Abstract
White chocolate is often considered as an unhealthy product with low phenolic content and antioxidant activity since it does not contain cocoa liquor. In this study, investigation on the phytochemical composition of cinnamon essential oil as well as its potential use to improve the antioxidant activity of white chocolate were carried out. The effect of the essential oil incorporation on the quality attributes of white chocolate was also examined. The results show that cinnamon essential oil was rich in cinnamaldehyde and exhibited antioxidant activity. The incorporation of cinnamon essential oil at a level of 0.1% (w/w) increased the antioxidant activity of the white chocolate more than twofold without significant effect on its hardness, melting properties and colour. However, a slight alteration on the flow behaviour of the white chocolate was observed. This study clearly shows that natural cinnamon essential oil could be an alternative to synthetic additives in foods to improve their antioxidant activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research interests on the exploration of bioactive compounds especially for their use in food processing have been increasing significantly due to their potential health benefits (Granato et al. 2016). Several medicinal plants traditionally used for enhancing health or healing different kinds of diseases around the world have been known to be promising for their application in functional food formulations. The genus Cinnamomun (cinnamon) has been reported as a potential source of bioactive compounds, such as cinnamaldehyde, eugenol, linalool and limonene. A considerable amount of literature has correspondingly shown its great potency as an anti-cancer, anti-Alzheimer’s, anti-inflammatory, anti-diabetic, anti-bacterial agent and antioxidant agent (Ribeiro-Santos et al. 2017; Muhammad and Dewettinck 2017; Muhammad et al. 2019b). Previous studies have shown that cinnamon has been successfully used to improve the health-promoting properties of coffee and yoghurt (Durak, et al. 2014; Shori and Baba 2011).
It is widely known that cocoa beans (Theobroma cacao L.), the main ingredients of chocolate, are rich in biological active compounds. However, they decrease significantly during cocoa bean processing namely, fermentation and drying as well as during chocolate manufacturing, particularly the roasting, mixing and conching processes (Żyżelewicz et al. 2016; Evina et al. 2016; Carrillo et al. 2014). Fermentation, for instance, in one hand is useful to develop the flavour profile of chocolate (Kongor et al. 2016), but in the other hand results in a loss of the contents of monomeric flavan-3-ols due to the enzymatic browning processes (Oracz et al. 2015).
White chocolate is made by mixing cocoa butter, milk powder, sugar and lecithin. Due to the absence of cocoa mass, white chocolate has significantly lower polyphenol content and antioxidant activity compared to milk and dark chocolate (Meng et al. 2009), and thus is often considered as unhealthy food by consumer. In the context of product stability, the lack of antioxidant compounds in white chocolate induce the rapid development of brown colour as a result of non-enzymatic browning reaction and oxidation process which limits the shelf-life of white chocolate (Jardim et al. 2011). Thus, improvement of antioxidant activity of white chocolate is required.
This study therefore aims to investigate the improvement of antioxidant activity of white chocolate using cinnamon. In our previous studies, the improvement of phenolic content and antioxidant activity of cocoa derived products, including milk chocolate, cocoa drink and cocoa powder using cinnamon extract have been carried out (Ilmi et al. 2017; Muhammad et al. 2017; Muhammad et al. 2018; Praseptiangga et al. 2019; Muhammad et al. 2019a). However, the use of cinnamon essential oil to improve the antioxidant activity of white chocolate is still under-investigated. As fortification of food with novel ingredients can change the characteristics of the food, research on the quality attributes of the white chocolate incorporated with cinnamon essential oil is also required.
Materials and methods
Preparation of C. burmannii Blume essential oil
Cinnamon barks (C. burmannii Blume) were collected from Kerinci, Sumatra (Indonesia). Cinnamon essential oil was obtained by hydro-distillation according to the protocol of Li et al. (2013). The initial ratio of the cinnamon and water in the distillation chamber was 1:10 (w/v). The chamber was constantly heated for 8 h to boil the water and to obtain the distillate. Afterwards, the distillate was moved to a conical flask and subjected to a phase separation. The water was removed to obtain the essential oil. The yield of the essential oil was approximately 4 mL per kg of raw material. The cinnamon essential oil was kept at 4 °C for further analysis.
Preparation of cinnamon essential oil-enriched white chocolate
Molten white chocolate (Barry Callebaut Belgium NV) was mixed with the cinnamon essential oil at a concentration of 0.1% (w/w) using a Stephan Universal Machine UMC 5 (Stephan Food Service Equipment GmbH, Hameln, Germany). The mixing temperature was kept at 35 °C. After being manually tempered by a trained chocolatier, the tempered chocolate was each moulded and cooled (12 °C, 2 h). The chocolate was then removed from the mould and kept at 20 °C for 14 days to ensure proper maturation before analysis.
GC–MS analysis of cinnamon essential oil
A Shimadzu GCMS-QP2010 SE instrument equipped with a Restek RTX-5MS low-bleed fused-silica capillary column (length 30 m and inner diameter 0.25 mm) with a film thickness of 0.25 µm was used for the analysis of the odour profile of the cinnamon essential oil. Samples (0.1 µL) were injected for odour profile analysis. The injector and the interface temperature were kept at 200 °C and 300 °C, respectively. Initial oven temperature was programmed at 60 °C and was then gradually increased to 300 °C. The effluent of the capillary column was introduced directly into the ion source of the mass spectrometer which had a temperature of 200 °C. The sector mass analyser was set to scan from 30 to 400 amu, while the linear velocity of the helium carrier gas was 0.75 mL/min at a split ratio of 153:1. Components of essential oil were identified by comparing mass spectra of each peak with those of authentic samples in a mass spectra library. The result was expressed as relative content (%) based on the result of area percentage obtained from the mean value of two injections.
Determination of antioxidant activity
Extraction of antioxidant compounds from white chocolate
Extraction of antioxidant compounds from the white chocolate is required prior the antioxidant activity analysis. The extraction was based on the work of Belščak et al. (2009). Exactly 20 g of white chocolate was washed three times with 100 mL of n-hexane to remove the fat content. The washing was conducted by a stirring process using a magnetic stirrer for 10 min with a speed of 1000 rpm. Afterwards, the defatted chocolate was air-dried in a dark condition (with the absence of light) for 24 h to remove n-hexane residue. The defatted chocolate (10 g) was well-mixed with 25 ml of solvent containing acetone (70%), distilled water (29.8%), and acetic acid (0.2%). The mixture was then maintained in an ultrasonic bath (Elmasonic P30H Elma Schmidbauer GmBH, Germany) for 30 min at 25 °C at a frequency of 80 kHz, followed by centrifugation for 10 min at 3000 rpm (Sigma 4K15 Sartorius AG, Germany) and filtration to remove the residual particles. This procedure was repeated twice, and then the supernatant was collected.
Ferric reducing antioxidant power assay
The ferric reducing antioxidant power (FRAP) analysis of cinnamon essential oil and white chocolate was carried out according to the method of Udayaprakash et al. (2015). A phosphate buffer (0.2 M, pH 7) was prepared by mixing Na2HPO4 (61 ml, 0.2 M) and NaH2PO4 (39 ml, 0.2 M). About 2.5 mL of phosphate buffer was mixed with the sample (1 mL) and 1% potassium ferricyanide (2.5 mL). After incubation at 50 °C for 30 min, 2.5 mL of 10% trichloroacetic acid was added to the mixture and centrifuged at 6500 rpm for 10 min. The absorbance was measured at 700 nm after the supernatant (4 mL) was added with 4 mL of distilled water and 0.8 mL of 0.1% FeCl3. A standard plot of ascorbic acid was used to measure the FRAP activity of the sample.
Phosphomolybdenum method
The method of Udayaprakash et al. (2015) was used as the reference for measuring the antioxidant activity of cinnamon essential oil and white chocolates. The phosphomolybdenum reagent was made by equally mixing sulphuric acid (0.6 M), sodium phosphate (28 mM), and ammonium molybdate (4 mM). Samples (0.5 mL) was added to 4.5 mL of the reagent, and then the solution was kept in a water bath at 95 °C for 90 min. The absorbance was then measured at 695 nm using a UV–visible spectrophotometer (Varian Cary 50 Bio, Agilent Technology). The antioxidant activity was expressed as micrograms of tannic acid equivalent per gram of the sample (µg TAE/g sample).
DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity analysis
DPPH radical scavenging activity of the essential oil and its main constituent was investigated using two protocols. The first protocol was adopted from Singh et al. (2007), while the second protocol followed the method of Skroza et al. (2015). In the first method, the essential oil (20–100 µL) was added to 4 mL of DPPH 0.01 mM. The absorbance was measured at 517 nm after 30 min incubation in the dark. In the second method, 100 µL of each samples was added to a DPPH solution system (4 mL), and then the absorbance was continuously monitored at 517 nm for 60 min. By this method, the TIC50 value (the time needed to inhibit 50% radical reaction) of the sample could be observed. For each method, the DPPH radical scavenging activity was calculated using Eq. (1).
Determination of quality attributes of white chocolate
Flow behaviour analysis
An AR2000 rheometer (TA Instruments, New Castle, USA) equipped with concentric DIN cylinder (cylinder: 42.00 mm; rotor outer radius: 14.00 mm; stator inner radius: 15.00 mm; geometry gap: 5920 mm) was used to determine the rheological properties of white chocolate following the protocol of ICA46. Briefly, 20 g of molten chocolate was placed in the cylinder and sheared at 5 s−1 at 40 °C for 15 min before the test. The measurement of the shear stress (τ) was conducted by increasing the shear rate (γ) from 2 to 50 s−1 (ramp up) and by holding for 16 s per shear rate level. After holding the shear rate at 50 s−1 for 60 s, decreasing the shear rate from 50 to 2 s−1 (ramp down) was conducted by holding for 16 s per shear rate level. The data were then fitted to the Casson model as shown in Eq. (2) to determine the Casson yield stress (τCA) and Casson viscosity (ηCA). The thixotropy was determined by measuring the difference between the ramp up and ramp down at the shear stress of 5 s−1.
Hardness measurement
The textural properties of white chocolate with a dimension of 100 mm × 24 mm × 10 mm was analysed using the Instron 5942 Universal Testing Machine (Norwood, MA, Canada) following the method of Saputro et al. (2016). The measurement was carried out by penetration with a needle-shaped probe (Φ = 1 mm) to a depth of 5 mm at a rate of 2 mm/s after a pre-load of 0.2 N. The hardness (N) defines the maximum force recorded during the probe penetration to the white chocolate.
Colour measurement
The lightness component (L*), green to red component (a*) and blue to yellow component (b*) values of the white chocolate within Specular Component Excluded (SCE) category were determined using a colorimeter (Minolta Model CM-2500D Spectrophotometer, Konica Minolta Sensing, Tokyo, Japan). The value of chroma (C*) and whiteness index (WI) of white chocolates were calculated using Eqs. (3) and (4), respectively while the degree of difference between the chocolate containing cinnamon essential oil and chocolate control (ΔE*) were calculated using Eq. (5).
Melting profile analysis
The melting profile of the white chocolates were analysed using a differential scanning calorimeter (DSC Q1000 TA Instruments, New Castle, USA) equipped with a refrigerated cooling system. Based on the work of Saputro et al. (2016), the chocolates were first sliced into small pieces, and then approximately 5 mg of the chocolate slices were put in hermetically sealed aluminium cups. The samples were heated up from 20 to 200 °C at a rate of 5 °C/min. The melting peak integration was conducted using a linear baseline in the Universal Analysis 2000 software version 4.7A (TA Instruments).
Statistical analysis
Statistical analyses were performed using SPSS Statistics 22. The Independent Samples T test was used to test the statistical differences between two samples (white chocolate control and white chocolate with cinnamon essential oil) and the differences were considered significant at p < 0.05.
Results and discussion
Volatile compounds and antioxidant properties of C. burmannii Blume essential oil
Typically, cinnamon essential oil is yellow in colour with hydrophobic characteristics and has a distinct scent. Various volatile constituents of various classes were identified in the cinnamon essential oil by GC–MS. They belong to various classes of organic compounds such as terpenes, aldehydes, esters and ketones (Table 1).
Cinnamaldehyde was recorded as the component with the highest area percentage (56.02%). This finding is in accordance with previous studies by Singh et al. (2007) and Li et al. (2013), who identified cinnamaldehyde as the typical volatile compound in the essential oil extracted from cinnamon bark. The high cinnamaldehyde content resulted in a spicy cinnamon odour (Narbona et al. 2010). Aside from its role as flavouring agent, cinnamaldehyde has a strong anti-microbial activity and has been reported as a major contributing factor in the decline of degenerative diseases as shown in some in vivo and in vitro studies (Chuang et al. 2012; Farrokhfall et al. 2014; Muhammad and Dewettinck 2017).
Besides cinnamaldehyde, some other volatile compounds also contributed to the odour profile of the C. burmannii Blume essential oil. Pleasant odours of the cinnamon essential oil, such as citrus-like, sweet-floral, flower and spicy-balsamic, can be attributed to the presence of limonene, linalool, 4-terpineol, and cinnamyl acetate, respectively (Jirovetz et al. 2002; Mahattanatawee et al. 2005; Ugliano and Moio 2008; Brechbill 2007). It was identified that the relative content of those compounds in the cinnamon essential oil were 1.59%, 0.35%, 1.33% and 3.99%, respectively (Table 1). Even though their presence in cinnamon is minor, those compounds might contribute to the aroma profile of the essential oil, since their threshold concentrations are much lower than that of cinnamaldehyde. Threshold concentrations is defined as the minimum concentration of a compound that is perceivable by the human sense of smell.
The cinnamon essential oil exhibited an antioxidant activity, as could be concluded from results of the FRAP assay and phospomolybdenum method which was 328 µmol L−1 ascorbic acid equivalent and 597 µg tannic acid equivalent, respectively. A kinetic study in DPPH solution was carried out to further study the antioxidant activity of the essential oil and its major constituent (i.e. cinnamaldehyde). The kinetic study on the antioxidant activity of bioactive compounds can be used to understand the behaviour of the bioactive compound in inhibiting radical reactions (Muhammad et al. 2017).
As shown, a gradual increase of DPPH radical reaction inhibition was observed, in conjunction with the increasing amount of essential oil (Fig. 1a). The radical scavenging activity of essential oil was higher than that of pure cinnamaldehyde, but lower than that of BHA (100 µg/mL). This study showed that cinnamaldehyde contributed to the antioxidant activity of the essential oil. Nevertheless, the antioxidant activity cannot be solely attributed to cinnemaldehyde because some other compounds may have contributed to the antioxidant activity of the essential oil, indicated by the fact that cinnamaldehyde was much less effective to inhibit the radical reaction than the essential oil. The difference in inhibition between the pure cinnamaldehyde and the essential oil is big, suggesting the contribution of other compounds.
Figure 1b illustrates the kinetic evolution of the reaction of DPPH by the antioxidant compounds. This method is useful to investigate the TIC50 of an antioxidant compound. TIC50 is defined as the time needed to inhibit 50% of the radical reaction. The TIC50 value is inversely related to its radical inhibition activity, which means that the compound having a lower TIC50 value has a higher antioxidant activity. The TIC50 value of the essential oil was more than 30 min, whereas cinnamaldehyde could not reach 50% radical inhibition even after 60 min of measurement. This result confirms that not only cinnamaldehyde but also some other compounds contributed to the antioxidant activity of the cinnamon essential oil. Figure 1b also confirms that the cinnamon essential oil had a lower antioxidant activity than BHA as indicated by a higher TIC50 value (34 min).
Antioxidant activity and quality attributes of white chocolate enriched with cinnamon essential oil
Antioxidant activity
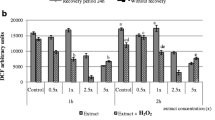
Due to its antioxidant potential, cinnamon essential oil is hypothesised to improve the antioxidant activity when incorporated in white chocolate. In this study, the incorporation of cinnamon essential was conducted at a level of 0.1% (w/w) as at this concentration, the flavour of the cinnamon essential oil-enriched chocolate was still acceptable to the consumer as shown in our previous study (Dwijatmoko et al. 2016; Ilmi et al. 2017). At a higher level of cinnamon essential oil, the chocolate was perceived too spicy and thus unacceptable. Figure 2 clearly shows that the incorporation of cinnamon essential oil at the level of 0.1% significantly improved the FRAP activity and total antioxidant activity of white chocolate. The increase was approximately twofold from the initial FRAP activity and total antioxidant activity. The antioxidant activity of cinnamon essential oil-enriched white chocolate in different test systems indicates that the chocolate has an ability to transfers electron to reduce any target compound [i.e., from Fe3+ to Fe2+; from Mo(VI) to Mo (V)]. Even though essential oil is volatile and sensitive to heat, the existence of the antioxidant activity in the cinnamon-enriched chocolate originating from the essential oil indicates the essential oil was relatively stable during chocolate production. This can be understood since the chocolate in this study was made by a consecutive step of mixing, tempering and moulding which did not involve a heat treatment. Thus, the incorporation of cinnamon essential oil could significantly improve the antioxidant activity of white chocolate. As aforementioned, the supplementation of a novel ingredient in food may alter its characteristics. Therefore, this current study was continued by investigating the quality attributes of the cinnamon essential oil-enriched white chocolate. Rheological and textural properties as well as melting profile and appearance are the crucial quality attributes of chocolate.
Flow properties
Knowledge on the flow behaviour of chocolate is highly important. In terms of processing, rheological characteristic determines the pumping and moulding properties of the chocolate while in a sensorial viewpoint, it contributes to the mouth feel of the chocolate. As such, during consumption, a chocolate with a high viscosity may stick to the teeth and palate making it unacceptable for the consumer (Saputro et al. 2016).
Figure 3 illustrates the effect of cinnamon essential oil on the rheological behaviour of white chocolate. In this study, Casson viscosity and Casson yield value were evaluated. Casson viscosity can be defined as the internal friction of the chocolate determining coating thickness among others, while Casson yield value can be defined as the amount of stress required to initiate fluid flow. It was shown that the presence of cinnamon essential oil slightly increased the Casson yield stress and the thixotropy, but not the Casson viscosity of white chocolate. Particle–particle interaction of the ingredients is a key factor affecting flow behaviour (Saputro et al. 2016). Cinnamon essential oil may create a high particle interaction in the white chocolate matrix, and thus the amount of stress required to initiate chocolate flow become higher as indicated by a high Casson yield stress value. A high particle interaction can form aggregation and gritty lump resulting in a high Casson viscosity (Saputro et al. 2016). However, in this study a gritty lump was not formed. After flowing, the cinnamon essential oil did not change the internal friction of particles in the white chocolate matrix as indicated by a stable Casson viscosity value. This phenomenon may be because the small amount of cinnamon essential oil added to the chocolate formula was not sufficient to give a significant impact on the internal friction during chocolate flow. During the shear, the particle interaction broke, and accordingly the difference between the shear rate value at 5 s−1 during the ramp up and the ramp down treatment became higher, resulting in a high thixotrophy value.
Hardness and melting profile
Hardness and melting profile are essential quality attributes of chocolate intensely related to organoleptic perception. Hardness describes the stiffness and the degree of ‘snap’ of chocolate while melting profile demonstrates the melting behaviour of chocolate in the mouth (Saputro et al. 2016; Muhammad et al. 2018). Figure 4a shows that the incorporation of cinnamon essential oil at a level of 0.1% did not alter the textural properties and the melting profile of white chocolate. Even though cinnamon essential oil may create a particle–particle interaction in the white chocolate matrix, the level of interaction was insufficient to alter the hardness of the white chocolate. Figure 4b illustrates the typical melting profile of the white chocolate control and the white chocolate incorporated with cinnamon essential oil. Three endothermic peaks were observed which are attributed to the melting point of milk fat, cocoa butter and sugar (Muhammad et al. 2018). Based on the data integration, no remarkable difference was observed on the value of Tonset (the temperature at which a specific crystal form starts to melt), Tmax (the temperature at which melting rate is highest), width (peak width at half height) and enthalpy (the amount of latent heat absorbed during melting) between the two types of chocolates. Both type of chocolates had a Tmax in the range of 32–34 °C indicating that the chocolates were well-tempered, and thus it had a good melting behaviour in the mouth.
Appearance
Colour and appearance is a pivotal parameter in chocolate since it give the first impression on the quality of the product to the consumer and thus strongly influences the product acceptability (Popov-Raljic and Lalicic-Petronijevic 2009). Table 2 shows that the incorporation of cinnamon essential oil resulted in no remarkable change in the colour properties of white chocolate, particularly on the L* and b* values. A slight difference was observed on the a* value, and thus resulted in a slight alteration on the whiteness index. Nonetheless, the discrepancy between the colours of those two samples would not be observable by consumers since they had a relatively similar chroma value. Moreover, it was shown that the degree of difference (ΔE) was only at a level of 0.9. According to Muhammad et al. (2018), colour differences are not obvious for the human if the ∆E is lower than 3.
Conclusion
Adding cinnamon essential oil is a strategically and technologically feasible approach to improve antioxidant activity. The essential oil of cinnamon (C. burmannii Blume) contains a wide range of typical volatile compounds constituting the cinnamon aroma, such as cinnamaldehyde, α-pinene, α-copaene, bornyl acetate, limonene and linalool, and exhibited antioxidant activity. There is no remarkable difference between the white chocolate control and the white chocolate containing cinnamon essential oil in terms of hardness, melting profile and appearance. Even though there were some deviating behaviour of the enriched chocolate in rheological properties, the differences were not significant and are not insuperable in industrial application. The antioxidant activity of cinnamon essential oil might contribute to retard the oxidation process of white chocolate, and thus might help to extend the shelf-life of the chocolate. To challenge this hypothesis, further studies focusing on the oxidation process of white chocolate enriched with cinnamon essential oil are required. Since essential oil is volatile, it would have been interesting to study the shelf-stability of the white chocolate in relation to antioxidant activity in the function of time. It might be also interesting to investigate the effect of cinnamon essential oil incorporation on the sensorial properties and the consumer acceptance of white chocolate as cinnamon essential oil might alter the odour profile of white chocolate.
References
Belščak A, Komes D, Horžić D, Ganić KK, Karlović D (2009) Comparative study of commercially available cocoa products in terms of their bioactive composition. Food Res Int 42:707–716
Benzo M, Gilardoni G, Gandini C, Caccialanza G, Finzi PV, Vidari G, Layedra P (2007) Determination of the threshold odor concentration of main odorants in essential oil using gas chromatography–olfactometry incremental dilution technique. J Chromatogr A 1150:131–135
Brechbill GO (2007) Classifying aroma chemicals. Fragrance Book Inc., New Jersey
Carrillo LC, Londoño-Londoño J, Gil A (2014) Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia. Food Res Int 60:273–280
Chuang LY, Guh JY, Chao LK, Lu YC, Hwang JY, Yang YL, Huang JS (2012) Anti-proliferative effects of cinnamaldehyde on human hepatoma cell lines. Food Chem 133:1603–1610
Durak A, Gawlik-Dziki U, Pecio Ł (2014) Coffee with cinnamon—Impact of phytochemicals interactions on antioxidant and anti-inflammatory in vitro activity. Food Chem 162:81–88
Dwijatmoko MI, Praseptiangga D, Muhammad DRA (2016) Effect of cinnamon essential oils addition in the sensory attributes of dark chocolate. Nusant Biosci 8:301–305
Evina VJE, De Taeye C, Niemenak N, Youmbi E, Collin S (2016) Influence of acetic and lactic acids on cocoa flavan-3-ol degradation through fermentation-like incubations. LWT Food Sci Technol 68:514–522
Farrokhfall K, Khoshbaten A, Zahediasl S, Mehrani H, Karbalaei N (2014) Improved islet function is associated with anti-inflammatory, antioxidant and hypoglycemic potential of cinnamaldehyde on metabolic syndrome induced by high tail fat in rats. J Funct Foods 10:397–406
Granato D, Santos JS, Maciel LG, Nunes DS (2016) Chemical perspective and criticism on selected analytical methods used to estimate the total content of phenolic compounds in food matrices. TrAC Trends Anal Chem 80:266–279
Ilmi A, Praseptiangga D, Muhammad DRA (2017) Sensory attributes and preliminary characterization of milk chocolate bar enriched with cinnamon essential oil. In: IOP conference series: materials science and engineering, vol 193, no 1. IOP Publishing, p 012031
Jardim DCP, Orseb AG, Efraima P, de Mouraa SCSR (2011) Kinetic of white chocolate color loss. Proc Food Sci 1:1026–1030
Jirovetz L, Buchbauer G, Ngassoum MB, Geissler M (2002) Aroma compound analysis of Piper nigrum and Piper guineense essential oil from Cameroon using solid-phase microextraction—gas chromatography, solid-phase microextraction–gas chromatography–mass spectrometry and olfactometry. J Chromatogr A 976:265–275
Kongor JE, Hinneh M, Van de Walle D, Afoakwa EO, Boeckx P, Dewettinck K (2016) Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—a review. Food Res Int 82:44–52
Kraujalytė V, Leitner E, Venskutonis PR (2012) Chemical and sensory characterisation of aroma of Viburnum opulus fruits by solid phase microextraction-gas chromatography–olfactometry. Food Chem 132:717–723
Li YQ, Kong DX, Wu H (2013) Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Ind Crops Prod 41:269–278
Mahattanatawee K, Goodner KL, Baldwin EA (2005) Volatile constituents and character impact compounds of selected Florida’s tropical fruit. Proc Fla State Hort Soc 118:414–418
Meng CC, Jalil AMM, Ismail A (2009) Phenolic and theobromine contents of commercial dark, milk and white chocolates on the Malaysian market. Molecules 14:200–209
Muhammad DRA, Dewettinck K (2017) Cinnamon and its derivatives as potential ingredients in functional foods—a review. Int J Food Prop 20:2237–2263
Muhammad DRA, Praseptiangga D, Van de Walle D, Dewettinck K (2017) Interaction between natural antioxidants derived from cinnamon and cocoa in binary and complex mixtures. Food Chem 231:356–364
Muhammad DRA, Saputro AD, Rottiers H, Van de Walle D, Dewettinck K (2018) Physicochemical properties and antioxidant activities of chocolates enriched with engineered cinnamon nanoparticles. Eur Food Res Technol 244:1185–1202
Muhammad DRA, Gonzalez CG, Doost AS, Van de Walle D, Van der Meeren P, Dewettinck K (2019a) Improvement of antioxidant activity and physical stability of chocolate beverage using colloidal cinnamon nanoparticles. Food Bioprocess Technol 12:976–989
Muhammad DRA, Doost AS, Gupta V, Bin Sintang MD, Van de Walle D, Van der Meeren P, Dewettinck K (2019b) Stability and functionality of xanthan gum–shellac nanoparticles for the encapsulation of cinnamon bark extract. Food Hydrocol. https://doi.org/10.1016/j.foodhyd.2019.105377
Narbona E, García-García E, Vázquez-Araújo L, Carbonell-Barrachina ÁA (2010) Volatile composition of functional ‘a la Piedra’turrón with propolis. Int J Food Sci Technol 45:569–577
Oracz J, Zyzelewicz D, Nebesny E (2015) The content of polyphenolic compounds in cocoa beans (Theobroma cacao L.), depending on variety, growing region, and processing operations: a review. Crit Rev Food Sci Nutr 55:1176–1192
Pino JA, Mesa J (2006) Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Frag J 21:207–213
Pino JA, Queris O (2011) Analysis of volatile compounds of mango wine. Food Chem 125:1141–1146
Popov-Raljić JV, Laličić-Petronijević JG (2009) Sensory properties and color measurements of dietary chocolates with different compositions during storage for up to 360 days. Sensors 9:1996–2016
Praseptiangga D, Invicta SE, Khasanah LU (2019) Sensory and physicochemical characteristics of dark chocolate bar with addition of cinnamon (Cinnamomum burmannii) bark oleoresin microcapsule. J Food Sci Technol 56:4323–4332
Qian MC, Wang Y (2005) Seasonal variation of volatile composition and odor activity value of ‘Marion’(Rubus spp. hyb) and ‘Thornless Evergreen’(R. laciniatus L.) blackberries. J Food Sci 70:C13–C20
Ribeiro-Santos R, Andrade M, de Melo NR, dos Santos FR, de Araújo Neves I, de Carvalho MG, Sanches-Silva A (2017) Revisiting an ancient spice with medicinal purposes: cinnamon. Trends Food Sci Techol 62:154–169
Saputro AD, Van de Walle D, Aidoo RP, Mensah MA, Delbaere C, De Clercq N, Van Durme J, Dewettinck K (2016) Quality attributes of dark chocolates formulated with palm sap-based sugar as nutritious and natural alternative sweetener. Eur Food Res Technol 243:177–191
Selli S, Cayhan GG (2009) Analysis of volatile compounds of wild gilthead sea bream (Sparus aurata) by simultaneous distillation–extraction (SDE) and GC–MS. Microchem J 93:232–235
Shori AB, Baba AS (2011) Cinnamomum verum improved the functional properties of bioyogurts made from camel and cow milks. J Saudi Soc Agric Sci 10:101–107
Singh G, Maurya S, Catalan CAN (2007) A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oil, oleoresins and their constituents. Food Chem Toxicol 45:1650–1661
Skroza D, Mekinić IG, Svilović S, Šimat V, Katalinić V (2015) Investigation of the potential synergistic effect of resveratrol with other phenolic compounds: a case of binary phenolic mixtures. J Food Compost Anal 38:13–18
Udayaprakash NK, Ranjithkumar M, Deepa S, Sripriya N, Al-Arfaj AA, Bhuvaneswari S (2015) Antioxidant, free radical scavenging and GC–MS composition of Cinnamomum iners Reinw. ex Blume. Ind Crops Prod 69:175–179
Ugliano M, Moio L (2008) Free and hydrolytically released volatile compounds of Vitis vinifera L. cv. Fiano grapes as odour-active constituents of Fiano wine. Anal Chim Acta 621:79–85
Yang Z, Kinoshita T, Tanida A, Sayama H, Morita A, Watanabe N (2009) Analysis of coumarin and its glycosidically bound precursor in Japanese green tea having sweet-herbaceous odour. Food Chem 114:289–294
Żyżelewicz D, Krysiak W, Oracz J, Sosnowska D, Budryn G, Nebesny E (2016) The influence of the roasting process conditions on the polyphenol content in cocoa beans, nibs and chocolates. Food Res Int 89:918–929
Acknowledgements
The authors also would like to express their profound gratitude to Cacaolab bvba (Evergem, Belgium) for the great help to produce chocolates and to the Ministry of Research, Technology, and Higher Education, Republic of Indonesia for the support through SAME Program 2019
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muhammad, D.R.A., Lemarcq, V., Alderweireldt, E. et al. Antioxidant activity and quality attributes of white chocolate incorporated with Cinnamomum burmannii Blume essential oil. J Food Sci Technol 57, 1731–1739 (2020). https://doi.org/10.1007/s13197-019-04206-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-04206-6