Abstract
Bacillus cereus is a human pathogenic bacterium that produces emetic and diarrheal foodborne diseases. This study evaluated the genetic and toxigenic diversity in B. cereus group isolates from powdered foods collected in public educational institutions, bakeries and powdered food companies located in Medellín, Colombia. B. cereus was detected in 35 of 305 (11%) powdered food samples and 52 B. cereus were isolated. The presence of ten toxin genes, hblCDAB, nheABC, cytK2, entFM and cesB, was evaluated in the isolates by multiplex PCR. The nheABC operon was found in all isolates (100%), hblCDAB in 22 (42%), hblCDA in 8 (15%) and hblCD in 3 (6%); the cytK2 gene was detected in 32 isolates (62%) and entFM in 32 (62%). Notably, the cesB gene was not detected. According to the presence of toxin genes, fifteen profiles were identified. The predominant toxigenic profile contained all toxin genes but cesB. A large genetic diversity was observed by GTG5 fingerprinting with 46 isolates grouped in seven clusters and the remaining six clustering individually. There was no relationship between toxigenic profiles and genetic clusters, but some genetic clusters seemed to be related to particular powdered food types. In general, the results evidenced high genetic and enterotoxigenic diversity among the B. cereus group isolates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Bacillus cereus group or Bacillus cereus sensu lato (s.l.) comprises nine species characterized by a high degree of genetic similarity, B. cereus sensu stricto (s.s.), Bacillus thuringiensis, Bacillus mycoides, Bacillus pseudomycoides, Bacillus anthracis, Bacillus weihenstephanensis, Bacillus cytotoxicus and Bacillus toyonensis (Jimenez et al. 2013), and the recently identified B. wiedmannii (Liu et al. 2017). These Gram-positive, spore forming, human pathogens are naturally found in soil. Therefore, soil contaminated food products carrying these bacteria constitute risk factors for foodborne infection or intoxication, mostly because their spores can survive cooking or pasteurization processes (De Jonghe et al. 2008).
Bacillus cereus causes two types of foodborne diseases, an emetic syndrome and a diarrheal syndrome (Stenfors-Arnesen et al. 2008). The emetic syndrome is caused by emetic toxin, cereulide, produced by a nonribosomal peptide synthetase encoded by the polycistronically transcribed cereulide synthetase (ces) gene cluster, located on a megaplasmid (Rajkovic et al. 2008). The diarrheal syndrome is caused by one or a combination of three enterotoxin types, hemolysin BL (HBL) encoded by the hblCDA or hblCDAB operons, non-hemolytic enterotoxin (NHE) encoded by the nheABC operon (Granum et al. 1999), and the cytotoxin K encoded by the cytK gene, a single protein similar to the β-toxin of Clostridium perfringens (Logan 2012). Two variants have been described to cytK gene, cytK1 gene encoding the more toxic variant of the CytK toxin while cytK2 gene, is the most common variant and least toxic (Stenfors-Arnesen et al. 2008). HBL and NHE toxins are both tripartite proteins. HBL contains two lytic components L2 and L1, and a binding component B, encoded by hblC, hblD and hblA, respectively (Ryan et al. 1997). In addition, an open reading frame, hblB, according to sequence analysis, was generated by duplication of hblA gene and is considered a pseudogene (Stenfors-Arnesen et al. 2008). NHE also contains a cytolytic protein NheA and two binding components NheB and NheC, encoded by nheA, nheB and nheC, respectively (Fagerlund et al. 2008). In addition, most B. cereus strains are potential producers of enterotoxin FM (EntFM), codified by the entFM gene (Stenfors-Arnesen et al. 2008).
Studies in distinct geographic locations showed high incidences of enterotoxin genes in B. cereus s.l. strains isolated from foods, as marketed food products, rice and spices, respectively (Fogele et al. 2018; Kim et al. 2014; Samapundo et al. 2011). A few studies have been performed in Colombia to identify toxin genes of B. cereus s.l. in DNA extracted directly from powdered foods, and the results showed that the enterotoxin genes were by far, more frequent than the emetic gene (Sánchez et al. 2014a, b). Knowledge about the genetic and toxigenic diversity of the B. cereus s.l. isolates contributes important epidemiological information, such as the differentiation of their clinical potential, finding of contamination sources, tracking isolates along the food chain and define strains distribution within foods (De Jonghe et al. 2008). Furthermore, B. cereus toxin genes have been detected in Colombia where it causes one third of the total foodborne illnesses (INS 2011). Therefore, the aim of this study was to evaluate the genetic and toxigenic diversity of B. cereus s.l. isolated from infant formula, flours and powdered milk in Medellín, Colombia.
Materials and methods
Food samples and B. cereus s.l. isolation
The samples evaluated were powdered food products collected in six public educational institutions, seven bakeries and two powdered food companies located in Medellín, Colombia (designated as A to O). They included, infant formula (n = 75), powdered milk (n = 75), wheat flour (n = 79) and corn starch (n = 76). B. cereus isolation from powdered foods was performed according to ISO 7932:2004, for the enumeration of presumptive B. cereus cells (ISO 2004). Briefly, 25 g of sample were dissolved on 225 ml of peptone water and serial dilutions were performed (10−1–10−3); a 100 μl sample from each dilution was spread on mannitol egg yolk polymyxin agar (MYP) (Merck Millipore, Darmstadt, Germany) using a sterile triangular cell spreader, and performed in triplicates in order to reduce experimental error, followed by incubation at 37 °C for at least 48 h. The colonies obtained were counted in plates that showed growth of 30–300 CFUs. Presumptive colonies were analyzed using biochemical tests, according to standard protocols that included catalase, motility, Voges-Proskauer reaction, glucose, xylose and arabinose utilization; starch, casein and gelatin hydrolysis; β-hemolytic activity on blood agar plates. Positive colonies to catalase, motility, Voges-Proskauer reaction, glucose utilization, β-hemolytic activity and, starch, casein and gelatin hydrolysis were considered B. cereus s.l. The isolates were preserved in cryotubes containing Tryptic Soy Broth and 15% (v/v) glycerol, and stored at − 80 °C. Emetic B. cereus NVH 1257 and F4810/72 and enterotoxigenic B. cereus ATCC 14579 and ATCC 10987 served as reference strains for phenotypic and molecular assays, and were kindly provided by Dr. Niels Hendriksen and Dr. Per E. Granum. All strains were kept as glycerol stocks at − 80 °C until required.
DNA extraction and toxin gene determination among B. cereus s.l. isolates
Bacteria strains were cultured in Luria–Bertani (LB) medium (Difco, Detroit, Mich., USA) at 37 °C overnight for DNA extraction (D’Alessandro et al. 2007). DNA concentration and purity were checked in a NanoDrop 2000 UV/VIS spectrophotometer (Thermo Fisher Scientific, Massachusetts, USA). The DNA was stored at − 20 °C for the following experiments. The B. cereus isolated from powdered foods were screened for the presence of the genes that encode the toxins cereulide (cesB), non-hemolytic enterotoxin (nheA, nheB, and nheC), hemolytic BL (hblC, hblD, hblA, and hblB), enterotoxin FM (entFM), and cytotoxin K (cytK2) by three multiplex PCR assays according to previously described methodology (Sánchez et al. 2020).
Bacillus cereus s.l. (GTG)5 fingerprinting
The B. cereus group isolates from powdered foods were analyzed to establish their genetic diversity according to amplification of repetitive element palindromic based-polymerase chain reaction (rep-PCR) using the single (GTG)5 primer (De Jonghe et al. 2008). Briefly, a total reaction volume of 20 μl containing 0.6 μM (GTG)5 primer, 200 μM dNTPs mix, 3.5 mM MgCl2, 1.6 U of Taq DNA polymerase (Thermo Fisher Scientific) and 50 ng of DNA template. PCR amplifications were performed under the following conditions, an initial denaturation of 5 min at 94 °C, followed by 30 cycles of denaturation at 94 °C for 1 min, annealing at 45 °C for 1 min and elongation at 65 °C for 8 min, and a final extension at 65 °C for 16 min. Amplification was carried out in a DNA thermocycler MyCycler (Bio-Rad, California, USA). PCR products were electrophoresed in a 1.5% (w/v) agarose gel (Amresco, Ohio, USA) with ethidium bromide for 2.5 h at a constant voltage of 120 V in 1X TBE buffer (0.1 M Tris base, 0.09 M boric acid, 0.01 M EDTA, pH 8.4), at 4 °C. The gene ruler 1 Kb DNA ladder (Thermo Fisher Scientific) was used as a size standard. The rep-PCR profiles were visualized under ultraviolet light, followed by digital capture with UVP Gel Doc system (UVP, California, USA). The resulting fingerprints were analyzed using the BioNumerics 6.6 software package (Applied Maths Inc. St Martems, Belgium), with 1% optimization and 1% position tolerance. Genetic similarity among digitized profiles was calculated using the Pearson correlation coefficient and the Unweighted Pair Group Method with Arithmetic average (UPGMA) was used to obtain a dendrogram of the profiles, using a 94% similarity value for cluster analysis.
Results and discussion
Incidence of B. cereus s.l. in powdered foods
The B. cereus count was below 103 cfu/g or ml in all powdered food samples, therefore, these foods are considered safe for human consume. B. cereus were detected in 35 of 305 (11%) powdered food samples and 52 B. cereus group isolates were recovered from these samples. The B. cereus incidence in powdered foods was similar, in powdered milk was 13%, followed by corn starch (12%), infant formula (11%) and wheat flour (10%). Although, infant formula only presented an 11% incidence, the highest number of B. cereus isolates (19) was recovered from this powdered food (Table 1).
The incidence of B. cereus s.l. in food products greatly vary; compared to those reports, the B. cereus incidences in powdered foods found in this study, are lower. For example, higher incidence was found in a Korean study that reported incidence of 78.3% B. cereus in rice samples (Kim et al. 2014). Variable B. cereus s.l. incidences were reported in a study carried out in food products marketed in Belgium (5% to 100%), such as rice, sauces, lasagna and cooked pasta (Samapundo et al. 2011). In Scotland, B. cereus s.l. were reported varying within 20–88% in soil, feces, raw and processed vegetables (Altayar and Sutherland 2006). In studies accomplished in Korea, Iran and New Zealand, B. cereus was identified in 40% of dried foods as raw rice (Jang et al. 2006); infant foods (Rahimi et al. 2013) and dehydrated potato (King et al. 2007), respectively. The results of the above studies are higher to the ones of the present work in which a low B. cereus incidence was found in powdered milk, corn starch, infant formula and wheat flour samples. A possible reason for this low incidence of B. cereus s.l. in these powdered foods is the low humidity present in these samples that prevents microbial growth and only sporulating bacteria can survive in these food types.
Distribution of toxin gene profiles among B. cereus s.l. isolates
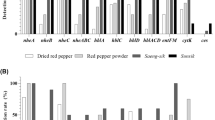
A total of 52 B. cereus s.l. were isolated from powdered foods. The distribution of toxin genes was as follows, the three genes of the nhe operon were detected in the 52 B. cereus s.l. (100%). All four genes of the hbl operon were found in 22 isolates (42%). The hbl operon missing the hblB gene in eight isolates (15%); notably, three isolates lacked the hblAB genes, indicating that these strains would not produce a functional hemolysin BL. The cytK2 and entFM genes were found in 32 isolates (62%) (Fig. 1). None of the 52 B. cereus group isolates contained the cesB gene.
These results showed that the enterotoxin genes were widely distributed among the B. cereus s.l. isolated from powdered foods. The nhe operon was highly conserved, with 100% of B. cereus harboring all three genes (nheABC) that encode the non-hemolytic enterotoxin. In general, studies have reported that B. cereus strains isolates from foods and soil carried all three genes of the nhe operon (Chaves et al. 2011; Kim et al. 2014; Ngamwongsatit et al. 2008). All this information and evidence from bioinformatic analysis suggest that the nhe operon is highly conserved, because of the vertical inheritance, which is possibly caused by a second, unknown but fitness relevant function of nhe operon (Böhm et al. 2015).
Regarding the B. cereus s.l. isolates that carried the hblCDAB operon, a wide genetic diversity was observed. The complete operon (hblCDA) was detected in 30 B. cereus isolates (57%), from which 22 isolates (42%) harbored the hblB pseudogene. Three B. cereus s.l. isolates (6%) carried only the hblCD genes. Similar hbl operon frequencies have been previously reported, describing that hblCDA operon is generally present in 45–65% of B. cereus strains (Thaenthanee et al. 2005). Studies carried out in Denmark and Brazil reported that 50–70% of B. cereus isolates harbored hblCDA operon (Chaves et al. 2011; Hansen and Hendriksen 2001). Higher frequencies (84–100%) of B. cereus strains carrying the hbl genes were reported in studies accomplished in Belgium (Samapundo et al. 2011) and Korea (Kim et al. 2014). Additionally, it has been found that 43% of B. cereus environmental/food strains carried hbl operon, as isolated strains in this study, while that a higher proportion of clinical strains (81%) harbored the hbl genes (Ghelardi et al. 2007). Previous studies have reported the lack of one or two components of the hbl operon (Kim et al. 2009; Wehrle et al. 2009). Similarly, in some isolates of the present study, the hblAB genes were absent; this polymorphisms of the hbl operon is suggestive of an inactive hemolysin BL in these isolates. Polymorphism of enterotoxin genes has been observed in other food-related B. cereus isolates (Chaves et al. 2011; Guinebretière et al. 2002). Further studies will be necessary to evaluate which factor(s) is/are responsible for the observed lack of hbl operon components.
The cytK2 gene was found in 62% of B. cereus s.l. isolates. These results and those of other studies reporting frequencies varying within 45.4%—88.81% (Chaves et al. 2011; Guinebretière et al. 2002; Ngamwongsatit et al. 2008; Samapundo et al. 2011) indicate that cytK2 gene is widely spread among the B. cereus s.l. strains. Similar to cytK2 gene, entFM gene was found in 32 isolates (62%), and results obtained previously in other studies suggest that entFM gene frequency and distribution among B. cereus s.l. strains varies greatly (45 to 100%) (Carter et al. 2018; Kim et al. 2009; Osman et al. 2018; Rahimi et al. 2013).
On the other side, none of the 52 B. cereus s.l. isolates carried the cesB gene required for the production of emetic toxin cereulide. B. cereus emetic strains are not frequently found (Altayar and Sutherland 2006), as shown in other studies in which the ces gene is not detected; for example in B. cereus strains from Brazil (Chaves et al. 2011). However, in Argentina a highly cytotoxic B. cereus strain isolated from cooked chicken harbored the ces gene (López et al. 2015), and in Korea was also reported B. cereus emetic strain isolated from rice (Kim et al. 2014). Notably, emetic strains have been isolated mainly from rice dishes, rice paddy fields and its processing environment in Asian countries, which suggests an association between B. cereus emetic strains and rice (Logan 2012).
According to the presence or absence of the toxin genes, hblCDAB, nheABC, cytK2, entFM and cesB, in 52 B. cereus s.l. isolates from powdered foods, fifteen different toxin gene profiles were identified (Table 2). Regarding the toxin gene profiles, 22% of the isolates (11 strains) had the profile with all genes for hemolysin BL, non-hemolytic enterotoxin, cytotoxin K and enterotoxin FM, and 18% of them had the hblB pseudogen (profile I), while in the rest, 4% (2 B. cereus) it was absent (profile II). This result is agreement to results obtained in other studies, which toxin gene profiles that included all enterotoxin genes were the most frequent profiles (Chaves et al. 2011; Kim et al. 2009; Ngamwongsatit et al. 2008; Samapundo et al. 2011). These results mean that only diarrheal syndromes can be caused by B. cereus s.l. isolated here, but more studies are needed to know if these strains can generate all toxins simultaneously to cause food poisoning.
(GTG)5 fingerprinting
Evaluation of the genetic diversity of B. cereus s.l. isolated from powdered foods by (GTG)5 genotyping showed that 46 isolates grouped into seven clusters and six clustered individually (Fig. 2). Interestingly, a relationship was found between particular clusters and specific powdered foods; for example, cluster 1 only contained isolates from infant formula, cluster 3 from corn starch, cluster 5 from wheat flour and cluster 7 from powdered milk. Regarding toxigenic profiles and genetic clusters, there were predominant toxigenic profiles in some clusters as clusters 2 (profile X) and 3 (profile I); however, the various toxigenic profiles were distributed among the 52 B. cereus group isolates. This observation indicates that the toxigenic profile is not related to the genetic background, as previously suggested (Chaves et al. 2011; Lee et al. 2012).
No association was found between cluster and place of powdered food collection, but there was a relationship between cluster and powdered food type. Notably, cluster 3 was formed by eight B. cereus isolates from corn starch exclusively, however five isolates carried all enterotoxin genes (profile I), and were isolated from a special corn starch used to cook Colombian buñuelos, which could indicate a contamination by a particular strain in this type of corn starch. Additionally, in cluster 2 the majority of B. cereus s.l. harbored the toxigenic profile X and were isolated from infant formula in different institutions, this may indicate a systematic contamination due to this particular powdered food is manufactured by the same factory.
In other studies, using (GTG)5 PCR fingerprinting, a relationship was observed between clusters and sample source. In South Africa, from 49 B. cereus strains obtained from extended shelf life, raw and pasteurized milk and filler nozzles, three groups comprised isolates exclusively from filler nozzles while other three groups were isolates from filler nozzles, raw and pasteurized milk (Mudagza and Buys 2017). In other study in Belgium, 61 of 80 B. cereus group isolated from food products marketed formed 15 distinct clusters and the remaining 19 were each clustered separately, the cluster 2 harbored mainly B. cereus group isolates from lasagna and bolognaise sauce, and cluster 6, 7 and 11, isolates from raw basmati rice, lasagna and béchamel sauce, respectively (Samapundo et al. 2011).
In general, the (GTG)5 fingerprint results revealed a high diversity among the 52 B. cereus group isolates obtained from infant formula, powdered milk, wheat flour and corn starch. The large genetic diversity obtained is similar to the high genetic diversity detected in Brazil among 97 foodborne B. cereus s.s. strains collected during three decades, which were grouped in 15 clusters (Chaves et al. 2011). Although a high genetic diversity was detected in B. cereus s.l. isolates from powdered foods, it was achieved to identify some contamination sources related to food type or to the producing food factory. This information contributes to the assessment of food safety/risk to aid decision-making in public health in Colombia.
Conclusion
The results indicate that B. cereus s.l. isolates obtained from powdered foods show a potential risk to cause diarrheal-type food poisoning as most of them contained all enterotoxin genes. According to B. cereus s.l. genetic diversity, no correlation was found between the genetic background and toxigenic profiles. However, the results suggest an association between B. cereus s.l. genetic background and food type, indicating that (GTG)5 fingerprinting is useful to identify contamination source in foods. Data on the incidence, toxigenic profiles and genetic diversity for B. cereus s.l. isolated from powdered foods constitute useful information that provide the bases for the assessment of the potential risk of this pathogen to cause food poisoning; it also contributes to a better understanding of the epidemiology of B. cereus s.l. in powdered food products.
References
Altayar M, Sutherland AD (2006) Bacillus cereus is common in the environment but emetic toxin producing isolates are rare. J Appl Microbiol 100:7–14
Böhm M, Huptas C, Krey V, Scherer S (2015) Massive horizontal gene transfer, strictly vertical inheritance and ancient duplications differentially shape the evolution of Bacillus cereus enterotoxin operons hbl, cytK and nhe. BMC Evol Biol 15:246. https://doi.org/10.1186/s12862-015-0529-4
Carter L, Chasea H, Giesekerb C, Hasbrouckb N, Stineb C, Khan A, Ewing-Peeples L, Tall B, Gopinath G (2018) Analysis of enterotoxigenic Bacillus cereus strains from dried foods using whole genome sequencing, multi-locus sequence analysis and toxin gene prevalence and distribution using endpoint PCR analysis. Int J Food Microbiol 284:31–39
Chaves JQ, Pires ES, Vivoni AM (2011) Genetic diversity, antimicrobial resistance and toxigenic profiles of Bacillus cereus isolated from food in Brazil over three decades. Int J Food Microbiol 147:12–16
D’Alessandro B, Antúnez K, Piccini C, Zunino P (2007) DNA extraction and PCR detection of Paenibacillus larvae spores from naturally contaminated honey and bees using spore-decoating and freeze-thawing techniques. World J Microbiol Biotechnol 23:593–597
De Jonghe V, Coorevits A, Vandroemme J, Heyrman J, Herman L, De Vos P, Heyndrickx M (2008) Intraspecific genotypic diversity of Bacillus species from raw milk. Int Dairy J 18:496–505
Fagerlund A, Lindbäck T, Storset AK, Granum PE, Hardy SP (2008) Bacillus cereus Nhe is a pore-forming toxin with structural and functional properties similar to the ClyA (HlyE, SheA) family of haemolysins, able to induce osmotic lysis in epithelia. Microbiology 154:693–704
Fogele B, Granta R, Valcin O, Berzins A (2018) Occurrence and diversity of Bacillus cereus and moulds in spices and herbs. Food Control 83:69–74
Ghelardi E, Celandroni F, Salvetii S, Ceragioli M, Beecher DJ, Senesi S, Wong A (2007) Swarming behavior of and hemolysin BL secretion by Bacillus cereus. Appl Environ Microbiol 73:4089–4093
Granum PE, O’Sullivan K, Lund T (1999) The sequence of the non-haemolytic enterotoxin operon from Bacillus cereus. FEMS Microbiol Lett 177:225–229
Guinebretière MH, Broussolle V, Nguyen-The C (2002) Enterotoxigenic profiles of food-poisoning and food-borne Bacillus cereus strains. J Clin Microbiol 40:3053–3056
Hansen BM, Hendriksen NB (2001) Detection of enterotoxic Bacillus cereus and Bacillus thuringiensis strains by PCR analysis. Appl Environ Microbiol 67:185–189
INS (2011) Perfil de riesgo Bacillus cereus en alimentos listos para el consumo no industrializados. Unidad de evaluación de riesgos para la inocuidad de los alimentos, Ministerio de la Protección Social. Instituto Nacional de Salud, Colombia
ISO (2004) ISO 7932:2004. Microbiology of food and animal feeding stuffs—Horizontal method for the enumeration of presumptive Bacillus cereus—Colony-count technique at 30 degrees C. International Organization for Standardization
Jang JH, Lee NA, Woo GJ, Park JH (2006) Prevalence of Bacillus cereus group in rice and distribution of enterotoxin genes. Food Sci Biotechnol 15:232–237
Jimenez G, Urdiain M, Cifuentes A, Lopez-Lopez AR, Blanch A, Tamames J, Kämpfer P, Kolstø AB, Ramón D, Martínez JF, Codoñer FM, Rosselló-Móra R (2013) Description of Bacillus toyonensis sp. Nov., a novel species of Bacillus cereus group, and pairwise genome comparisons of the species of the group by means of ANI calculations. Syst Appl Microbiol 36:383–391
Kim SK, Kim KP, Jang SS, Shin EM, Kim MJ, Oh S, Ryu S (2009) Prevalence and toxigenic profiles of Bacillus cereus isolated from dried red peppers, rice, and sunsik in Korea. J Food Prot 72:578–582
Kim B, Bang J, Kim H, Kim Y, Kim BS, Beuchat LR, Ryu J (2014) Bacillus cereus and Bacillus thuringiensis spores in Korean rice: prevalence and toxin production as affected by production area and degree of milling. Food Microbiol 42:89–94
King NJ, Whyte R, Hudson JA (2007) Presence and significance of Bacillus cereus in dehydrated potato products. J Food Prot 70:514–520
Lee N, Sun JM, Kwon KY, Kim HJ, Koo M, Chun HS (2012) Genetic diversity, antimicrobial resistance, and toxigenic profiles of Bacillus cereus strains isolated from Sunsik. J Food Prot 75:225–230
Liu Y, Du J, Lai Q, Zeng R, Ye D, Xu J, Shao J (2017) Proposal of nine novel species of the Bacillus cereus group. Int J Syst Evol Microbiol 67:2499–2508
Logan NA (2012) Bacillus and relatives in foodborne illness. J Appl Microbiol 112:417–429
López AC, Minnaard J, Pérez PF, Alippi AM (2015) A case of intoxication due to a highly cytotoxic Bacillus cereus strain isolated from cooked chicken. Food Microbiol 46:195–199
Mudagza DT, Buys EM (2017) Diversity of Bacillus cereus strains in extended shelf life. Int Dairy J 73:144–150
Ngamwongsatit P, Buasri W, Pianariyanon P, Pulsrikarn C, Ohba M, Assavanig A, Panbangred W (2008) Broad distribution of enterotoxin genes (hblCDA, nheABC, cytK, and entFM) among Bacillus thuringiensis and Bacillus cereus as shown by novel primers. Int J Food Microbiol 121:352–356
Osman K, Kappell A, Orabi A, Al-Maary K, Mubarak A, Dawoud T, Hemeg H, Moussa I, Hessain A, Yousef H, Hristova K (2018) Poultry and beef meat as potential seedbeds for antimicrobial resistant enterotoxigenic Bacillus species: a materializing epidemiological and potential severe health hazard. Sci Rep 8:11600. https://doi.org/10.1038/s41598-018-29932-3
Rahimi E, Abdos F, Momtaz H, Baghbadorani ZT, Jalali M (2013) Bacillus cereus in infant foods: prevalence study and distribution of enterotoxigenic virulence factors in Isfahan. Sci World J 2013:1–5
Rajkovic A, Uyttendaele M, Vermeulen A, Andjelkovic M, Fitz-James I, In’t Veld P, Denon Q, Vérhe R, Debevere J (2008) Heat resistance of Bacillus cereus emetic toxin, cereulide. Lett Appl Microbiol 46:536–541
Ryan PA, Macmillan JD, Zilinskas BA (1997) Molecular cloning and characterization of the genes encoding the L1 and L2 component of hemolysin BL from Bacillus cereus. J Bacteriol 179:2551–2556
Samapundo S, Heyndrickx M, Xhaferi R, Devlieghere F (2011) Incidence, diversity and toxin gene characteristics of Bacillus cereus group strains isolated from food products marketed in Belgium. Int J Food Microbiol 150(1):34–41
Sánchez JA, Correa MM, Aceves-Diez AE, Castañeda-Sandoval LM (2014a) Direct detection of toxigenic Bacillus cereus in dietary complement for children and cassava starch. Rev Col Quim 43(2):5–9
Sánchez J, Correa M, Aceves-Diez AE, Castañeda-Sandoval LM (2014b) Detección directa de genes toxigénicos de Bacillus cereus en fécula de maíz y harina de trigo mediante reacción en cadena de la polimerasa múltiple (mPCR). Medicina y Laboratorio 20(9–10):441–451
Sánchez J, Correa M, Aceves-Diez AE, Rasschaert G, Heyndrickx M, Castañeda-Sandoval LM (2020) Genomic and toxigenic heterogeneity of Bacillus cereus sensu lato isolated from ready-to-eat foods and powdered milk in day care centers in Colombia. Foodborne Pathog Dis 17(5):340–347. https://doi.org/10.1089/fpd.2019.2709
Stenfors-Arnesen LP, Fagerlund A, Granum PE (2008) From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev 32:579–606
Thaenthanee S, Wong AC, Panbangred W (2005) Phenotypic and genotypic comparisons reveal a broad distribution and heterogeneity of hemolysin BL genes among Bacillus cereus isolates. Int J Food Microbiol 105:203–212
Wehrle E, Moravek M, Dietrich R, Burk C, Didier A, Martlbauer E (2009) Comparison of multiplex PCR, enzyme immunoassay and cell culture methods for the detection of enterotoxinogenic Bacillus cereus. J Microbiol Methods 78:265–270
Acknowledgements
This research was funded by the Department of Science, Technology and Innovation -Colciencias- (Grant No. 386) and Committee for Research Development -CODI- at the University of Antioquia, Colombia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sánchez-Chica, J., Correa, M.M., Aceves-Diez, A.E. et al. Genetic and toxigenic diversity of Bacillus cereus group isolated from powdered foods. J Food Sci Technol 58, 1892–1899 (2021). https://doi.org/10.1007/s13197-020-04700-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04700-2