Abstract
The global shift from the usage of crude oil in bio-production is receiving much attention owing to environmental concern associated with fossil fuel. Lignocellulosic biomass (LB) is a good carbon candidate for bio-production because it is environmental-friendly. Corncob being one of such LB is rich in glucose and xylose, which can be utilized for bio-production. We co-utilize these sugars for the production of enzymes from Pichia pastoris GS115 (Wild Type: WT). Glucose utilization was efficient from synthetic and real hydrolysate but xylose utilization was very low, hence, the need for optimization. Mutants were selected upon Adaptive Laboratory Evolution to efficiently utilize xylose. As expected, all the mutants examined showed improved xylose utilization but surprisingly, there was only 1.8 g/l residual xylose in the 50th generation (GS50). The 30th evolutionary generation (GS30) compared well with the WT by completely utilizing the glucose and also accumulated 48 OD600 cell biomass, which is the highest among all the strains evaluated. More importantly, GS30 secreted 72.6 U/ml and 45.1 U/ml β-galactosidase and β-mannanase on hydrolysate respectively, which are higher than the titre for the WT. Conclusively, this study demonstrated the efficacy of corn corncob hydrolysate in biomanufacturing and gives insight for the optimization study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biomass (LB) is a cheap and abundant renewable feedstock from agricultural component for the production of valuable chemicals whose adoption immensely contributes to environmental sustainability as opposed to crude oil biorefinery. The development of renewable energy from LB as an alternative to fossil fuels is viewed as highly important for more sustainable economy (Qian, 2013). Significant efforts are been made to improve and produce new chemicals that are economically feasible (Ge et al., 2018), which has lead to the adoption of LB such as: corn fibre, red algae, coffee waste, corncob and corn stover among others with varying degrees of outcome (Kim et al., 2009; Du et al., 2020; Bhatia et al., 2018; Wang et al., 2017).
Corncob being one these LB types, is rich in glucose, xylose and a minor quantity of arabinose, galactose, rhamnose and insignificant quantity of mannose (Arumugam and Anandakumar, 2016) therefore, making it a promising, low-cost feedstock for bioproduction (Fu et al., 2020). Although, biomass hydrolyzation has been the major practice in presenting the feedstock for microbial bioconversion but inefficient microbial utilization of xylose, the component of LB, has been the major bottleneck in its bioconversion to valuable commodities (Van Vleet and Jeffries 2009). Hence, engineering the xylose utilization pathway both in native and non-native host bas been implemented to mitigate this barrier with interest in the production of fuels and chemicals (Chiang et al., 2013; Dmytruk et al., 2008).
Pichia pastoris is one of the most important eukaryotic systems for industrial production of enzymes due to its ability for efficient production both at laboratory and industrial scale (Macauley-Patrick et al., 2005; Bankefa et al., 2018a; Razaghi et al., 2016; Viera Gomes et al., 2018). Hence, optimization of the use of sustainable carbon sources by P. pastoris, specifically the engineering xylose metabolism in this cell factory is the topic of the study.
The emergence of Adaptive Laboratory Evolution (ALE) for system engineering has been helpful in developing and optimizing bio-systems, optimizing non-native pathways, increasing rate of substrate utilization and phenotypic fitness (Chatterjee and Yuan 2006; Fong et al., 2005; Wisselink et al., 2009). Harnessing this tool on P. pastoris cell factory for improving xylose utilization may therefore be the right step towards efficient carbon utilization in LB for low-cost production of enzymes.
β-galactosidase (lac) is an enzyme that catalyzes the hydrolysis of B-D-galactose residues in B-D-galactoside or transfer galactosyl residue to saccharide acceptors to yield galacto-oligosaccharide (GOS). β-mannanase (man) on the other hand, catalyzes the random hydrolysis of β-1,4-mannosidic linkages in mannan, galactomannan, glucomannan, and galactoglucomannan. These enzymes have a wide range of applications and have been produced in Aspergillus oryzae and Bacillus subtilis but the huge production cost stresses the need for developing other strategies that are more cost effective.
The high percentage of xylose in corncob is our concern in this study, since a previous study had demonstrated the utilization of synthetic xylose by Pichia pastoris for β-mannanase production (Li et al., 2015), we opined that enhancing xylose utilization capacity of P. pastoris system through ALE, could be a panacea for efficient utilization of the embedded xylose in corncob biomass and thereby aiding its conversion into β-galactosidase and β-mannanase.
Materials and methods
Adaptive laboratory evolution (ALE)
P. pastoris GS115 wild-type (WT) strain was used to start the Adaptive Laboratory Evolutionary processes. The shake flask sequential batch culture in aerobic condition was adopted. The xylose-based medium BMXY (8.7 g/l monopotassium phosphate, 13.4 g/l YNB, 0.4 mg/l biotin, 20 g/l peptone, 10 g/l yeast extract and 20 g/l xylose, pH 6.0) was used and subjected to 30 0C at 200 rpm. An aliquot of the culture was transferred to new BMXY medium upon reaching the stationary phase (3–5 days) to initiate the new batch process. The evolutionary process was allowed to continue until 70th generation. The samples collected at 30th, 50th, and 70th generations were tagged GS30, GS50 and GS70, respectively and were reported in this study.
Preparation of corncob hydrolysate
The method described by Santos et al. 2011 was adopted with minor modification. Briefly, the pre-treatment of corncob preceded its adoption for use as fermentation medium. Having removed any particles attached to the cob, they were dried at 70 0C for 12 H and thereafter milled in a hammer mill and fines were removed by intertwined 20 mesh sieves. The fine mills were subjected to heating by direct stream, after which 50 g of the mills were solubilized in diluted sulfuric acid by maintaining 115 0C for 21 min. Upon cooling, the pH of the acidified corncob was adjusted to 6.0 by NaOH and the slurries were thereafter separated into liquid (hemicellulosic hydrolysates) and solid (pre-treated). This study focused on the hemicellulosic hydrolysates and referred to it as corncob hydrolysate (CCH).
Construction of plasmids and strains
PCR fragments encoding the β-galactosidase gene (lac) from Aspergillus oryzae and β-mannanase gene (man) from Bacillus subtilis were amplified (Table 1) and double digested with BstBI and Not1 site and cloneed into an hygromycin based vector pPICHα (Bankefa et al., 2018a) to generate pPICHα-lac and pPICHα-man. The resulting plasmids were thereafter linearized by Sa1I and transformed with GS115 and GS30 competent cells by electroporation at 1500 charging voltage, 200 ohms Resistance and 50 μF Capacitance. The successful transformants were selected on YPD-hygromycin (YPD: 2% D-glucose, 2% peptone and 1% yeast extract) based medium and the positive transformants were tagged GS-lac, GS30-lac, GS-man and GS30-man respectively.
Shake flask fermentation
Shake-flask fermentation of the evolved strains (GS30, GS50, and GS70) and the GS115 wild-type were performed on a 72 H induction processes. All strains were pre-cultured in YPD medium until the cells reached stationary phase. Subsequently, an aliquot to make the initial cell density of 8 was transferred into 25 ml of BMXY (per litre: mono-potassium phosphate 8.7 g, YNB 13.4 g, biotin 0.4 mg, peptone 20 g, yeast extract 10 g, xylose 20 g; pH 6.0) at pH 6.0, 30 °C and 200 rpm. For the fed-batch fermentation set-up, after the 24 H into the processes 5 g/l of xylose or 3 ml of CCH in case of CCH fermentation was first introduced, followed by 12 H interval feeding of same carbon source. The cell biomass was determined at OD 600 nm while the residual xylose (RX) or glucose concentration as applicable were determined before subsequent feeding by HPLC using an Aminex HPX-87H ion-exchange column (7.8 × 300 mm) with 0.05 mmol/l sulfuric acid as mobile phase and 0.5 ml/minute flow rate.
Shake flask cultivation for expression of enzymes
The fermentation of strains harboring extracellular enzymes (GS30-lac, GS-lac, GS30-man and GS-man) on BMXY and CCH were carried out as previously described. They were pre-cultured in YPD medium until the cells reached stationary phase, after which an aliquot of 8 OD600 was transferred into 25 ml of CCH or BMXY. 3 ml of CCH or 5 g/l BMXY was added to respective culture setup in the shake flask until 84 H fermentation processes. Samples were taken every 12 H to determine the cell growth and enzyme activity.
β-Galactosidase activity
β-Galactosidase (lac) activity was determined by adding 100 μL reaction mixture containing 0.25 (w/v) oNPG in 0.1 mol/l sodium acetate buffer (pH 5.2) to 25 μL of fermentation supernatant and incubated at 60 °C for 10 min. 125 μL of 1 M Na2CO3 was added to terminate the reaction. The release of o-nitrophenol (oNP) was measured colorimetrically through the absorbance at 420 nm and one unit of β-Galactosidase was defined as the amount of enzyme that releases 1 μmol of oNP per minute.
β-Mannanase activity
β-Mannanase (man) activity was determined as described by Ma et al. 2004. One unit of β -mannanase activity was defined as the amount of enzyme that liberated 1 μmol of reducing sugars per minute with locust bean gum as substrate.
Extracellular protein expression on SDS-PAGE
The expressed extracellular protein was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Fermented samples were centrifuged at 10,000 g for 5 min and 20 μL of the supernatant was carefully aspirated and mixed with 2 × SDS loading buffer in a 1.5 ml tube and denatured at 160 °C for 5 min. 10 μL of the samples and the Protein ladder Marker were thereafter laid in the wells of SDS gel and subjected to 80 V to ease the protein flow through the interface and then 120 V upon subsequent flow into the lower gel to complete the electrophoresis until the total flow through into the SDS-running buffer. The gel was stained by a 30 min immersion in staining buffer and thereafter decolorized in a de-staining buffer.
Results
Effects of adaptive evolution on cell growth of GS115
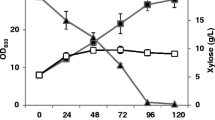
The Adaptive Laboratory Evolution was carried out on xylose based medium in order to strengthen the xylose utilization efficiency of GS115 wild-type (WT). Three different evolved strains were investigated in the study; GS30 (mutant of 30th generation), GS50 (mutant of 50th generation), and GS70 (mutant of 70th generation). Findings revealed that all the mutants had varying growth pattern on xylose-complex medium. Compared to the WT, GS70 exhibited a 5% growth decline while both GS50 and GS30 exhibited 25% and 13% improved growth under same condition (Fig. 1a). All the evolved strains also showed improved xylose utilization compared to control strain. However, not the final evolved strain (GS70), but the GS50 strain was optimal in terms of xylose utilization and biomass yield. After 72 H of fermentation only 1 g/l of residual xylose (RX) was detected from GS50 while approx. 5 g/l was observed for GS70 evolved strain (Fig. 1b). In addition, the xylose consumption between 48 to 72 H surprisingly did not lead to biomass accumulation, in fact a 11% decline in biomass was observed in fed batch fermentation (Fig. 1c).
Effect of Adaptive evolution on xylose utilization in BMXY complex media. a Biomass accumulation in batch cultivation. b Residual xylose concentration. c Biomass accumulation in complex based medium in fed-batch phase cultivation. GS30: 30th generation of evolved strain, GS50: 50th generation of evolved strain, GS70: 70th generation of evolved strain, GS115: wild-type. Three parallel flasks are tested for each strain at 30 °C. Error bars represent deviations (n = 3). Statistically significant differences (P\0.05) were determined by student’s t test
Shake flask growth on corncob hydrolysate
Next we analyzed the performance of evolved strains on corncob hydrolysate (CCH) since it contains carbon source other than xylose and owing to relative abundance of carbon sugars in the hydrolysate. We opined that mutant with ability to efficiently co-utilize the carbon sources in the hydrolysate can be selected for bioconversion into value added commodities hence, all the mutants were investigated on CCH. GS30 showed the highest biomass production on CCH (similar to the WT), whereas GS50 and GS70, showed 21% and 33% decline in biomass accumulation, respectively compared to the WT. Surprisingly, despite low concentration of carbon source present at 48 H of the fermentation processes, a continued cell growth was observed for GS50 until 72 H contrary to the stationary phase experienced by other mutants and the control strain (Fig. 2a). Investigation into the xylose utilization of the strain in CCH-based medium also followed the same trend as observed in BMXY complex-based medium with the WT exhibiting highest residual xylose concentration of 4.5 g/l at 72 H of the fermentation processes (Fig. 2b). Study into the residual glucose concentration also revealed different pattern as GS30 and WT completely utilized glucose in CCH at 48 H of the culturing processes while 2.3 g/l and 5.5 g/l were observed for GS50 and GS70, respectively under same condition (Fig. 2c).
Batch cultivation on corncob hydrolysate for ALE-derived and wild-type strain a Biomass accumulation in batch cultivation. b Residual xylose concentration. c Residual glucose concentration. GS30: 30th generation of evolved strain, GS50: 50th generation of evolved strain, GS70: 70th generation of evolved strain, GS115: wild-type. Three parallel flasks are tested for each strain at 30 °C. Error bars represent deviations (n = 3). Statistically significant differences (P\0.05) were determined by student’s t test
Fed-batch growth of strains on CCH
Next, strains were exposed to fed-batch fermentation on CCH. GS30 exhibited higher growth compared to other evolved strains and the WT. Interestingly, 16% higher yield was observed in GS30 at 72 H compared to the WT. While GS50, which accumulated higher cell density on BMXY medium showed 6% decline in biomass accumulation when compared to the WT on CCH. The lowest cell density of 35 was observed for GS70 at the end of the fermentation processes (Fig. 3).
Fed-batch cultivation on corncob hydrolysate for ALE-derived and wild-type strain, GS30: 30th generation of evolved strain, GS50: 50th generation of evolved strain, GS70: 70th generation of evolved strain, GS115: wild-type. Three parallel flasks are tested for each strain at 30 °C. Error bars represent deviations (n = 3). Statistically significant differences (P\0.05) were determined by student’s t test
Enzyme secretion
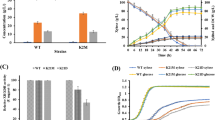
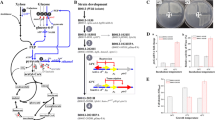
Since GS30 efficiently utilized both glucose and xylose compared to other mutants in CCH for cellular accumulation, the strain was harnessed for enzyme production. Resulting recombinant plasmid pPICHα-lac expressing β-galactosidase and pPICHα-man expressing β-mannanase (Fig. 5a) were transformed into GS115 and GS30, thus generating four P. pastoris strains GS-lac, GS-man, GS30-lac and GS30-man, respectively. Correct clones were confirmed by the PCR detection of 1.3 kb and 2.9 kb bands for β-mannanase and β-galactosidase, respectively (Fig. 5b and c). Analysis of these strains revealed that bioconversion of CCH into functional enzyme is possible although not as efficient as those obtained in synthetic media (Fig. 4). For instance, β-galactosidase production from GS30 reached 118 U/ml and 73 U/ml on BMXY synthetic media and CCH, respectively. However, about 50% and 42% improvement in enzyme secretion were observed in GS30 compared to the WT on BMXY-medium and CCH, respectively. Secretion of β-mannanase also followed same trend on BMXY-complex medium and CCH as about 35% and 53% increase in enzyme secretion level were respectively observed for GS30 compared to the WT. We further confirmed proper enzyme expression, as we detected for GS30 upon 72 H fermentation on CCH correct band sizes of 60 KDa and 130 KDa by SDS-PAGE for β-mannanase and β-galactosidase, respectively (Fig. 5d and e).
Enzyme secretion of ALE-derived and wild-type strain. a β-galactosidase secretion on BMXY complex medium. b β-galactosidase secretion on CCH hydrolysate. c β–mannanase secretion on BMXY complex medium. d β–mannanase secretion on CCH hydrolysate. GS115-lac: Wild type strain integrated with β-galactosidase gene, GS30-lac: 30th generation of evolved strain integrated with β-galactosidase gene, GS115-man: Wild type strain integrated with β-mannanase gene, GS30-man: 30th generation of evolved strain integrated with β-mannanase gene. Three parallel flasks are tested for each strain at 30 °C. Error bars represent deviations (n = 3). Statistically significant differences (P\0.05) were determined by student’s t test
a Cloning vector map for β-galactosidase and β-mannanase from Aspergillus oryzae and Bacillus subtilis. b Confirmation of correct clones for β-mannanase. c Confirmation of correct clones for β-galactosidase. d Protein expression of β-mannanase from GS30 on SDS-PAGE. e Protein expression of β-galactosidase from GS30 on SDS-PAGE
Discussion
Concerted efforts are being made to efficiently utilize lignocellulosic biomass (LB) in order to avert environmental concern associated with consumption of fossil fuels in bio-productions, with key interest in harnessing the enriched carbon sugars present in LB towards production of bio-based commodities (Bilal et al., 2017). Corncob is one of such LB that have been implicated to contain sugars, which microbial cell factories can convert into value added commodities, and hence, be of significant economy value. Efforts are being made towards utilizing all abundant sugars in this biomass (Arumugam and Anandakumar 2016), but the concern of inefficient utilization of the principal carbon source (xylose) by cell factories (Jefferies 2016) continue to plaque its fuller adoption. In this study we adopt ALE towards improving xylose utilization, and examined the efficiency of resultant strains for enzyme production on corncob hydrolysate (CCH). As expected, all the mutants displayed improvement in xylose utilization over the GS115 wild-type (WT) strain, although surprisingly the evolved strains GS50 and GS30 exhibited improved substrate utilization over a later generated GS70 evolved strain on BMXY complex media. In addition to this, GS30 exhibited higher biomass accumulation more evidently in fed-batch fermentation, which could be directly linked to higher assimilation of xylose. The sudden decline in the cell growth of GS70 between 48 and 72 H, despite uptake of xylose was unexpected and cannot be affirmatively explained; however, we equally shared from the view that the uptake of xylose might be directed to other cellular activities, which are not growth related. More importantly, this finding is a pointer that “efficiency of strain for any trait is not necessarily a function of prolonged evolution period”. Hence, strain selection upon evolution must be on case to case evaluation (Drlica, 2003).
The abundant glucose concentration of the hydrolysate also suggests that any strain with potential to efficiently co-utilize both glucose and xylose will be a good candidate for bio-based production. Hence, xylose domestication interference on glucose assimilation of evolved strains was then evaluated. The evolved strains took another dimension as the WT demonstrated its supremacy over the evolved strains in glucose utilization and biomass yield on glucose, which thus implies that the evolution for selection of a particular trait may invariably affect other functions and hence, “selection of mutants for a particular trait is specific” and that inclusion of other factors, which indirectly have influence on strain improvement whose effects are difficult to predict may be attributed (Bulter et al., 2003).
The progressive decline in glucose assimilation upon advancement in evolution further corroborates the earlier statement that “selection of particular trait may invariably affect other functions of the system” as xylose assimilation, which was core in evolution was even “timely” favoured. Therefore, for efficient co-utilization of hydrolysate sugars a “balanced” mutant with ability to optimally utilize the substrates is vital, and hence, should be mutant of choice, as the selection of any mutant, which optimally utilize the principal sugar component will only “timely” favour its utilization but affect overall efficiency.
The multi-carbon sources of CCH could also be responsible for the enhanced growth observed in GS30 as against the GS50 on BMXY complex medium, which thus indicates that evolution highly enhanced xylose assimilation in GS50 compared to GS30 but its overall efficiency in co-utilizing the multi-carbon sugars was less pronounced, compared to its GS30 counterpart. Bankefa et al. 2018b reported that utilization of multi-carbon sugars by P. pastoris improved enzyme production and any system with ability to utilize carbon source efficiently is expected to improve protein secretion (Cos et al., 2006). In this study, GS30 was considered a “balanced” mutant among other mutant strains evaluated, owing to its efficient utilization of CCH multi-carbon sugars, and hence, was considered as mutant of choice for harnessing bioconversion of lignocellulosic hydrolysate into enzyme secretion.
β-galactosidase and β-mannanase were used as model proteins for assessing the bioconversion ability of CCH. Upon cloning of the enzymes in both the WT and GS30, findings revealed that CCH can be harnessed for enzymes production as both GS30 and WT secreted certain levels of model enzymes, although the secretions were lower than those obtained in the synthetic medium contrary to the report by Castrillo et al. 2015. The reasons for this outcome may be attributed to pure chemical compositions of the complex media. The efficient utilization of xylose by GX30 improved the enzyme secretions in CCH compared to the WT, which is an indication that improved biomass accumulation is key towards optimizing enzyme secretion and also supports the claim that balanced flux of metabolic pathways aided the activity of host system. Therefore, the genomic and transcriptional study of the evolved strain is recommended to understand the regulation pattern of key genes for substrates utilization and enzyme secretion as guide to optimization processes. The study also revealed that strain optimization by ALE is a key tool to enhance strain traits and that when engineering a particular strain for specific carbon utilization in a multi-carbon source, there is a need for careful consideration of other carbon sources or traits in order to achieve a “balance strain” that will be efficient for the desired function. More importantly, we conclude that the abundant sugars in corncob hydrolysate can be harnessed for production of industrially useful enzymes by unlocking the bottleneck of substrate utilization in P. pastoris and that the system can further be engineered for the said function.
Data availability
The datasets supporting the conclusions of this article are included in the main manuscript.
References
Arumugam N, Anandakumar S (2016) Mini review on corncob biomass: a potential resource for value-added metabolites. Euro J Exp Bio 6:9–13
Bankefa OE, Wang M, Zhu T, Li Y (2018b) Enhancing the secretion pathway maximizes the effects of mixed feeding strategy for glucose oxidase production in the methylotrophic syeast Pichia pastoris. Bioresour Bioprocess 5:25. https://doi.org/10.1186/s40643-018-0211-y
Bankefa OE, Wang M, Zhu T, Li Y (2018a) Hac1p homologues from higher eukaryotes can improve the secretion of heterologous proteins in the yeast Pichia pastoris. Biotechnol Lett 40(7):1149–1156. https://doi.org/10.1007/s10529-018-2571-y
Bhatia SK, Kim JH, Kim MS, Kim J, Hong JW, Hong YG, Kim HJ, Jeon JM, Kim SH, Ahn J, Lee H, Yang YH (2018) Production of (3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymer from coffee waste oil using engineered Ralstonia eutropha. Bioprocess Biosyst Eng 41:229–235. https://doi.org/10.1007/s00440-017-1861-4
Bilal M, Asgher M, Iqbal HMN, Hu H, Zhang X (2017) Biotransformation of lignocellulosic materials into value-added products-A review. Int J Bio Macromol 98:447–458. https://doi.org/10.1016/j.ijbiomac.2017.01.133
Bulter T, Alcalde M, Sieber V, Meinhold P, Schlachtbauer C, Arnold FH (2003) Functional expression of a fungal laccase in Saccharomyces cerevisiae by directed evolution. Appl Environ Microbiol 69:987–995. https://doi.org/10.1128/AEM.69.2.987-995.2003
Castrilo M, Bich G, Kramer G, Velazquez M, Rodriguez J, Zapata P, Villalba I (2015) Evaluation of synthetic and semi-synthetic culture media for Endo-1,4- β-glucanases secretion by Trichoderma koningiopsis. Proced Mats Sci 8:786–792. https://doi.org/10.1016/j.mspro.2015.04.136
Chatterjee R, Yuan L (2006) Directed evolution of metabolic pathways. Trends Biotechnol 24:28–38. https://doi.org/10.1016/j.tibtech.2005.11.002
Chiang CJ, Lee HM, Guo HJ, Wang ZW, Lin LJ, Chao YP (2013) Systematic approach to engineer Escherichia coli pathways for co-utilization of a glucose–xylose mixture. J Agric Food Chem 61:7583–7590. https://doi.org/10.1021/jf401230r
Cos O, Ramon R, Montesinos JL, Valero F (2006) Operational strategies, monitoring and control of heterologous protein production in the methylotrophic yeast Pichia pastoris under different promoters. Microb Cell Fact 5(1):5–17. https://doi.org/10.1186/1475-2859-5-17
Dmytruk OV, Voronovsky AY, Abbas CA, Dmytruk KV, Ishchuk OP, Sibirny AA (2008) Overexpression of bacterial xylose isomerase and yeast host xylulokinase improves xylose alcoholic fermentation in the thermo-tolerant yeast Hansenula polymorpha. FEMS Yeast Res 8:165–173. https://doi.org/10.1111/j.1567-1364.2007.00289.x
Drlica K (2003) The mutant selection window and antimicrobial resistance. J Antimicrol Chemo 52:11–17. https://doi.org/10.1093/jac/dkg269
Du C, Li Y, Zong H, Yuan T, Yuan W, Jiang Y (2020) Production of bioethanol and xylitol from non-detoxified corn cob via-two stage fermentation strategy. Bioresour Technol 310:123427. https://doi.org/10.1016/j.biotech.2020.123427
Fong SS, Burgard AP, Herring CD, Knight EM, Blattner FR, Maranas CD, Palsson BO (2005) In silico design and adaptive evolution of Escherichia coli for production of lactic acid. Biotechnol Bioeng 9:20542. https://doi.org/10.1002/bit.20542
Ge X, Chang C, Zhang L, Cui S, Luo X, Hu S, Qin Y, Li Y (2018) Conversion of lignocellulosic biomass into platfform chemicals for biobased polyurethane application. Adv Bioener 3:161–213. https://doi.org/10.1016/bs.aibe.2018.03.002
Jeffries TW (2016) Engineering yeasts for xylose metabolism. Curr Opin Biotechnol 17:320–326. https://doi.org/10.1016/j.copbio.2006.05.008
Kim TH, Ngbiem NP, Hicks KB (2009) Pretreatment and fractionation of corn stover by soaking in ethanol and aqueous ammonia. Appl Biochem Biotechnol 153:1–3. https://doi.org/10.1007/s12010-009-8524-0
Li P, Sun H, Chen Z, Li Y, Zhu T (2015) Construction of efficient xylose utilizing Pichia pastoris for industrial enzyme production. Microb Cell Fact 14:0206–0208. https://doi.org/10.1186/s12934-015-0206-8
Ma Y, Xue Y, Dou Y, Xu Z, Tao W, Zhou P (2004) Characterization and gene cloning of a novel mannanase from alkaliphilic Bacillus sp. N16–5. Extremoph 8:447–454. https://doi.org/10.1007/s00792-004-0405-4
Macauley-Patrick S, Fazenda ML, McNeil B, Harvey LM (2005) Heterologous protein production using the Pichia pastoris expression system. Yeast 22:249–270. https://doi.org/10.1002/yea.1208
Qian EW (2013) Pretreatment and Saccharifiction of lignocellulosic biomass. Res Appro Sust Biom Sys 2013:181–204. https://doi.org/10.1016/B978-0-12-404609-2.00007-6
Razaghi A, Tan E, Lua LHL, Owens L, Karthikeyan OP (2016) Is Pichia pastoris a realistic platform for industrial production of recombinant human interferon gamma? Biologicals 2016:1–9. https://doi.org/10.1016/j.biologicals.2016.09.015
Santos VT, Esteves PJ, Milagres AM, Carvalho W (2011) Characterization of commercial cellulases and their use in the saccharification of a sugarcane bagasse sample pretreated with dilute sulfuric acid. J Ind Microbiol Biotechnol 38:1089–1098. https://doi.org/10.1007/s10295-010-0888-1
Van Vleet JH, Jeffries TW (2009) Yeast metabolic engineering for hemicellulosic ethanol production. Curr Opin Biotechnol 20:300–306. https://doi.org/10.1016/j.copbio.2009.06.001
Viera Gomes AM, Carmo TS, Carvalho LS, Bahia FM, Parachin NS (2018) Comparison of Yeasts as Host for Recombinant Protein Production. Microorganisms 6:38. https://doi.org/10.3390/microorganisms6020038
Wang X, Salvachua D, Nogue VS, Michener WE, Bratis AD, Dorgan JR, Beckham GT (2017) Propionic acid production from corn stover hydrolysate by Propionibacterium acidipropionici. Biotechnol Biofuels 10(1):1–13. https://doi.org/10.1186/s13068-017-0884-z
Wisselink HW, Toirkens MJ, Wu Q, Pronk JT, van Maris AJA (2009) Novel evolutionary engineering approach for accelerated utilization of glucose, xylose, and arabinose mixtures by engineered saccharomyces cerevisiae strains. Appl Environ Microbiol 74(4):907–914. https://doi.org/10.1128/AEM.02268-08
Acknowledgements
The authors thank Nico J Claassens, Laboratory of Microbiology, Wageningen University, The Netherlands, for his feedback and critical reading of this manuscript. We also acknowledge the support of Biosolution Technnologies, Akure, Nigeria.
Funding
This work was supported by Tertiary Education Trust Fund (TETFUND) Research Project Intervention for the Federal University Oye-Ekiti. The sponsor has no influence in submitting the manuscript to Journal of Food Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Consent for publication
All the authors approved the consent for publishing the manuscript in Journal of Food Science and Technology and if accepted for publication, it will not be published elsewhere in the same form or in any other language.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bankefa, O.E., Samuel-Osamoka, F.C. & Oladeji, S.J. Improved enzyme production on corncob hydrolysate by a xylose-evolved Pichia pastoris cell factory. J Food Sci Technol 59, 1280–1287 (2022). https://doi.org/10.1007/s13197-021-05135-z
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-021-05135-z