Abstract
This study was conducted to investigate the effects of nanoemulsions prepared with various concentrations of olive oil on the sensory, chemical and microbiological quality of rainbow trout fillets. The results showed that the shelf life of rainbow trout fillets were determined as 10 days for the control, 12 days for tween 80 group, 14 days for the 15% olive oil nanoemulsion group (O15) and 16 days for both 30% (O30) and 45% olive oil nanoemulsion groups (O45). It was determined that the use of olive oil suppressed the fish smell and improved the organoleptic quality of fish fillets, extending shelf life. Best sensory results were obtained from O30 and O45 treatment groups. The use of nanoemulsion had a positive effect on the biochemical parameters (TVB-N, PV, FFA, TBARs and pH), as well as inhibiting bacterial growth compared to the control group. Among nanoemulsion groups, the lowest bacteria content was found in O45 group. As a result, O30 and O45 treatment groups can be recommended for the preservation of fish fillets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquaculture industry has arisen as one of the most promising and rapidly growing industries, providing nutritious, high quality animal protein and providing income and employment around the world. Especially rainbow trout stands out for both Turkey and the world aquaculture. According to Turkish Statistical Institute (TUIK 2017), annual trout production in Turkey is around 109.657 tons. Thus, longer shelf life is essential for a species with such economic importance to the consumers.
Since fish spoil very quickly compared to other fresh food, the oxidative spoiling of aquaculture products, due to their rich unsaturated fatty acid content, is one of the most important issues for both the food industry and the consumers. As a result of oxidative spoiling, the shelf life of the product is reduced, and unwanted changes occur in the flavor, aroma and color of the product (Singh et al. 2005). To reduce the occurrence of rancid taste and smell, synthetic antioxidants and antimicrobials are used widely.
Recently, interest in natural additives has increased, due to the negative effects of synthetic additives on human health (Amorati et al. 2013). Among natural additives, vegetable oils are widely preferred (Ozogul et al. 2016). Olive oil is an important oil in food conservation. Because olive oil is a natural and effective additive, it is the perfect alternative to meet the consumer demand for fresh and chemical preservative-free aquaculture products. For the application of natural additives to aquaculture products, researchers are developing new nanotechnological preservation methods. In this sense, nanoparticles, nanofibers, different bioactive material(s)-loaded nano-scale materials, nanoparticles integrated nanofibers, and nanoemulsions currently are being used in order to delay the microbiological growth, chemical and physical deterioration in the fish fillets and products (Ceylan 2018; Ceylan et al. 2018; Durmuş and Ozogul 2018). The use of nanoemulsions to improve the shelf life of aquaculture products without affecting the taste of products is among these new methods. Since nano-food have ability to improve production, shelf-life, quality and safety of product, they have generated policy debates in many countries (Gruère 2011). European Commission obligated to label nano-food products. The attitude to regulation, labelling and approval of products of food nanotechnology varies from country to country. There is also need for regulatory guidelines in relation to potential use of nanotechnology in food science.
Nanoemulsions are heterogeneous emulsions where droplets of a liquid are distributed in another, non-mixable liquid. Nanoemulsions limit microorganisms’ access to water with their water-binding property (Al-Adham et al. 2000) and thus have a suppressive effect on bacteria (Bortoleto et al. 1998), fungi (Hamouda et al. 1999) and viruses. Many studies show that nanoemulsions are suitable for use in food products as preservatives (Al-Adham et al. 2000; Durmuş and Ozogul 2018).
While there are studies on the effect of nanoemulsions on improving the shelf life of seafood products (Joe et al. 2012; Ozogul et al. 2016, 2017; Durmuş and Ozogul 2018), no information available on the use of nanoemulsion based on different concentrations of olive oil for rainbow trout. For this reason, this study was conducted to investigate the effect of nanoemulsions prepared with different levels of olive oil on the quality of trout fillets stored at 4 ± 2 °C.
Materials and methods
Nanoemulsion preparation
Nanoemulsion preparation was carried out using the method stated by Joe et al. (2012) with minor modifications. In the study, to create an oil phase in the oil nanoemulsion in water; 15% (O15), 30% (O30) and 45% (O45) commercial vegetable oil (olive oil), 3% ethanol and 3% surfactant-Tween 80, (GRAS = considered safe-harmless in general) were used. These oil phase components were mixed and kept in the oven at 86 °C for 1 h. The mixture was removed from the oven and cooled to room temperature, and then sterilized pure water was added to the cooled mixture. The mixture was then placed in an ice-filled beaker and homogenized for 15 min in an ultrasonic homogenizator (Optic Ivymen System CY-500, Barcelona, Spain) for 15 min at 72 AMPL. Thermodynamic stability, nanoemulsion droplet size, viscosity and surface tension of the nanoemulsions were determined. The average particle size of droplets in the emulsions was measured using a Mastersizer 2000 (Malvern, UK) based on laser diffraction. Viscosity was measured using by a rheometer (TA Instruments ARES Rheometer). Thermodynamic stability was determined according to Shafiq et al. (2007). Surface tension was measured by gonyometer (Attension Theta, Biolin Scientific, Espoo, Finland).
Sample preparation
Rainbow trout (Oncorhynchus mykiss), an economically valuable and widely cultivated species, was used in this study. The fish were purchased from a local fish farm in the Çamlıyayla district of Mersin, Turkey. Immediately after harvesting, the fish were transported to Department of Seafood Processing Technology Laboratories at Faculty of Fisheries, Cukurova University without breaking the cold chain in ice filled Styrofoam containers. Average length and weight of the fish were 29.01 ± 1.35 cm and 312.17 ± 19.87 g respectively. They were immediately gutted, filleted with skin on, and divided into 5 lots. A total of 405 fish were used for this work. Each fillet was approximately 75–80 g. One lot (162 fillets; the reference samples) was stored on plates (2 fillets per plate) wrapped with permeable stretch film (Prima Gıda Iht. Mad. Ambalaj. San, Mersin, Turkey). Other lots were treated with nanoemulsion through immersion. Fillets were kept in the nanoemulsion for 4 min. After that, they were put in a plate (2 fillets for each plate) and wrapped with stretch film. For each analysis day, 3 plates (total 6 fillets) were randomly selected for each group. All samples were stored at 4 ± 2 °C. Data were obtained from fillets from three plates (triplicate) treated separately with duplicate measurements of the appropriate sample from each plate (n = 6). There were 5 groups including the control (C), tween 80, 15% (O15), 30% (O30) and 45% (O45) in cold storage. Sensory, chemical and microbiological testing was done on day 0, 2, 4, 6, 8, 10, 12, 14 and 16 of storage.
Proximate analysis
Protein contents of fish was carried out using the Kjeldahl method (AOAC 1984). Percent protein was determined by using a Kjeldahl conversion factor (N × 6.25). Lipid level was determined according to the method of Bligh and Dyer (1959). AOAC Method 920.153 (2002) and AOAC Method 950.46 (2002) were applied for the determination of ash and moisture respectively.
Sensory analysis
Quality Index Method (QIM) scheme improved by Bonilla et al. (2007) was used for sensory analysis of raw fish. The scheme contained quality parameters (e.g., skin brightness, skin mucus, flesh texture, flesh-blood, odour, colour, brightness, and gaping). For each of these parameters, the scheme had 4 simple descriptors, scoring demerit points from 0 to a maximum of 3, where 0 represented best quality and higher scores (e.g., 3) indicated poorer quality. Ten experienced panellists (who were familiar with sensory assessment) participated in the sensory assessment. Training session included the objective of the tests and discussion of the procedures. During training, fresh and very spoiled fish samples were used as references. A completely randomised sampling scheme was adopted using coded samples. Sensory assessment was done on each test day. Samples were assessed under natural day light conditions at 24 °C. At each sampling time, three fish for each group were randomly chosen to evaluate their sensorial attributes for both raw and cooked. Water was given between tests to clean palates.
The freshness evaluation of the cooked fish (odour, taste, texture, general acceptability and colour) was evaluated with a hedonic scale (from 9 to ≤ 1). A score of 9 indicate ‘very good’ while ≤ 1 demonstrate ‘very bad’. Microwave (Model: Siemens HF24G241, Munich, Germany) was used for cooking of fish at 600 W for 2 min.
Chemical analyses
TVB-N (total volatile basic nitrogen) content of fish was determined by Antonocopoulus (1973) method. The method is based on the extraction of TVB-N using alkaline solution and titration of the recovered ammonia using steam distillation. TBARs (the thiobarbituric acid reactive substances) value was carried out by the method of Tarladgis et al. (1960) and expressed TBARs as mg of malondialdehyde (MA) per kg of fish sample. AOCS method Ca 5a-40 (1997) was applied for free fatty acid (FFA) analysis and expressed as the percentage of oleic acid. FFA determination is based on a titration method with a standard alkali (0.1 M NaOH) using phenolphthalein as an indicator. AOCS Method Ja 8-87 (1994) was used for peroxide value (PV) and expressed in meq of peroxide oxygen per kg fat. The peroxide value is determined by measuring the amount of iodine which is formed by the reaction of peroxides with the iodide ion. The liberated iodine is titrated with sodium thiosulphate using starch as an indicator. The pH was measured using a pH meter (315i/SET, Weilheim, Germany). The sample was homogenised using an Ultraturrax (T25, Ika-Werke, Staufen, Germany) in distilled water at a ratio 1:10 (w/v) at its highest setting.
Microbiological analysis
Triplicate samples (one per storage plate) with duplicate measurements from each group (n = 6) were taken to estimate mesophilic aerobic bacteria, Enterobacteriaceae and psychrophilic viable counts from each of the different treatments. Fish muscle (10 g) was mixed with 90 ml of sterile Ringer solution (1/4 strength) and then Stomached (Masticator Nr S18/420, IUL Instruments, Barcelona, Spain) for 3 min. Further decimal dilutions were made, and then 0.1 ml of each dilution was pipetted onto the surface of plate count agar (Fluka 70152, Steinheim, Switzerland) plates in triplicate. The total viable counts (TVC) for each experimental group were performed by the spread plate method (ICMSF 1982). Plates were incubated for 2 days at 30 °C for mesophilic bacteria and 10 days at 10 °C for psychrophilic bacteria. For total Enterobacteriaceae, violet red bile agar (VRBA) were used and the pour plate method was performed (FDA 1998).
Statistical analysis
All experiments were carried out in triplicate and the results are reported as the mean and standard deviation of these measurements. A one-way analysis of variance (ANOVA) was run using the SPSS version 22 software (SPSS, Chicago, Illinois, USA) and the Duncan’s multiple range test comparisons at P value of < 0.05 were run to determine significant differences.
Results and discussion
Properties of nanoemulsions
Physical characteristics of nanoemulsions prepared using different concentrations of olive oil including thermodynamic stability, droplet size, surface tension and viscosity were determined (Table 1). No differences were observed in thermodynamic stability between the groups of nanoemulsions but there were significant differences (P < 0.05) in surface tension. Thermo-dynamic stability of nanoemulsions prepared from olive oil showed similarity with the studies of other researcher (Ozogul et al. 2016). Yazgan et al. (2017) reported that the surface tension of nanoemulsions based on sunflower oil was 41.53 nm while the surface tension of nanoemulsions prepared from olive oil was reported as 30.16 nm (Ozogul et al. 2016). Yazgan et al. (2017) found different viscosity value for sunflower based nanoemulsion compared to those of viscosity of olive oil based nanoemulsion in this study. The lowest viscosity was observed in O15 group while the highest viscosity was observed in O45 groups. Statistically significant differences with regards to droplet size were observed between groups of nanoemulsions prepared using different concentrations of olive oil. The group with the lowest droplet size was O15, whereas the largest value belonged to O45. Joe et al. (2012) reported that the droplet size of nanoemulsions prepared using sunflower seed, castor, coconut, peanut, sesame and similar commercial oils vary between 72.52 and 875.22 nm. Ozogul et al. (2016) found the droplet sizes of nanoemulsions prepared using sunflower seed, hazelnut, canola, soybean, corn and olive oils as 212, 185, 299, 250, 250 and 275 nm respectively. The physical characteristics of nanoemulsions prepared in this study were similar or different from previous studies. These differences are thought to arise from the types of oils used to prepare nanoemulsions, surfactants and the differences in the methods used to prepare nanoemulsions.
Proximate analysis
The average protein, lipid, moisture and ash levels of rainbow trout fillets were determined to be 18.90, 4.84%, 74.92% and 1.43%, respectively. Many studies reported the protein, lipid, moisture and ash content of rainbow trout as 17.4–22.9%, 2.7–16.5%, 63.4–73.4% and 1.0–1.6% respectively (Gökoǧlu et al. 2004; Oğuzhan et al. 2006; Fallah et al. 2011). In this study, all parameters aside from moisture were found to be similar to the other studies. Nutritional composition of the fish vary with fish species, gender, age, environmental conditions, fishing season and region (Ozyurt and Polat 2006; Durmus et al. 2014).
The nutritive value of fish can be affected by storage conditions, processing and cooking methods. Deterioration in the quality of fish muscle has frequently occurred during storage due to changes in lipid and proteins (Sikorski 1980). These changes in fish muscle are of great commercial importance since they determine the storage life of fish, especially in texture, flavor, and color, which results from lipid oxidation or protein degradation (Jiang and Lee 1985).
Sensory analyses
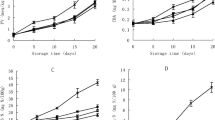
Figure 1a demonstrated the change in sensory characteristics of raw trout fillets treated with nanoemulsions during the period of cold storage. Significant differences were observed between the control and the treatment groups with regards to sensory parameters (Fig. 1a). Compared to the control group, nanoemulsion treatment had a positive effect on sensory quality of rainbow trout by removing strong fishy odour during storage period, thus extending the shelf life of fish. Nanoemulsion treated fillets were preferred more by the panelists compared to the control group, and nanoemulsion treatment improved the shelf life of trout. The shelf life of the trout was determined as 10 days for the control group, 12 days for the tween 80 group, 14 days for O15 group and 16 days for O30 and O45 groups. Ozogul et al. (2017) determined that shelf lives of ice stored trout fillets without or with nanoemulsions based on rosemary, laurel, thyme and sage essential oils were 14 days and 17 days respectively. According to the sensory evaluation, it was determined that shelf life of nanoemulsion groups stored on ice was extended and the fish quality was preserved during storage.
Raw trout fillets with or without nanoemulsions were cooked every 2 days during the 16 days storage period and offered to panellists for sensory (odour, taste and texture) evaluation (Fig. 1b–d, respectively). Nanoemulsion application did not affect the flavor and aroma of the fish negatively. Cooked trout fillets had similar shelf life as raw ones. Odour scores of trout fillets were observed to decrease during storage (Fig. 1b). Even though no difference was observed among nanoemulsion groups, differences were observed between the treatment groups and the control. At the end of the storage period, the lowest scores among nanoemulsion groups were observed in O15 group, while the highest scores were observed in O30 and O45 groups. During storage, flavor values steadily decreased across all groups, just like odour values. A faster decrease in sensory scores was observed in the control group compared to the treatment groups. Among nanoemulsion groups, the best scores were observed in O30 group. It was determined that nanoemulsion treatment groups were more preferable than the control and tween 80 group. Many researchers reported that nanoemulsion treatment had a positive sensory effect on fish fillets (Ozogul et al. 2017; Yazgan et al. 2017; Ozogul et al. 2018).
Fish meat with soft tissue is undoubtedly unacceptable for customers, and as such considered a quality loss for fish processing plants (Cheng et al. 2014). In this study, decreases in texture scores were observed (Fig. 1d), as observed in odour and flavor scores. It was determined that the texture score of the control group is lower than all other groups until the last day of storage. Throughout the storage period, nanoemulsion treatment groups were preferred by panelists and received higher scores for taste, smell and texture compared to the control. Nanoemulsion treatment had a positive effect on the taste, odour and texture of trout.
Chemical analyses
The increase in TVB-N amount is caused by the activation of spoiling bacteria and endogenous enzymes (Ozogul et al. 2004). It is well known that nanoemulsions are self-preserving antimicrobials since they have ability to bound the water found in their structure, thus restricting access to the water by microorganisms (Al-Adham et al. 2000). At the beginning of storage, the TVB-N values were 14.85 mg/100 g for tween 80 and 15.87 mg/100 g for the control (Table 2). Over the storage period, fluctuations were observed in TVB-N values. Rehbein and Oehlenschlager (1982) reported that TVB-N value increased over the storage period. However, studies performed on different fish species showed that TVB-N values can vary with species as well (Rehbein and Oehlenschlager 1982). At the end of storage, the lowest TVB-N value was determined in O45 treatment group with 17.01 mg/100 g, while the highest value was obtained from the control group (25.05 mg/100 g). Recommended TVB-N value for fish meat at the end of storage is under 35 mg/100 g (ECC 1995). In this study, all groups were found to remain under 35 mg/100 g value. It was found that nanoemulsion treatment delayed TVB-N formation compared to the control group. In other studies, Joe et al. (2012) studied the effects of sunflower seed oil nanoemulsions on the shelf life and quality of mackerel, and determined that TVB-N values were lower compared to the control group.
It has also been reported that application of nanoemulsion based on oils reduced lipid oxidation parameters of fish (Yazgan et al. 2017; Shadman et al. 2017; Ozogul et al. 2017). Hydroperoxides, measured by peroxide value (PV), form as a result of the oxidation of fats. At the beginning of storage, PV value of rainbow trout fillets was found in the range of 2.36 and 3.11 meq O2 kg−1. Over the storage period, the PV values of trout fillets treated with olive oil nanoemulsion were found to be lower than those of the control and tween 80 groups. At the end of the storage period, the control group had the highest PV value with 16.15 meq O2 kg−1, while the lowest value was observed in O45 nanoemulsion group with 2.11 meq O2 kg−1 (Table 2). This could be attributed to effects of nanoemulsion on prevention of lipid oxidation. Some studies suggest that fish meat becomes bitter at 10–20 meq O2 kg−1 peroxide level (Bonnell 1994). In a very good material, the peroxide value is under 4 meq O2 kg−1, and in a good material it should not exceed 10 meq O2 kg−1; threshold value for consumption is reported as 20 meq O2 kg−1 (Ozogul et al. 2006). In this study, it was determined that PV value did not exceed the limit value for consumption in all groups. Similar results were observed in previous studies (Ozogul et al. 2016; Yazgan et al. 2017).
At the beginning of storage, TBARs value of rainbow trout fillets was found to be in the range of 0.33 and 0.38 mg MA kg−1 (Table 2). Overall, an increase in the TBARs value of all groups was observed during the storage period. The control group reached the highest TBARs value on day 16 of storage with 2.56 mg MA kg−1. Statistically significant differences in TBARs values were observed between groups during the storage period (P < 0.05). At the end of the storage period, the TBARs values of nanoemulsion treatment groups were found to be lower than those of the control and tween 80 groups. This could be due to the prevention effects of nanoemulsion on lipid oxidation. The oxidation level in fish meat varies depending on species, fat content, other chemical characteristics, the processing technology used and storage conditions. Auto-oxidative decay of fats cause observed changes in important parameters for food quality such as color, smell, taste, texture and nutritional values (Fernandez et al. 1997). It was reported that TBARs should be below 3 mg MA kg−1 in a very good material and not more than 5 mg MA kg−1 in a good material, while the consumption threshold is between 7 and 8 mg MA kg−1 (Varlık et al. 1993). In this study TBARs values are within consumption threshold values.
As free fatty acid content of fish meat increases over storage, it is known that there is a relationship between free fatty acid amount and loss of freshness in fish (Ozogul et al. 2005). On day 0 of storage, FFA value was found to be between 1.83 and 3.20%. Over the storage period, nanoemulsion groups demonstrated lower FFA values compared to the control group. On the last day of storage, the control group was found to have the highest value (6.06%) while the lowest level was obtained from O45 group (2.81%). Ozogul et al. (2017) reported initial FFA value in trout fillets stored on ice as 4.92%. Even though the FFA value fluctuated in all groups, nanoemulsions groups had lower FFA values than those of control. However, Rezaei and Hosseini (2008) reported that the initial FFA value for rainbow trout was 1.50% and reached 2.89% at the end of the 20 days storage period. In this study, it was determined that olive oil based nanoemulsion caused FFA values to decrease in rainbow trout fillets treated with nanoemulsions compared to the control group. This could have resulted from prevention effects of nanoemulsion on of lipid hydrolysis.
In the current study, the lowest pH value was observed in O30 treatment group (6.52) while the highest pH value was observed in the control group (6.71). Statistically significant differences were observed in pH values between the control group and the treatment groups during the storage period (P < 0.05). Gülyavuz and Ünlüsayin (1999) stated that in processed aquaculture products, a pH value at and over 7 is considered spoiled. Ozyurt et al. (2007) indicated that depending on storage temperature, pH values over 7.1 showed a state of spoilage in fish. Based on the results obtained from this study, the pH values were lower than these values. In fish, the breakup of nitrogen compounds creates volatile compounds, which causes an increase of the pH value. Olive oil based nanoemulsion groups were determined to have lower pH values than those of the control group. This is attributed to the result of use of nanoemulsions having a negative effect on microorganism growth and thus delaying the creation of volatile compounds.
Microbiological analyses
Changes in microbiological quality of trout fillets with or without nanoemulsions during the 16 days storage period are shown in Fig. 2. At the beginning, the highest aerobic mesophilic bacteria count was observed in the control group with 2.85 log cfu g−1 whereas the lowest value was observed in the tween 80 group (2.15 log cfu g−1). In the studies with trout, the initial mesophilic bacteria counts ranging between 3 and 4 log cfu g−1 were reported (Ozogul et al. 2017). During the storage period, the total mesophilic bacteria count (TMBC) in all groups increased. ICMSF (1986) stated that the acceptable TMBC is 7–8 log cfu g−1. In this case, the control group exceeded the acceptable microbial threshold on day 10 of storage (7.02 log cfu g−1) while tween 80 treatment group went over the threshold on day 14 of storage (7.72 log cfu g−1). Among the nanoemulsion groups, O15 gave higher value (7.50 log cfu g−1) than the acceptable threshold value on the last day of storage day 16 while O30 and O45 treatment groups did not exceed the threshold value during the storage period (6.11 log cfu g−1 and 6.69 log cfu g−1 respectively). Overall, the TMBC values of nanoemulsion treatment groups were found to be lower than both the control and tween 80 treatment groups. This showed that nanoemulsion inhibited bacterial growth.
The initial Enterobacteriaceae count for all groups was found to be in the range of 1.00–1.15 log cfu g−1 (Fig. 2). Ozogul et al. (2017) reported the initial Enterobacteriaceae count for trout as 2.27 log cfu g−1. Even though an increase was observed in all groups during storage, the fastest and highest increase were observed in the control and tween 80 treatment groups. Among nanoemulsion groups, the lowest Enterobacteriaceae count was observed in O45 group. This showed that higher olive oil concentration had a higher inhibitor effect on the Enterobacteriaceae group. As the total Enterobacteriaceae count recommended for food is 5 log cfu g−1, the control group exceeded the threshold on day 10 of storage (5.66 log cfu g−1), tween 80 group on day 12 (6.41 log cfu g−1), and all olive oil treatment groups on the last day of storage (O15 with 7.28 log cfu g−1, O30 with 5.65 log cfu g−1, O45 with 5.64 log cfu g−1). Ozogul et al. (2017) reported that at the point the trout treated with nanoemulsions were rejected by panelists, the total Enterobacteriaceae count was 5.81 log cfu g−1 for the control group on day 14, and in the range of 5.09–5.32 for thyme, rosemary, bay leaf and sage groups. Similarly, Ozogul et al. (2016) and Yazgan et al. (2017) reported similar results for sea bass and bream.
At the beginning of storage, the total psychrophilic bacteria counts (TPBC) for the control and tween 80 treatment groups were observed as 2.15 log cfu g−1 and 2.08 log cfu g−1 respectively, while on the initial and day 2 of storage no aerobic psychrophilic bacteria were found on the trout fillets treated with nanoemulsions. Rezaei et al. (2007) reported the initial TPBC of trout as 2.3 log cfu g−1 and their final TPBC count at the end of storage as 6.1 log cfu g−1. In all groups, TPBC counts reached similar levels on day 6 of storage, and then a linear increase was observed for the rest of storage period. From day 10 of storage, the control group reached higher TPBC levels than all other groups. ICMSF (1986) acceptable threshold value of 7 log cfu g−1 was exceeded by the control group on day 12 of storage (7.51 log cfu g−1), by tween 80 and O15 on day 14 (7.82 log cfu g−1 and 7.40 log cfu g−1 respectively), and by O30 and O45 groups on the last day of storage (7.51 log cfu g−1 and 7.39 log cfu g−1 respectively). Among nanoemulsion groups, it was determined that higher concentrations of olive oil were more effective on TPBC. Ozogul et al. (2017) reported that TPBC were 6.91 log cfu g−1 for the control group on day 14, 7 log cfu g−1 for the thyme oil based nanoemulsion, 7.18 log cfu g−1 for rosemary, 7.16 log cfu g−1 for laurel and 7.58 log cfu g−1 for sage on day 17.
Conclusion
This study showed that the quality of trout fillets can be better preserved using nanoemulsions based on olive oil. From a sensory perspective, it was determined that nanoemulsions suppressed fishy odour during the storage period. Thus, the use of nanoemulsions extended the shelf life of fish by 6 days, and the chemical and microbial parameters were observed to be lower than those of the control during the storage period. It was concluded that olive oil at the concentration of 30% and 45 can be used in formation of nanoemulsions as a preservative for fish.
References
Al-Adham I, Khalil E, Al-Hmoud N, Kierans M, Collier P (2000) Microemulsions are membrane-active, antimicrobial, self-preserving systems. J Appl Microbiol 89:32–39
Amorati R, Foti MC, Valgimigli L (2013) Antioxidant activity of essential oils. J Agric Food Chem 61(46):10835–10847
Antonocopoulus N (1973) Bestmmung des flüchhtigen basensticktoofs. In: Ludorf W, Meyer V (eds) Fische und fischerzeugnisse. Aulage Verlag Paul Parey, Berlin und Hamburg, pp 224–225
AOAC (1984) Official methods of analysis of the association of the official analysis chemists. Association of Official Analytical Chemists, Washington, DC
AOAC Official Method 920.153 (2002) Ash content. In: Official methods of analysis, 17th edn. Association of Official Analytical Chemists, Gaithersburg
AOAC Official Method 950.46 (2002) Moisture content in meat. In: Official methods of analysis, 17th edn. Association of Official Analytical Chemists, Gaithersburg
AOCS (1997) Official method Ca 5a-40. Free fatty acids. In: Firestone DE (ed), Official methods and recommended practices of the American Oil Chemists’ Society, 5th edn. AOCS Press, Champaign
AOCS Method Ja 8-87 (1994) Peroxide value. In: Firestone DE (ed) Official methods and recommended practices of the American Oil Chemists’ Society, 4th edn. AOCS Press, Champaign
Bligh EC, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:913–917
Bonilla AC, Sveinsdottir K, Martinsdottir E (2007) Development of quality index method (QIM) scheme for fresh cod (Gadus morhua) fillets and application in shelf life study. Food Control 18:352–358
Bonnell AD (1994) Introduction. Quality assurance in seafood processing: a practical guide. Springer, Berlin, pp 1–16
Bortoleto RK, De Oliveira AHC, Ruller R, Arni RK, Ward RJ (1998) Tertiary structural changes of the α-hemolysin from Staphylococcus aureuson association with liposome membranes. Arch Biochem Biophys 351:47–52
Ceylan Z (2018) Use of characterized chitosan nanoparticles integrated in poly(vinyl alcohol) nanofibers as an alternative nanoscale material for fish balls. J Food Saf 38:1–5
Ceylan Z, Sengor GFU, Yilmaz MT (2018) Nanoencapsulation of liquid smoke/thymol combination in chitosan nanofibers to delay microbiological spoilage of sea bass (Dicentrarchus labrax) fillets. J Food Eng 229:43–49
Cheng JH, Sun DW, Han Z, Zeng XA (2014) Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality. Compr Rev Food Sci Food Saf 13:52–61
Durmuş M, Ozogul Y (2018) The effects of nanoemulsions on the fatty acid profiles of sea bass fillets during storage at 2 ± 2 °C. EgeJFAS 35(3):227–235
Durmus M, Polat A, Öz M, Ozogul Y, Ucak I (2014) The effects of seasonal dynamics on sensory, chemical and microbiological quality parameters of vacuum-packed sardine (Sardinella aurita). J Food Nutr Res 53:344–352
EEC (1995) Total volatile basic nitrogen (TVB-N) limits values for certain categories of fishery products and specifying the analysis methods to be used. Commission Decision 95/149/EEC of 8 March 1995. Off J Eur Commun L97:84–87
Fallah AA, Siavash Saei-Dehkordi S, Nematollahi A (2011) Comparative assessment of proximate composition, physicochemical parameters, fatty acid profile and mineral content in farmed and wild rainbow trout (Oncorhynchus mykiss). J Food Sci Tech 46(4):767–773
FDA (1998) Enumeration of Escherichia coli and the coliform bacteria, bacterial analytical manual, revision A, 8th edn. AOAC International, Washington, DC
Fernandez J, Perez-Alvarez JA, Fernandez-Lopez JA (1997) Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem 59(3):345–353
Gökoǧlu N, Yerlikaya P, Cengiz E (2004) Changes in biogenic amine contents and sensory quality of sardine (Sardina pilchardus) stored at 4 ºC and 20 ºC. J Food Qual 27(3):221–231
Gruère GP (2011) Labeling nano-enabled consumer products. Nano Today 6(2):117–121
Gülyavuz H, Ünlüsayin M (1999) Su ürünleri işleme teknolojisi. Süleyman Demirel Üniversitesi Egirdir Su Ürünleri Fak. ders kitabı, Ankara
Hamouda T, Hayes MM, Cao Z, Tonda R, Johnson K, Wright DC, Baker JR Jr (1999) A novel surfactant nanoemulsion with broad-spectrum sporicidal activity against Bacillus species. J Infect Dis 180(6):1939–1949
ICMSF (1982) Microorganisms in foods. Their significance and methods of enumeration, 2nd edn. Univ. Toronto Pres, London
ICMSF (1986) Microorganisms in foods. The international commission on microbiological specifications for foods of the international union of biological societies. Blackwell, Oxford, pp 181–196
Jiang ST, Lee TC (1985) Changes in free amino acids and protein denaturation of fish muscle during frozen storage. J Agric Food Chem 33(5):839–844
Joe MM, Chauhan PS, Bradeeba K, Shagol C, Sivakumaar PK (2012) Influence of sunflower oil based nanoemulsion (AUSN-4) on the shelf life and quality of Indo-Pacific king mackerel (Scomberomorus guttatus) steaks stored at 20 °C. Food Cont 23:564–570
Oguzhan P, Angiş S, Haliloğlu HI, Atamanalp M (2006) Gökkuşağı Alabalığı (Oncorhynchus mykiss) filetolarında sıcak tütsüleme sonrası kimyasal kompozisyon değişimleri. EgeJFAS 23:465–466
Ozogul F, Polat A, Özogul Y (2004) The effects of modified atmosphere packaging and vacuum packaging on chemical, sensory and microbiological changes of sardines (Sardina pilchardus). Food Chem 85(1):49–57
Ozogul F, Gökbulut C, Ozyurt G, Ozogul Y, Dural M (2005) Quality assessment of gutted wild sea bass (Dicentrarchus labrax) stored in ice, cling film and aluminium foil. Eur Food Res Technol 220:292–298
Ozogul Y, Özogul F, Kuley E, Özkutuk AS, Gökbulut C, Köse S (2006) Biochemical, sensory and microbiological attributes of wild turbot (Scophthalmus maximus), from the Black Sea, during chilled storage. Food Chem 99(4):752–758
Ozogul Y, Durmus M, Ucar Y, Ozogul F, Regenstein JM (2016) Comparative study of nanoemulsions based on commercial oils (sunflower, canola, corn, olive, soybean, and hazelnut oils): effect on microbial, sensory, and chemical qualities of refrigerated farmed sea bass. Innov Food Sci Emerg Technol 33:422–430
Ozogul Y, Yuvka I, Ucar Y, Durmus M, Kösker AR, Oz M, Ozogul F (2017) Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme and sage) on sensory, chemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. LWT Food Sci Technol 75:677–684
Ozogul Y, Durmus M, Kuley Boga E, Uçar Y, Ozogul F (2018) The function of emulsions on the biogenic amine formation and their indices of sea bass fillets (Dicentrarchus Labrax) stored in vacuum packaging. J Food Sci 83(2):318–325
Ozyurt G, Polat A (2006) Amino acid and fatty acid composition of wild sea bass (Dicentrarchus labrax): a seasonal differentiaton. Eur Food Res Technol 222:316–320
Ozyurt G, Polat A, Tokur B (2007) Chemical and sensory changes in frozen (− 18 °C) wild sea bass (Dicentrarchus labrax) captured at different fishing seasons. Int J Food Sci Technol 42(7):887–893
Rehbein H, Oehlenschlager J (1982) Zur Zusammensetzung der TVB-N-Fraktion (Fulchtige Basen) in Sauren Extrakten und
Rezaei M, Hosseini SF (2008) Quality assessment of farmed rainbow trout (Oncorhynchus mykiss) during chilled atorage. J Food Sci 73:93–96
Rezaei M, Montazeri N, Langrudi HE, Mokhayer B, Parviz M, Nazarinia A (2007) The biogenic amines and bacterial changes of farmed rainbow trout (Oncorhynchus mykiss) stored in ice. Food Chem 103(1):150–154
Shadman S, Hosseini SE, Langroudi HE, Shabani S (2017) Evaluation of the effect of a sunflower oil-based nanoemulsion with Zataria multiflora Boiss. Essential oil on the physicochemical properties of rainbow trout (Oncorhynchus mykiss) fillets during cold storage. LWT Food Sci Technol 79:511–517
Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M (2007) Design and development of oral oil in water ramipril nanoemulsion formulation: in vitro and in vivo assessment. J Biomed Nanotechnol 3(1):28–44
Sikorski Z (1980) Advances in fish sciences and technology, Connell edn. Fishing News (Books) Ltd., London, p 78
Singh G, Marimuthu P, Murali HS, Bawa AS (2005) Antioxidative and antibacterial potentıals of essentıal oıls and extracts ısolated from varıous spıce materıals. J Food Saf 25:130–145
Tarladgis B, Watts BM, Yonathan M (1960) Distillation method for determination of malonaldehyde in rancid food. J Am Oil Chem Soc 37:44–48
TUIK (Turkish Statistical Institute) (2017) Turkish fisheries statistics. http://www.tuik.gov.tr/PreTablo.do?alt_id=1005
Varlık C, Uğur M, Gökoğlu N, Gün H (1993) Principles and methods of quality control of marine products. Gıda Teknolojisi Derneği Yayın (17)
Yazgan H, Ozogul Y, Durmuş M, Balikçi E, Gökdoğan S, Uçar Y, Aksun ET (2017) Effects of oil-in-water nanoemulsion based on sunflower oil on the quality of farmed sea bass and gilthead sea bream stored at chilled temperature (2 ± 2 °C). J Aquat Food Prod Technol 26(8):979–992
Funding
This study was supported by the University of Cukurova (Project No. FBA-2017-7405).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Durmuş, M., Ozogul, Y., Köşker, A.R. et al. The function of nanoemulsion on preservation of rainbow trout fillet. J Food Sci Technol 57, 895–904 (2020). https://doi.org/10.1007/s13197-019-04122-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-04122-9