Abstract
Dissipation behavior and degradation kinetics of fenamidone + mancozeb (Sectin 60 WG) and iprovalicarb + propineb (Melody Duo 66.75 WP) in tomato were studied at recommended dose (RD) and double dose (DD) of application. The analysis of the field samples were carried out by employing liquid chromatography tandem mass spectrometry (LC–MS/MS) for fenamidone and iprovalicarb residues and gas chromatography mass spectrometry for mancozeb and propineb residues after thorough validation of the extraction methods. The dissipation of residues best followed 1st + 1st order for all the test fungicides. The half-life period for fenamidone, mancozeb, iprovalicarb and propineb were 2, 2, 1.5 and 2 days for RD and 3, 2.5, 2 and 3 days for DD, respectively. The pre-harvest intervals were not applicable for iprovalicarb, fenamidone and mancozeb (at RD) as the residues at 0 day were below maximum residue limit set by European Union, and it was 1 day for DD of iprovalicarb, 3.5 days for DD of fenamidone, 3 days for DD of mancozeb, 3 and 7 days for propineb at RD and DD, respectively. A PHI of 4 and 7 days are proposed for fenamidone + Mancozeb and iprovalicarb + propineb, respectively. Dietary exposure calculated for all the pesticides were safe on all the sampling days except for propineb residues for which it was safe after first day of the double dose application. The study will be useful for promoting effective residue management and safe use of these chemicals for controlling fungal diseases in tomato crop.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tomato (Solanum lycopersicum L.) is a widely grown vegetable crop throughout the world and constitutes an important part of our diet. It is consumed either raw or cooked in the form of salads, purees, sauces, and drinks (Gupta et al. 2011). In India, the area under cultivation for tomato crop is 0.789 million ha with an average annual production of 19.759 million tones (Horticulture Statistics at a Glance 2018). Fungal diseases like early blight (Alternaria solani) and late blight (Phytophthora infestans) are adversely affecting the quality and yield of tomatoes all over the world. There are several fungicides which are being commercially available in the market as either singular or combination formulations for the control of fungal diseases.
Iprovalicarb [N-[(1S)-2-Methyl-1-[[[1-(4-ethylphenyl) ethyl] amino] carbonyl] propyl] carbamic acid 1-methylethyl ester], is a systemic fungicide having high biological activity against downy mildew (Horvat 2001). It is used for controlling the oomycetes class of pathogenic fungi in grapes, potatoes, tomatoes and other crops. Propineb ([[(1-methyl-1, 2-ethanediyl) bis [carbamodithioato]](2-)]-zinc homopolymer), is a basic foliar dithiocarbamate fungicide used especially on oomycetes, ascomycetes, basidiomycetes and fungi imperfecti (Sharma et al. 1994; Tomlin 1994). Melody duo® is a systemic preventive water dispersible granule fungicide formulation of iprovalicarb and propineb used to control late blight (Phytophthora infestans) on potatoes and tomatoes (http://www.bayercropscience.co.za/products/Product.aspx?p_id=909). Mancozeb ([[1,2-ethanediylbis-[carbamodithioato]](2-)] manganese, mixture with [[1,2-ethanediylbis- [carbamodithioato]]-(2-)]zinc), is a broad spectrum dithiocarbamate fungicide for controlling a number of fungal diseases, such as anthracnose, leaf spot, and downy mildew (Chaudhury et al. 2009) on various crops. Sectin® 60WG is a combination formulation in which mancozeb is combined with fenamidone. Fenamidone [(5S)-3-Anilino-5-methyl-2-(methylsulfanyl)-5-phenyl-3, 5-dihydro-4H-imidazol-4-one], a broad-spectrum fungicide which belongs to the imidazolinone class of chemicals. It is used to control alternaria leaf spot and downy mildew in vegetables, purple early and late blight in potatoes and tomatoes (Gary et al. 2010), downy mildew of vines (Lacombe et al. 2001) and melon (Gengotti et al. 2009). Fenamidone 10% + mancozeb 50% as combination formulation have been reported to be effective in controlling of downy mildew of grapes (Prakash et al. 2007; Prabhu 2007).
Use of fungicides in combination mixtures enhance the disease controlling efficiency as well as help in combating development of disease resistance to the existing fungicides. But, at the same time, in view of chances of accumulation of residues of these agrochemicals in the final product and rigid maximum residue limit (MRL) regulations, necessitate applying these chemicals carefully with prior knowledge of their residue dynamics. Being a multiple picking crop, the residue issue further aggravates. However, concentration of pesticide residues in fruits at below the MRL could only be ensured; if adequate pre-harvest intervals estimated through GAP (good agricultural practices) trials, are maintained between the last application and the harvest. Maity and Mukherjee (2009) has conducted an assessment of iprovalicarb residue in cabbage (Brassica oleracea var. capitata) and reported the detection of residue up to 15 days in both cabbage head and leaves at both recommended and double doses of application. There have been various reports of dissipation studies of these compounds in different crops (Angioni et al. 2012). However, information of dissipation behavior of these compounds when applied in combination mixture in tomato fruits is not available. Therefore, a field study was undertaken to investigate the dissipation kinetics of individual component and to determine the half-life and PHIs of the ready mix formulations Melody duo 66.75 WP and Sectin 60 WG in tomato fruit to ensure their safe use with respect to the EU-MRLs (EU Pesticide Database, Regulation (EC) No 396/2005). Further, considering the multiple picking nature, the day-wise residue levels were employed for safety evaluation based on acceptable daily intake of these pesticides. The study would be useful for registration of these combination products; so that the farmers can have more options to control the fungal diseases in tomato crop.
Materials and methods
Chemicals and reagents
The combination formulation of Melody Duo 66.75 WP (iprovalicarb 5.5% + propineb 61.25%) and Sectin 60 WG (fenamidone 10% + mancozeb 50%) were obtained from Bayer Crop Science Ltd., Mumbai, India. The certified reference standards of iprovalicarb (98% pure), fenamidone (97.5% pure) and thiram (99.5% purity for validation study) were purchased from Dr Ehrenstorfer GmbH (Augsburg, Germany). Carbon disulfide (CS2) with 99.9% purity was procured from Sigma-Aldrich (Poole, UK). Tin (II) chloride, hydrochloric acid LR (35%) was purchased from SD Fine-Chemicals Ltd. (Mumbai, India). Formic acid (98% pure), acetic acid (99% pure), gradient grade methanol, and ethyl acetate (AR) were obtained from Merck India Ltd. (Mumbai, India). HPLC grade water was obtained through Sartorius water purification system (Gottingen, Germany).
The reaction mixture used for the analysis of mancozeb and propineb was prepared by dissolving 30 g of Tin (II) chloride into 1L of HCl (35%) and the solution obtained was slowly added in 1 L of water to obtain a clear solution which was stored in a polypropylene container.
The stock solution of CS2 (2000 µg mL−1) was prepared by accurately pipetting out 79 µl of CS2 into a volumetric flask (certified A class, 50 mL) containing approximately 45 mL of isooctane, which was made up to 50 mL with isooctane. The CS2 stock solution was kept in refrigerator at 20 °C and used within 2 days of preparation. CS2 working standard solutions of 200 and 20 µg mL−1 concentrations (10 mL each) were prepared by serial dilution of the stock solution with isooctane. The stock solution of thiram was prepared by weighing 10 (± 0.05) mg into a 10 mL volumetric flask (certified A class) and dissolved in ethyl acetate up to the mark to get a stock solution of ≈ 1000 µg mL−1 concentration. A 100 µg mL−1 thiram working standard was prepared from the stock solution by serial dilution in isooctane.
Field experiments
Field experiments were conducted in two separate fields at a farm located in the Manjri village of Pune district (Maharashtra State, India) as per the EU guidelines for crop field trials (EC regulation 2014). In the first field, Melody Duo 66.75 WP (Iprovalicarb 5.5% + propineb 61.25%) formulation was applied at recommended dose (RD; 2250 g ha−1) and double the recommended dose (DD; 4500 g ha−1) in separate plots at fruit formation stage which were repeated after 7 days. Similarly, in the second field, Sectin 60 WG (fenamidone 10% + mancozeb 50%) was applied at RD of 1500 g ha−1 and DD of 3000 g ha−1 in separate plots (4 × 3 m2 each). Each treatment, including the untreated control, was replicated thrice in randomized block design. The average maximum and minimum temperatures during the field experiment were 21.3 and 27.7 °C, respectively with average relative humidity ranging between 76 and 87%. There was no rainfall during the study and the crop was grown following a recommended package of practice.
Sampling and preparation of laboratory sample
Around 2 kg of tomato fruit samples from each replicate were harvested on 0 (2 h after second spray), 1, 2, 3, 5, 7, and 10 days after final application of fungicides. The fruits showing signs of infestation of insect pests, diseases or any physiological disorder were not considered during sampling. All the samples were transported to the laboratory and immediately stored at 0 °C until analysis to prevent any degradation losses of the residues.
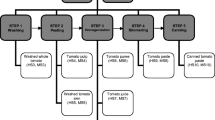
For preparation of laboratory sample, the entire sample (2 kg) was chopped into pieces of approximately 1 cm × 1 cm size and divided into two parts of 1 kg each. For the analysis of iprovalicarb and fenamidone, one part (1 kg) was crushed thoroughly in a blender from which approximately 200 g of the crushed sample was further homogenized. For analysis of dithiocarbamate compounds, the chopped sample was used for extraction.
Sample preparation for iprovalicarb and fenamidone
For sample preparation of iprovalicarb and fenamidone residue analysis, the method reported by Jadhav et al. (2015), in fruits and vegetables was used with slight modifications and after thorough validation. Homogenized sample (10 g) was drawn into a 50 mL polypropylene centrifuge tube. The sample was extracted with ethyl acetate (10 mL) plus anhydrous sodium sulfate (10 g) by homogenization at 15,000 rpm for 2 min (a high-speed homogenizer DIAX-900, Heidolph, Germany) followed by centrifugation at 5000 rpm for 5 min. A 5 mL aliquot of supernatant was cleaned using 50 mg primary secondary amine (PSA) and 150 mg Na2SO4. For analysis by LC–MS/MS, 2 mL of the aliquot was evaporated to dryness under a gentle stream of nitrogen in a low volume concentrator (TurboVap LV, Caliper Life Sciences, Massachusetts, USA) at 35 °C. The residues were re-dissolved in 2 mL methanol: 0.1% acetic acid (1:1), centrifuged (5000 rpm for5 min), filtered through 0.2 µm N-6,6 membrane filters, and analyzed by LC–MS/MS.
Sample preparation for mancozeb and propineb
For sample preparation of mancozeb and propineb analysis, SnCl2/HCl acid hydrolysis method estimating CS2 concentration was employed (Mujawar et al. 2014; Shabeer et al. 2015b). In brief, the extraction was carried out as follows. Chopped tomato sample (25 g) was taken in a glass bottle of 250 mL capacity (Scott Duran, Hattenbergstrae, Germany) and to it reaction mixture (75 mL) was added which was followed by addition of isooctane (25 mL). The bottle was immediately closed with screw cap and placed in a water bath maintained at 80 °C for 1 h with intermittent shaking after every 20 min. The bottle was then cooled to < 20 °C by placing in ice cold water bath. From the upper isooctane layer, 1 mL of aliquot was transferred into a microcentrifuge tube and centrifuged at 5000 rpm for 5 min at 10 °C. The supernatant was transferred into GC auto sampler vial and the residues of mancozeb and propineb were estimated in terms of CS2 by injecting into GC–MS. Quantification was done against the linear calibration standards of CS2.
LC–MS/MS analysis
The analysis of iprovalicarb and fenamidone residue was carried out using high performance liquid chromatography (HPLC) (Waters 2695 separation module) hyphenated to triple quadrupole (Quattro Premier, Waters Corporation, Milford, USA) mass spectrometer equipped with electron spray ionization (ESI) probe. The HPLC separation was carried out by injecting 10 μl onto a C18 column (Lichrocart® 55 mm × 2 mm, 3 μm; Merck India Ltd., Mumbai) with mobile phase flow rate of 0.4 mL min−1. The mobile phase was composed of A—methanol:water (20:80, v/v) with 5 mM ammonium formate and B—methanol:water (90:10, v/v) with 5 mM ammonium formate; with a gradient programme as follows: 0–0.5 min 20% B, 0.5–3.0 min- 98% B, 3.0–9.0 min 98% B, 9.0–10.0 min 20% B, 10–15.0 min 20% B. The column oven temperature was maintained at 35 °C. The LC–MS/MS analysis was done in positive polarity by multiple reaction monitoring (MRM) and the mass transitions (Q1 (precursor ion) > Q3 (product ion)) for iprovalicarb were 321 > 119 and 321 > 203 as quantifier and qualifier, respectively. In case of fenamidone, the respective quantifier and qualifier mass transitions were 312 > 65 and 312 > 92. The ratios of the peak area of two daughter ions were 1.6 and 2.9 for iprovalicarb and fenamidone, respectively. The corresponding ion ratio in the positive samples were determined and confirmed in compliance with the European Commission (EC) guidelines (DG-SANTE/11813/2017). The retention time (tR) for fenamidone was 5.85 min whereas, for iprovalicarb tR was 6.1 min (Fig. 1).
GC–MS analysis
For CS2, GC–MS analysis was conducted on a Trace GC Ultra equipped with Triplus auto sampler hyphenated to ITQ 900 mass spectrometer (Thermo Fisher Scientific, Austin, TX, USA) operated at selected ion monitoring (SIM) mode. The separation of CS2 was performed on a DB-5MS (5% diphenyl, 95% dimethylpolysiloxane, 30 m × 0.25 mm ID, and 0.25 µm film thickness) capillary column. Ultra-pure grade helium was used as carrier gas and maintained at flow rate of 1 mL min−1. The oven temperature was programmed with an initial temperature of 40 °C (hold for 5 min), ramped at the rate of 40 °C min−1 up to 200 °C (hold 5 min) for a run time of 14 min. The selected ions for CS2 estimation were 75.9 and 77.9 amu. The retention time of CS2 was 0.84 min.
Method validation
A single laboratory method validation (as per SANTE/11813/2017) was performed with respect to linearity, limit of detection (LOD), limit of quantification (LOQ), matrix effect, accuracy and repeatability (SANTE 2017).
Calibration curves and linearity
The linear response with respect to concentration was evaluated by establishing 7 point calibration curve with calibration standards in the range of 0.0025–0.25 µg mL−1 prepared in solvent i.e. methanol: water (1:1, v/v) and in matrix (control) extract for fenamidone and iprovalicarb. Similarly, a six point calibration curve was established for CS2 by injecting standard solutions of CS2 at concentration level ranging between 0.04 and 1.3 µg mL−1 in solvent and control matrix. The calibration curve was obtained by plotting the peak area response against the concentration.
Selectivity and sensitivity
The selectivity of the method was evaluated by comparing the chromatograms of spiked matrix samples with those of blank samples. The limit of detection (LOD) of the test compounds was determined by considering a signal to noise ratio (S/N) of 3 with reference to the background noise obtained for the blank sample, whereas, the limits of quantification (LOQ) were determined by considering a S/N ratio of 10 pertaining to the quantifier MRM and S/N ratio of 3 of qualifier ion.
Matrix effect
The Matrix effects (ME) of iprovalicarb and fenamidone were evaluated by comparing the peak area response of the solvent standard with that of matrix matched standard at 0.025 µg mL−1. Similarly, matrix effect for CS2 was evaluated by comparing peak area response of CS2 at 0.04 µg mL−1 level in solvent and matrix matched standard. The matrix effect was calculated using the equation: (Peak area of post extraction spike × 100/Peak area of solvent standard).
The ME values above 100% indicated matrix induced signal enhancement whereas, below 100% indicated signal suppressions.
Accuracy and precision
Accuracy of the method was established through the recovery study in untreated tomatoes. For evaluation of % recovery of iprovalicarb and fenamidone, homogenized tomato samples (10 g) were fortified at three concentrations levels viz. 0.01, 0.025 and 0.1 mg kg−1 in a set of six replicates each and kept undisturbed for 15 min. After extraction as per the given protocol, and analysis by LC–MS/MS, the % recovery was determined against the matrix matched calibration standard. For validation of dithioocarbamate compounds, the recoveries of thiram measured in terms of CS2 equivalent of thiram (1 mol of thiram = 0.6323 mol of CS2) at 0.04, 0.16, and 1.3 mg kg−1 levels were evaluated in six replicates. The spiked samples were extracted as per the given protocol, analysed by GC–MS and the quantification of the residues was performed using matrix matched calibration standards of CS2.
Precision of the method was estimated in the conditions of repeatability by measuring % relative standard deviation (% RSD) of the replicate analysis at each spiked levels.
Data analysis
The residue dissipation data of the studied fungicides were analyzed using the curve fitting software Table curve 2D v5.01 (Sabale et al. 2014). Equation parameters, regression equation, and half-life were calculated by the software. The different models used are listed below:
In the above equations, [A]t is the concentration (μg g−1) of A at time t (days) and [A]1, [A]2 are the initial concentrations of A at time 0 degraded through 1st or 1st + 1st order process. The symbols k1 and k2 are the degradation rate constants 1 and 2. Since the 1st + 1st order model cannot be described in a differential form, DT50 could only be calculated by an iterative procedure. For estimation of PHI values, the EU-MRLs of 3.0 mg kg−1 for mancozeb and propineb, 0.7 mg kg−1 for iprovalicarb and 1.0 mg kg−1 for fenamidone for tomato (EU Pesticide Database 2018) were considered.
Safety evaluation
The food safety of the studied fungicides was evaluated by comparing their dietary exposure i.e. theoretical maximum daily intake (TMDI) vis-a-vis the maximum permissible intake (MPI). The values of the dietary exposure were calculated by multiplying the residue levels in each sample (mg kg−1) with an average per capita consumption of 0.0179 kg person child−1 day−1 of tomato (Saha et al. 2014).
Results and discussion
Method validation
The coefficient of determination (R2) for the calibration curve for iprovalicarb and fenamidone were > 0.99 in solvent and matrix matched standards. The LODs for iprovalicarb and fenamidone were 0.001 mg kg−1 with corresponding LOQs of 0.0025 mg kg−1. The matrix effect study indicated signal suppression for iprovalicarb (5%) and fenamidone (15%). The average recovery (%) at 0.01, 0.025 and 0.100 mg kg−1 fortification levels were 86 ± 6%, 82 ± 4% and 83 ± 2% for iprovalicarb and 86 ± 6%, 94 ± 5% and 80 ± 3% for fenamidone, respectively.
In case of mancozeb and propineb, linearity with R2 value > 0.99 was obtained in both solvent and matrix matched standards. With the optimized GC conditions, the LOD and LOQ were 0.01 and 0.04 mg kg−1, respectively which is well below the MRL of 3.0 mg kg−1. The signal enhancement for CS2 was less than 10% indicating that the matrix effect was not significant. The recoveries of fungicides (in terms of CS2) in tomato at 0.04, 0.16, and 1.30 mg kg−1 were 74 ± 9%, 83 ± 7% and 80 ± 5%, respectively.
Dissipation of residues in field experiment and determination of PHI
The dissipation pattern for the extractable residues of iprovalicarb and propineb and fenamidone and Mancozeb in tomato are presented in Figs. 2 and 3, respectively. The relative standard deviations (RSDs) for the concentration of the samples collected in replicates were within 20% for both the RD and DD of applications.
Iprovalicarb and propineb
The initial (2 h after application) residue deposits were 0.653 (RD) and 1.083 (DD) mg kg−1 for iprovalicarb and 4.24 (RD) and 10.08 (DD) mg kg−1 for propineb, respectively. In case of iprovalicarb, more than 50% of the initial deposits dissipated within 1.5 (RD) and 2 (DD) days of field applications with excellent fit of residue data to both 1st and 1st + 1st order model giving R2 value of > 0.986 (≈ 0.99). Whereas, the residue data of propineb best fits into 1st + 1st order model compared to 1st order model for both the RD and DD treatments with respective R2 value of 0.984 and 0.987 in 1st + 1st order model as compared to 0.921 and 0.986 in 1st order model. Our previous published literature also reported 1st + 1st order dissipation kinetics for dimethomorph, famoxadone, cymoxanil, pyraclostrobin, metiram, pyraclostrobin in grape and raisin preparation (Sabale et al. 2014; Shabeer et al. 2015a, b) and fipronil and difenconazole in okra (Hingmire et al. 2015). The DT50 values of propineb according to the 1st + 1st order model were 2 and 3 days with PHI of 3 and 7 days at RD and DD, respectively against EU MRL of 3 mg kg−1 (Table 1). No PHI was applicable to iprovalicarb for RD pertaining to the residues less than MRL value of 0.7 mg kg−1, whereas, PHI value of 2 day was applicable for DD of application. Hence, a PHI of 7 days is recommended for the combination fungicide iprovalicarb 5.5% + propineb 61.25%.
Fenamidone and mancozeb
The initial (2 h after spraying) residue deposits were 0.46 and 0.69 mg kg−1 for fenamidone and 1.66 and 4.65 mg kg−1 mancozeb at RD and DD, respectively. The dissipation rate of both fungicides were initially faster, and the speed slowed down with time indicating a nonlinear pattern which reflects that simple 1st order kinetics (uniform rate of dissipation) might not be appropriate to explain the dissipation behavior of the residues. More than 50% of the initial deposits of fenamidone dissipated within 2 (RD) and 3 (DD) days after spraying with a best fit of residue data at RD and DD to 1st + 1st order model (R2 >0.99). In case of mancozeb, the residue data were best fit into 1st + 1st order model as compared to 1st order model for both the RD and DD treatments with respective R2 value of 0.991 and 0.990 in 1st + 1st order model as compared to 0.934 and 0.984 in 1st order. In case of mancozeb, the half-life values were 2.0 and 2.5 days for RD and DD, respectively with corresponding PHI of 0 and 3.5 days (Table 1). PHI was not applicable for fenamidone as the initial residue (on 0 day) deposit itself was below the EU-MRL of 1.0 mg kg−1. Hence, an overall PHI of 4 days is recommended for the combination fungicide fenamidone 10% + mancozeb 50%.
Safety evaluation
The acceptable daily intake (ADI) for iprovalicarb, fenamidone, mancozeb and propineb are 0.015, 0.03, 0.05, 0.007 mg kg−1 body weight day−1, respectively (apps.who.int/pesticide-residues-jmpr-database/Document/203; http://www.fao.org/docrep/W8141E/w8141e0d.htm). Multiplying the ADI by the body weight of an average child (16 kg), the MPIs were estimated at 0.24, 0.48, 0.48, and 0.112 mg person−1 day−1 for iprovalicarb, fenamidone, mancozeb and propineb, respectively. The dietary exposure of all the pesticides on each sampling day based on average daily consumption of 0.0179 kg tomato were less than the MPI both at the RD and DD of applications (Tables 2, 3). Dietary exposure of propineb was higher than the MPI in 0th and 1st day of samples at DD of application and attained safer levels from 2nd day of the final application.
Conclusion
The ready mix formulations like Melody Duo 66.75 WP (Iprovalicarb 5.5% + propineb 61.25%) and Sectin 60 WG (Fenamidone 10% + mancozeb 50%) can be recommended for use on tomato, as they did not leave any harmful residues on tomato fruits after the required waiting period (PHI) and strictly following recommended dose of application. Considering the multi-picking nature of the crop, the recommended PHI should be strictly followed for the MRL compliance.
References
Angioni A, Porcu L, Dedola F (2012) Determination of famoxadone, fenamidone, fenhexamid and iprodione residues in greenhouse tomatoes. Pest Manag Sci 68(4):543–547
Chaudhury U, Iqbal J, Mustafa A (2009) Efficacy of different fungicides for the control of downy mildew of cuccumbers. J Anim Plant Sci 19(4):202–204
EC regulation (2014) Document 7029/VI/95 rev.5. General recommendations for the design, preparation and realization of residue trials. http://ec.europa.eu/food/plant/protection/resources/app-b.pdf
EU Pesticide Database (2018) http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=pesticide.residue.selection&language=EN. Accessed June 2018
Gary V, Ken P, Natalia P, Richard R, Pam R, Shouan Z (2010) Florida plant disease management guide: chemical control guide for diseases of vegetables. https://edis.ifas.ufl.edu/pg047
Gengotti S, Sbrighi C, Antoniacci L (2009) New fungicides to control downy mildew on melon. Inf Agrar 65(21):50–52
Gupta S, Gajbhiye VT, Sharma RK, Gupta RK (2011) Dissipation of cypermethrin, chlorpyriphos, and profenofos in tomato fruits and soil following application of pre-mix formulations. Environ Monit Assess 174:337–345
Hingmire S, Oulkar DP, Utture SC, Shabeer ATP, Banerjee K (2015) Residue analysis of fipronil and difenoconazole in okra by liquid chromatography tandem mass spectrometry and their food safety evaluation. Food Chem 176:145–151
Horticulture Statistics at a Glance- Database-2018 (2018) Ministry of Agriculture, Government of India. http://agricoop.nic.in/sites/default/files/Horticulture%20Statistics%20at%20a%20Glance-2018.pdfnhb.gov.in/area-pro/database-2011.pdf
Horvat A (2001) New systemic fungicides against diseases caused by fungi: Melody Duo and Melody Combi, composed for a healthy crop. Slovenian National AGRIS Centre, Biotechnical Faculty, University of Ljubljana http://www.agroweb.bf.uni-lj.si/ no. 182
Jadhav MR, Oulkar DP, Shabeer ATP, Banerjee K (2015) Quantitative screening of agrochemical residues in fruits and vegetables by buffered ethyl acetate extraction and LC-MS/MS analysis. J Agric Food Chem 63(18):4449–4456
Lacombe JP, Patty L, Steiger D (2001) La fenamidone: antimildiou de la vigneet de la pomme de terre. Phytoma 535:42–46
Maity A, Mukherjee I (2009) Assessment of iprovalicarb, a systemic fungicide in/on cabbage (Brassica oleracea var. capitata). Bull Environ Contam Toxicol 83:341–347
Mujawar S, Utture S, Fonseca E, Matarrita J, Banerjee K (2014) Validation of a GC–MS method for the estimation of dithiocarbamate fungicide residues and safety evaluation of mancozeb in fruits and vegetables. Food Chem 150:175–181
Prabhu HV (2007) Biological efficacy of new combination products against downy mildew of grapes. Ann Biol 23(1):53–56
Prakash VR, Eswaran AK, Kumar S, Usharani S (2007) Effectiveness of novel combination fungicide against downy mildew incidence, fruit quality, shelf life and post-harvest pathogens of grapevine in India. Plant Arch 7(2):775–780
Sabale R, Shabeer ATP, Utture CS, Banerjee K, Jadhav MR, Oulkar DP, Adsule PG, Deshmukh MB (2014) Dissipation kinetics, safety evaluation, and assessment of pre-harvest interval (PHI) and processing factor for kresoxim methyl residues in grape. Environ Monit Assess 186(4):2369–2374
Saha S, Shabeer ATP, Jadhav MR, Loganathan M, Banerjee K, Rai AB (2014) Bioefficacy, residue dynamics and safety assessment of the combination fungicide trifloxystrobin 25% + tebuconazole 50%-75 WG in managing early blight of tomato (Lycopersicon esculentum Mill.). J Environ Sci Health (B) 49(2):134–141
SANTE (2017) Guideline document on analytical quality control and method validation procedures for pesticide residue analysis in food and feed, SANTE document no. SANTE/11813/2017
Shabeer ATP, Banerjee K, Jadhav M, Girame R, Utture S, Hingmire S, Oulkar D (2015a) Residue dissipation and processing factor for dimethomorph, famoxadone and cymoxanil during raisin preparation. Food Chem 170:180–185
Shabeer ATP, Girame R, Hingmire S, Banerjee K, Sharma AK, Oulkar D, Utture S, Jadhav M (2015b) Dissipation pattern, safety evaluation, and generation of processing factor (PF) for pyraclostrobin and metiram residues in grapes during raisin preparation. Environ Monit Assess 187(2):31–34
Sharma ID, Nath A, Dubey JK (1994) Persistence of mancozeb (Dithane M-45) in some vegetables and efficacy of decontamination processes. J Food Sci Technol 31(3):215–220
Tomlin C (1994) The pesticide manual, 10th edn. BCPC/RSC, Crop Protection Publications, Farnham
Acknowledgements
The authors are grateful to the Directors of ICAR-National Research Centre for Grapes, Pune, India and ICAR-Indian Institute of Vegetable Research, Varanasi, India for their ardent support during the tenure of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, Y.B., Shabeer, T.P.A., Jadhav, M. et al. Analytical method validation, dissipation and safety evaluation of combination fungicides fenamidone + mancozeb and iprovalicarb + propineb in/on tomato. J Food Sci Technol 57, 2061–2069 (2020). https://doi.org/10.1007/s13197-020-04240-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04240-9