelines of the Canadian Council on Animal Care, and animal welfare and conditions were considered in the use of the experimental animals. Subsequently, 30 g of 0 h samples were rapidly frozen in liquid nitrogen. Another 180 g of muscle samples was transferred to our laboratory in ice bags within 45 min and vacuum packed in pouches. Samples were washed with PBS to remove any blood and contaminants on the surface. Further, a sample from each yak was snap-frozen immediately in liquid nitrogen for 5 min. The remainder of each of the 30 g of muscle pieces was subdivided into two fractions and distributed to two treatments as follows: one section did not receive any treatment (as the control group) and the other samples were injected with AC-DEVD-CHO (200 mmol/L) in the ratio of 1:1 (w/v) and then stored at 4 C for 6, 12, 24, 72, 120, and 168 h. At the end of each storage period, the samples were obtained and stored at −80 °C until needed.
Caspase-3, caspase-8, and caspase-9 activities
The activities of caspase-3, caspase-8, and caspase-9 proteases were determined using the Enzchek® Protease Assay Kits (Molecular Probes, Beijing, China) according to the method described by Ross et al. (Underwood et al. 2008). Briefly, 200 mg samples in cracking fluid (0.5 mL 100 mol/L HEPES pH 7.5, 10% sugar, 0.1% NP-40 10 mol/L DTT) were centrifuged at 3800 × g for 5 min. The pellets were suspended in 1.5 mL of PBS (137 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4, 2 mmol/L KH2PO4, pH 7.8), frozen at − 20 °C, sonicated in an ice bath by using a microtip sonicator until they were thawed, and immediately refrozen. This procedure should be repeated three times. The cell extract was then centrifuged (18,000 × g, 30 min) to remove any debris, and the supernatant was transferred to a clean microtube. The Bradford Protein Assay Kit (Beyotime Institute of Biotechnology, Shanghai, China) was used to determine the total protein concentration of the samples. Caspase-3, caspase-8, and caspase-9 activities were quantified using the Enzchek® Caspase-3 Assay, Caspase-8 Assay, Caspase-9 Assay according to the manufacturer’s instructions. The cell extracts (50 µL) were incubated with the caspase substrates AC-DEVD-AMC, AC-IETD-AMC, and AC-LEHD-AMC (25 µmol/L, final concentration) for 1 h in the dark at room temperature. The appearance upon enzymatic cleavage of the nonfluorescent substrates AC-DEVD-AMC, AC-IETD-AMC, and AC-LEHD-AMC was subsequently assayed with a microplate reader (Molecular Device, M5, US) using an excitation wavelength of 485 nm and an emission wavelength of 538 nm. The background fluorescence signal from the negative controls without any enzyme was subtracted from the fluorescence of all of the samples.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis
Under the condition of all samples at 4 °C for 2 h, 12 h, 24 h, 72 h and 120 h sampling, we added 4 mL sodium dodecyl sulphate (SDS)-PBS (8.8 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, 0.24 g KH2PO4 distilled water to 1000 mL, adjust by Na2HPO4 pH 7.4). With the proposed materials and methods, we added the buffer (10 mL 2% SDS pH 7.0), and ground the samples, which were then centrifuged at 8000 × g for 5 min; 5 mL supernatant was obtained and then placed in − 80 °C.
We followed and slightly improved the method of Hopkins on protein denaturation electrophoresis production (Pandey et al. 2014). The concentration of separation gel was 12.5%, and the concentration of concentrated gel was 5%. To test the protein cryogenically refrigerated at − 80 °C, we mixed the sample buffer (60% Tris, 25% HCl, 14.4 g glycerin, 0.1% bromophenol blue, pH 6.8) with 4:1 mixture and boiled it in water for 5 min. After the protein cooled, it was centrifuged at 8000 × g for 5 min, and the trace sample was absorbed from 15 to 20 L. The electrophoretic voltage conditions were as follows: concentrated adhesive at 75 V, separation glue at 110 V, at time at about 4 h. After dyeing and decolorization, the separation gel is placed into a large medium, poured into the dye for 40 min, and gently shaken to color evenly. Afterward, a decolorizing agent was added to the decolorizing solution for 10 min. After the secondary decolorization, which was soaked for 10 h and then changed into distilled water to preserve the glue. The gels or membranes were scanned (GT-800F EPSON) at a resolution of 300 dpi, and the densities of targeted bands were quantified by Quantity One software (Bio-Rad).
Shear force
Using the method of Girard et al. to evaluate the shear force, we cooked the muscles in a water bath at 80 °C to an internal temperature of 78 °C (Underwood et al. 2008). The samples were cooled overnight at 4 °C before the shearing core of the samples was examined using a tenderometer (C-LM4, College of Engineering, Gansu Agricultural University, China). Three to five cores (1.27 cm diameter) were cut from the steaks, parallel to the orientation of the muscle fibers. Each sample was then sheared with the long axis of the fibers running perpendicular to the blade. The results were expressed as shear force (kg/cm2).
Myofibril fragmentation index (MFI)
Muscle fibrillation index (MFI) was determined as described in a previous study by Delgado with slight modifications (Chen et al. 2015). The samples were homogenized at 1000 × g for 30 s with 8 mL of buffer (20 mmol/L K3PO4, 100 mmol/L KCl, pH 7.1; 1 mmol/L MgCl2, 1 mmol/L EDTA, and 1 mmol/L NaN3) at 4 °C (Chen et al. 2015). The homogenized samples were centrifuged for 15 min at 1000 × g, and the supernatant was decanted. The sediment was resuspended in 8 mL of buffer (20 mmol/L K3PO4, 100 mmol/L KCl, pH 7.1; 1 mmol/L MgCl2, 1 mmol/L EDTA, and 1 mmol/L NaN3) and centrifuged again. The sediment with 10 mL of buffer was filtered using a mesh screen. The protein concentration was determined using biuret method with a BSA standard. The final protein concentration was determined to 0.5 mg/mL, and the absorbance was measured immediately at 540 nm with an ultraviolet spectrophotometer (UV2550, Shimadzu Corporation, Kyoto, Japan). MFI was calculated by multiplying readings by 200.
pH
Muscle pH was monitored using a portable pH meter (Mallinckrodt Chemicals, Phillipsburgh, NJ, USA). The pH meter was calibrated at pH 7.0 and 4.0 with standard buffers which was stored at room temperature (20 °C). Data with average values were recorded thrice.
ATP, ADP, AMP, and IMP activity assay method
The changes in ATP, ADP, AMP, and IMP contents in the postmortem muscle were determined by high-performance liquid chromatography. Using the method of Kawase., we removed 800 mg of frozen sample from liquid nitrogen; 3 mL of 7% perchloric acid (PCA) was added and centrifuged at 15,000 × g for 10 min (Church et al. 2010). The supernatant was neutralized with l.44 mL 0.85 mol/L K2CO3 and centrifuged at 15,000 × g for 10 min. ATP, ADP, AMP, and IMP were measured on an Agilent 1100 Liquid Chromatograph/HPLC (G1946A, USA) with a wavelength of 254 nm, a flow rate of 1 mL/min, and a temperature of 7 °C. The sample injection volume was at 10 µL, which comprise the reference substance injection volume of 2 µL buffer (12 mmol/L disodium hydrogen phosphate and 88 mmol/L sodium dihydrogen phosphate, pH 6.5) as mobile phosphate phase A and 8 µL methanols as mobile phase B. The ATP, ADP, AMP and IMP standard results were measured by external standard method. Qualitative and quantitative analysis results showed that the retention time of the reference substance and the peak area of the sample.
Statistical analysis
Data were presented as mean ± standard deviation and were analyzed with SPSS (SPSS 13.0, Chicago, IL, USA) through one-way ANOVA. Multiple comparisons were performed with Duncan method. Significance level was set at P < 0.05. Origin Pro 7.5 was used for mapping.
Results and discussion
Caspase-3, caspase-8, and caspase-9 activities
On the basis of previous findings, we verified the effect of caspase on the maturation of yak meat by adding AC-DEVD-CHO, which is a specific inhibitor of caspase-3. The changes in various parameters, including caspase-3, caspase-8 and caspase-9 activities, of yak meat after 2–120 h were tested to identify the alterations of caspase enzyme activity.
In Fig. 1-1, caspase-3 activities between the AC-DEVD-CHO group and the control group were significantly different. In the control group, the caspase-3 activity increased rapidly and then decreased 2–120 h after slaughter. The caspase-3 activity reached the highest at 12 h after slaughter and increased 107.45% (P < 0.01) compared with that at 6 h after slaughter. In the AC-DEVD-CHO group, the caspase-3 activity at 2–120 h postmortem was not significantly different (P > 0.05). In comparison with the AC-DEVD-CHO group, the caspase-3 activity of the control group was increased 6.08% without a significant difference (P > 0.05) at 6 h after slaughter. At 24 h after slaughter, the caspase-3 activity of the control group decreased by 11% compared with that of the AC-DEVD-CHO group (P > 0.05).
The caspase-8 activity in the AC-DEVD-CHO group and the control group were significantly different from 6 to 24 h after slaughter (P < 0.05). At 12 h after slaughter, the caspase-8 activity in the control group was increased by 92.26% (P < 0.01) compared with that in the AC-DEVD-CHO group (Fig. 1–2). At 6 h after slaughter, the caspase-8 activity of the control group increased 126.74% (P < 0.01) compared with that of the AC-DEVD-CHO group. After 24 h, the caspase-8 activity of the control group increased 87.28% (P < 0.01) compared with that of the AC-DEVD-CHO group. At 120 h after slaughter, the caspase-8 activity of the control group decreased 6.24% compared with that of the AC-DEVD-CHO group with no significant difference (P > 0.05).
In Fig. 1–3, the caspase-9 activity in the control group increased rapidly and then decreased slowly. The maximum value was reached at 6–12 h after slaughter. The activity at 6 h after slaughter significantly increased by 83.00% (P < 0.01) compared with that at 2 h. The caspase-9 activity in the AC-DEVD-CHO group decreased within 2–120 h after slaughter. The activity dropped to the lowest point at 12 h after slaughter. In comparison with 2 h after slaughter, the activity at 12 h was decreased 33.34% (P < 0.05). The activities of caspase-9 in the AC-DEVD-CHO group and the control group were significantly different at 6–120 h after slaughter (P < 0.05). At 6 h after slaughter, the activity in the control group increased significantly by 104.50% (P < 0.05) compared with that in the AC-DEVD-CHO group. At 12 h after slaughter, the activity in the control group increased significantly by 168.33% (P < 0.01) compared with that of the AC-DEVD-CHO group. At 120 h after slaughter, the activity in the control group increased significantly by 113.00% (P < 0.01) compared with that in the AC-DEVD-CHO group.
Herrera-Mendez suggest that meat tenderization is a multienzymatic process involving the well-studied systems of calpains, cathepsins, proteasomes, and caspases whose functions in postmortem muscle are unclear (Chen et al. 2011). This experiment showed the caspase-3 activity at 2–120 h after slaughter. The yak meat yielded at 12 h have the highest caspase-3 activity, and the AC-DEVD-CHO group did not show any changes in caspase-3 activity. Therefore caspase-3 inhibitor AC-DEVD-CHO can significantly decrease this induction. Kemp found that the caspase-3 activity of longissimus dorsi muscle in cattle after 12 h was the highest and then decreased significantly, which was consistent with the results of this experiment (Smit et al. 2002). Zhang found that in comparision with the control group, the caspase-3 inhibitor group showed significant variability (Kawase et al. 2004). Underwood have shown that in vitro incubation inhibited caspase-3 and protein degradation is still ongoing; this result is in contrast to the results of this trial; the difference may be due to the vitro incubation of samples (Dimri et al. 1995). In this study, after 2–6 h, the caspase-8 activity in the control group showed that the caspase-8 activity is earlier than that of caspase-3. Fritz found that the binding of caspase-8 to the apoptotic signal of AC-DEVD-CHO is in the outer membrane of the mitochondrion, and then mediates apoptosis through the death receptor pathway (Kemp et al. 2006). As the result, that normal caspase-3 activity can be suppressed by inhibiting caspase-8. Caspase-3 inhibition possibly affects caspase-8 in the apoptotic pathway and caspase-8 plays an important role in the apoptotic pathway. After 2–12 h, the caspase-9 activity in the control group was higher than that in the AC-DEVD-CHO group, and this activity was inhibited under the caspas-3 inhibotor. The caspase-9 activity affected and hindered the apoptotic pathway of endogenous cascade. Youle et al. found that the upstream caspase-9 of the mitochondrial pathway participates in the apoptotic cascade reaction in downstream caspase-3 and caspase-6 Shin and Sung (2001). Olie et al. found that caspase-9 is involved in caspase-3 activation (Fritz and Greaser 2010). Thus, our data suggested that caspase activation attenuates AC-DEVD-CHO induced early apoptotic death by caspase-3, caspase-8, and caspase-9.
SDS-PAGE
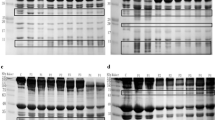
Figure 2 shows that the three maps are added to the yak meat caspase-3 inhibitor myofibrillar degradation. No significant difference was found between the AC-DEVD-CHO group and the control group at 2 h after slaughter, and no degradation product of troponin-T appeared at 30 kDa; the effect of the inhibitor was not significant. The AC-DEVD-CHO group significantly differed from the control group at 12 h after slaughter, and the inhibitory effect on myofibrillar proteins was significant. Figure 2 show that the protein band at 12 h is at the top of the concentration with large stains and scattered traces. After 72 h, the AC-DEVD-CHO group and the control group had different degrees of degradation; the effect of the inhibitor was lower than that of the control group. At 120 h after slaughter, the AC-DEVD-CHO group showed significant degradation of myofibrillar protein; the inhibitor almost had no inhibitory effect. Therefore, the inhibitory effect of caspase-3 inhibitor on protein degradation was significant at 12 h after slaughter.
Considering that caspases play a pivotal role in the apoptotic death of muscle fibers, we further characterized the role of AC-DEVD-CHO in apoptosis to determine whether caspases participate in this model. In muscle fibers exposed to AC-DEVD-CHO, the cleavage activity of myofibers increased, thereby suggesting that caspase-3-like proteases were activated. These findings prompted us to examine whether an inhibitor of caspase-3-like proteases blocks cell death triggered by caspase-3, caspase-8, and caspase-9. Although the inhibition of caspase-3 enzyme activity with AC-DEVD-CHO attenuated the death of myofibers up to 12 h, our data indicated that blocking caspase activities with peptide inhibitors did not provide any substantial long-term protection because AC-DEVD-CHO prevented myofiber degradation and cell death for an extended period and over a broad range of AC-DEVD-CHO concentrations. Thus, our data implied that only caspase activation is responsible for the attenuation of AC-DEVD-CHO-induced early apoptotic death stimulated by caspase-3, caspase-8, and caspase-9. This type of cell death is initially dependent on caspase activation, but another type of neuronal cell death independent of caspase-3 activation occurs at later time points. However, we could not formally exclude the role of an unidentified caspase family member that plays a more prominent role in the AC-DEVD-CHO-induced death of myofibers.
Samejima et al. studied the improvement of the hardness of myofibrils during the slaughter of chicken fibers (Youle and Strasser 2008). Hopkins investigated bovine and mutton protein electrophoresis and revealed that caspase-3 degrades muscle fibrin markers that are nTc-T 30 kDa degradation products (Pandey et al. 2014). Fritz et al. conducted beef muscle immunoblotting and found that myosin and troponin-T are first destroyed after slaughter destroyed the overall connection between muscle fibers (Kemp et al. 2006). Therefore, observation of degradation products can determine the degree of tenderness of yak meat. At 12 h after slaughter, with the addition of the inhibitor to yak meat, it did not show degradation products (Fig. 2), indicating that caspase did not play a role. In this study, caspase was significantly involved in the process of slaughtering yak meat by adding AC-DEVD-CHO, a specific inhibitor of apoptosis caspase-3. Figure 2 shows that the AC-DEVD-CHO inhibitory effect was significant (P < 0.05) at 12 h after slaughter. Huang et al. found that caspase-3 positively affects muscle protein degradation (Samejima and Wolfe 1976). Kemp analyzed the degradation of caspase-3 protein in vitro and differences in natural maturation. They observed that the effect of the apoptosis-related enzyme on yak meat is likely stronger than that on the meat of other animals (Samejima and Wolfe 1976).
Shear force
The shear force values obtained would classify the LD from the carcasses examined which was shown in Fig. 3. The relationships between shear force in usual samples and the change in caspase inhibitor treatment samples were investigated at 0 and 120 h in the early postmortem period. From 2 to 120 h, the shearing force of the AC-DEVD-CHO group was always higher than that of the CK group (35.34%, 21.34%, 23.35%, 47.50%, 56.80%, 60.19%) at 2–120 h, with significant differences (p < 0.05). In the CK group, from 2 to 120 h, the shear force increased slightly and then decreased rapidly. At 6 h, the shear force increased by 9.60% from 2 h, and at 24 h post-mortem the shear force decreased by 22.61% from 12 h. After 120 h, the shearing force decreased significantly by 45.34% (P < 0.01), and after 120 h, the shearing force decreased significantly by 42.16% (P < 0.01). In the AC-DEVD-CHO group, the shearing force gradually decreased from 2 to 120 h, and the shearing force at 12 h post-mortem decreased by 3.60% compared with 2 h (P > 0.05). The shearing force at 24 h was 7.45% lower than that at 12 h, the shearing force at 72 h was 16.67% lower than that at 24 h, and the shearing force at 120 h was significantly decreased by 31.54% (P < 0.01).
Sun studied the yak meat, pointing out that tenderness is an important factor to reflect the relationship between meat quality index and caspase (Huang et al. 2012a). Tenderness is related to the time of meat hanging. Underwood et al. studied on postmortem beef tenderness and found that the relationship between caspase and shear force was significant (Dimri et al. 1995). Huang showed that the meat tenderness of the meat were better at 5 days than at 2 days after slaughter, and the meat was tender with high quality for 14 days after slaughter (Kemp and Wheeler 2011). In our study, the shear force between the control group and the AC-DEVD-CHO group was very significant after 2–120 h postmortem interval. The shear force of the AC-DEVD-CHO group was significantly higher than that of the control group, which may be due to the inhibition of the caspase activity.
MFI
In Fig. 4, the MFI value of AC-DEVD-CHO group was significantly lower than that of CK group (26.96%, 20.46%, 58.89%, 63.69%, 65.51% and 61.69%) at 2–120 h, and the shear force value of AC-DEVD-CHO group was significantly different from CK group (P < 0.05). In the AC-DEVD-CHO group, the MFI increased slightly from 2 to 120 h after slaughter, and increased 38.74% (P < 0.05) from 12 h. The MFI value of CK group increased from 2 to 72 h, and decreased slightly after 72 h. The MFI value reached the maximum value at 72 h, increased 231.38% (P < 0.01) compared with 6 h, and increased 218.54% (P < 0.01) at 120 h compared with 2 h.
MFI is closely related to tenderness, and the changes of MFI can reflect the degradation degree of myofibrils. Thus, we chose MFI as the index for evaluating the effects of mitochondrial apoptotic activation mediated by yak meat tenderization in postmortem aging. Calkins pointed out that the degree of muscle fiber fragmentation in postmortem aging can reflect the degree of meat tenderness (Sun et al. 2014). Sawdy studied the waist muscle of beef after slaughter for 36 h, and the MFI increased after slaughter (Huang et al. 2012b). Barese studied the degradation of muscle fiber by caspase-3. The results showed that caspase inhibitor played an important role in the maturation of tenderness in the early stage of postmortem, and when the caspase was inhibited, even if other endogenous enzymes can play the role of degradation, the effect of tender decreased significantly. This conclusion is consistent with our experimental results. Therefore, the relationship between caspase and other endogenous tender factors is likely dependent on apoptosis-related enzymes.
pH
In our study, the pH of the control group changed slowly after 2–120 h postmortem (Fig. 5). After 12–24 h in the AC-DEVD-CHO group, the pH continued to decline. Hence, the inhibition of caspase, even if other endogenous enzymes exist, also leads to the process of tendering and the process of postmortem aging to slow down. This shows that the acid environment is conducive to the activation of caspases. The early pH decreases rapidly after slaughter, and the activity of μ-calpain is inhibited. The degradation rate of caspases is improved. This experiment shows that the early caspase degradation protein is high, which makes the meat tender. Fernanda et al. studied on wild boars and commercial pigs, the early rapid decline of pH, and calcium-activated enzyme inhibitory protein (calpastatin) that causes μ-calpain autolysis; μ-calpain has low activity (Sawdy et al. 2004).
The change of carcass pH directly regulates the intracellular acidity of carcass cells, triggering or inhibiting a series of intracellular changes, especially the changes of various endogenous enzyme activities. Mohrhauser and Underwood et al. found that decreased pH was associated with changes in caspase activity (Dimri et al. 1995). Immonen et al. showed that the final pH of pork meat affected the tenderness of pork; the pH of carcass was greater than 7, and the caspase-3 activity could be inhibited; the decomposition of residual glycogen and ATP consumption would be active again, until the formation of the maximum stiffness; moreover, the active stage of the enzyme is in the stagnation of the solution (Marchiori and Felício 2003).
ATP, ADP, AMP, and IMP activity assay method
In Fig. 6-1, the ATP activity of the AC-DEVD-CHO group was higher than that of the control group at 2–120 h after slaughter (P < 0.05). At 2–120 h, the control group showed a decreasing trend. At 24 h after slaughter, the activity decreased by 37.76% compared with that at 2 h after slaughter. The ATP activity of the control group reached the lowest point at 72 h after slaughter and decreased significantly by 73.79% (P < 0.05) compared with that at 24 h. The trend of the AC-DEVD-CHO group was observed to have irregular rise and decrease after 2–120 h postmortem. The ATP activity of the AC-DEVD-CHO group at 6 h after slaughter decreased by 29.70% compared with that at 2 h (P < 0.01). At 72 h after slaughter, the activity decreased by 41.71% compared with that at 12 h (P < 0.01). At 1230 h after slaughter, the ATP activity of the AC-DEVD-CHO group was slightly increased by 14.64% with no significant difference (P > 0.05).
In Fig. 6-2, the ADP activity of the AC-DEVD-CHO group was higher than that of the control group at 2–120 h after slaughter. The ADP activity of the AC-DEVD-CHO group shows a declining trend and then rise at 2–120 h after slaughter. The postmortem interval decreased by 32.45% at 6 h after slaughter compared with that at 2 h. At 120 h after slaughter, the interval decreased by 46.47% (P < 0.01) compared with that at 12 h. The control group at 2–120 h after slaughter showed a decreasing trend and reached the lowest point at 24 h after slaughter.
In Fig. 6-3, the AMP activity of the AC-DEVD-CHO group was higher than that of the control group at 2–24 h after slaughter (P < 0.05). The control group at 2–120 h showed a decreasing trend. The lowest point was reached at 12 h after slaughter, and decreased by 20.00% compared with that at 2 h after slaughter. At 120 h after slaughter, the activity decreased by 42.86% (P < 0.01). After 2–120 h postmortem in the AC-DEVD-CHO group, the trend had a slight increase and a gradual decrease. At 72 h after slaughter, the lowest level was reached; the activity decreased significantly by 68.89% (P < 0.01) compared with that at 2 h after slaughter. At 72 h after slaughter, the activity of the AC-DEVD-CHO group was significantly lower than that of the control group by 7.14% (P > 0.05). The activity of the AC-DEVD-CHO group at 120 h increased 20.29% than that in the control group.
At 2–120 h after slaughter, the IMP activity of the AC-DEVD-CHO group was always lower than that of the control group (P < 0.05; Fig. 6-4). In the control group, at 2–120 h after slaughter, the trend was gradually rising and then declining. At 24 h after slaughter, the IMP activity reached the highest point, which was significantly increased by 88.24% (P < 0.01). At 72 h after slaughter, the activity decreased by 27.87% compared with that at 24 h. At 120 h after slaughter compared with 72 h after slaughter slight, the activity increased 12.14% with no significant difference (P > 0.05). The IMP activity of the AC-DEVD-CHO group at 2–120 h after slaughter showed a slow rise in the trend. At 6 h after slaughter, the activity was gradually lower than 23.87% compared with that at 2 h. At 120 h after slaughter, the activity significantly increased 109.03% (P < 0.01) compared with that at 6 h after slaughter. At 120 h after slaughter, the activity significantly increased by 74.58% (P < 0.05) compared with that at 2 h after slaughter.
In our study, the changes of ATP activity in the control group and the AC-DEVD-CHO group were significantly different after 2–120 h. The ATP activity in the AC-DEVD-CHO group decreased significantly (P < 0.05) at 2–6 h after postmortem; this result may be due to the energy consumption of muscle contraction after postmortem, and the caspase-3 activity was blocontroled. An abnormal increase was observed at 6–12 h after slaughter; this observation may be a temporary increase in the inhibition of the amount of ATP at the same time of the glycolysis of yak meat. After 12–72 h, the ATP activity of the AC-DEVD-CHO group continuously decreased. Borges demonstrated that the ATP activity decreases significantly in the apoptosis of mouse muscle cells (Immonen and Puolanne 2000). Adrain et al. found that the inhibitor of caspases directly alters the ATP contents, and the role of caspase-3 degradation protein is limited (Borges et al. 2014). Samali et al. found that cell apoptosis is associated with the decrease in ADP and AMP contents (Adrain et al. 2001). Underwood observed that the production of apoptotic signals likely reduces ADP and AMP contents in hematopoietic cells. Dimri et al. (1995) Our results showed that the consumption of ADP in the control group and the AC-DEVD-CHO group significantly decreased. The ADP content in the AC-DEVD-CHO group was higher than that in the control group. The AMP activity of the control group and the AC-DEVD-CHO group showed a downward trend. At 72–120 h after slaughter, the two groups decreased to the same degree. The change in energy factor suggested that the caspase inhibitor significantly influenced the postmortem aging of yak meat. Fernanda et al. revealed that the occurrence of apoptosis in cells is accompanied by an increase in the IMP content (Samali et al. 2007). Our results demonstrated that the IMP activity in the control group and the AC-DEVD-CHO group increased, and the degree of the increase in IMP content in the AC-DEVD-CHO group was smaller than that in the control group.
Conclusion
In summary, the activities of Caspase-8 and caspase-9 were affected when AC-DEVD-CHO inhibited caspase-3 activity, so caspase-3 played an important role in the whole apoptosis pathway. AC-DEVD-CHO affect the process of degradation of muscle protein became slow, and the degradation of protein components was abnormal. At 72 h, caspase no longer dominated protein degradation after AC-DEVD-CHO injected. Compared to controls, samples treated with AC-DEVD-CHO, retarded structural disruption of muscle fibers. Therefore, calpains play a major role in tenderization of muscle. The correlation between energy metabolism, intracellular pH and caspase-3 activation in living cells and the dynamic changes during apoptosis. Induction of apoptosis was accompanied by a switch to energy production and caspase-3 activation. The results present a new insight into the basic mechanisms of programmed cell death and suggest new targets for the development of apoptisis in yak meat.
References
Adrain C, Creagh EM, Martin SJ (2001) Apoptosis-associated release of smac/diablo from mitochondria requires active caspases and is blocked by bcl-2. EMBO J 20(23):6627–6636
Borges BO, Curi RA, Baldi F, Feitosa FLB, Albuquerque LGD, Oliveira HND et al (2014) Polymorphisms in candidate genes and their association with carcass traits and meat quality in nellore cattle. Pesq Agrop Brasileira 49(5):364–371
Cao J, Sun W, Zhou G, Xu X, Peng Z, Hu Z (2010) Morphological and biochemical assessment of apoptosis in different skeletal muscles of bulls during conditioning. J Anim Sci 88(10):3439–3444
Chen L, Feng XC, Lu F, Xu XL, Zhou GH, Li QY et al (2011) Effects of camptothecin, etoposide and ca on caspase-3 activity and myofibrillar disruption of chicken during postmortem ageing. Meat Sci 87(3):165–174
Chen L, Feng XC, Zhang YY, Liu XB, Zhang WG, Li CB et al (2015) Effects of ultrasonic processing on caspase-3, calpain expression and myofibrillar structure of chicken during post-mortem ageing. Food Chem 177(12):280–287
Church C, Moir L, Mcmurray F, Girard C, Banks GT, Teboul L et al (2010) Overexpression of fto leads to increased food intake and results in obesity. Nat Genet 42(12):1086–1092
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C et al (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92(20):9363–9367
Fritz JD, Greaser ML (2010) Changes in titin and nebulin in postmortem bovine muscle revealed by gel electrophoresis, western blotting and immunofluorescence microscopy. J Food Sci 56(3):607–610
Herrera-Mendez CH, Becila S, Boudjellal A, Ouali A (2006) Meat ageing: reconsideration of the current concept. Trends Food Sci Technol 17(8):394–405
Huang M, Huang F, Ma H, Xu X, Zhou G (2012a) Preliminary study on the effect of caspase-6 and calpain inhibitors on postmortem proteolysis of myofibrillar proteins in chicken breast muscle. Meat Sci 90(3):536–542
Huang W, Cao JX, Wang DY, Wei-Min XU, Zhang MH (2012b) Impact of caspase-3 activation on the tenderness of duck skeletal muscle during postmortem conditioning. Sci Agric Sin 45(7):1372–1379
Immonen K, Puolanne E (2000) Variation of residual glycogen-glucose concentration at ultimate ph values below 5.75. Meat Sci. 55(3):279–283
Kauffman EC, Robinson VL, Stadler WM, Sokoloff MH, Rinker-Schaeffer CW (2003) Metastasis suppression: the evolving role of metastasis suppressor genes for regulating cancer cell growth at the secondary site. J Urol 169(3):1122–1133
Kawase E, Wong MD, Ding BC, Xie T (2004) Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the drosophila testis. Development 131(6):1365–1375
Kemp CM, Wheeler TL (2011) Effects of manipulation of the caspase system on myofibrillar protein degradation in vitro. J Anim Sci 89(10):3262–3271
Kemp CM, Bardsley RG, Parr T (2006) Changes in caspase activity during the postmortem conditioning period and its relationship to shear force in porcine longissimus muscle. J Anim Sci 84(10):2841–2846
Koohmaraie M, Geesink GH (2006) Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Sci 74(1):34–43
Marchiori AF, Felício PED (2003) Quality of wild boar meat and commercial pork. Sci Agric 60(1):1–5
Ouali A, Herrera-Mendez CH, Coulis G, Becila S, Boudjellal A, Aubry L et al (2006) Revisiting the conversion of muscle into meat and the underlying mechanisms. Meat Sci 74(1):44–58
Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V et al (2014) Negative regulation of cytochrome c-mediated oligomerization of apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J 19(16):4310–4322
Samali A, O’Mahoney M, Reeve J, Logue S, Szegezdi E, Mcmahon J et al (2007) Identification of an inhibitor of caspase activation from heart extracts; atp blocks apoptosome formation. Apoptosis 12(3):465–474
Samejima K, Wolfe FH (1976) Degradation of myofibrillar protein components during postmortem aging of chicken muscle. J Food Sci 41(2):250–254
Sawdy JC, Kaiser SA, St-Pierre NR, Wick MP (2004) Myofibrillar 1-d fingerprints and myosin heavy chain ms analyses of beef loin at 36 h postmortem correlate with tenderness at 7 days. Meat Sci 67(3):421–426
Shin S, Sung B, Cho Y, Kim H, Ha N, Hwang J et al (2001) An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7†. Biochemistry 40(4):1117–1123
Smit RH, Noat Y, Untiedt C, Lang ND, van Hemert MC, van Ruitenbeek JM (2002) Measurement of the conductance of a hydrogen molecule. Nature 419(6910):906–909
Sun Z, Feng X, Ling H, Zhao H, Yu Q (2014) Tenderness and apoptotic activity of yak meat during postmortem aging. Trans Chin Soc Agric Mach 45(1):191–202
Underwood KR, Means WJ, Du M (2008) Caspase 3 is not likely involved in the postmortem tenderization of beef muscle. J Anim Sci 86(4):960–966
Wolf SJ, Maloney GE, Shih RD, Shy BD, Brown MD (2017) Correction: correction to “clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute carbon monoxide poisoning” [annals of emergency medicine. Ann Emerg Med 69(1):98–107
Youle RJ, Strasser A (2008) The bcl-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9(1):47–59
Acknowledgements
We thank colleagues in the laboratory and our collaborators for their useful suggestions. This work was supported by the program for National Natural Science Foundation of China (No. 31760482), and the National Beef Cattle Industrial Technology System (CARS-37) from the Ministry of Agriculture of the People’s Republic of China. The Fostering Foundation for the Excellent Ph.D. Dissertation of Gansu Agricultural University (2018003).
Funding
This work was supported by the China Agriculture Research System (No. CARS-37), National Natural Science Foundation of China (Grant Nos: 31760482), and National Nonprofit AgroScientific Research (201203009).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the author.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yayuan, Y., Ling, H., Qunli, Y. et al. Effects of caspase activity of yak meat and internal environment changing during aging. J Food Sci Technol 59, 1362–1371 (2022). https://doi.org/10.1007/s13197-021-05145-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-021-05145-x