Abstract
The aim of this study was to use high hydrostatic pressure treatment to enhance the extraction efficiency of the active components from the fruiting bodies of Antrodia cinnamomea, and compare with those obtained by shake and ultrasonic extraction methods. The conditions of high pressure extraction (HPE) at 600 MPa, a liquid/solid ratio of 40:1, and 3 min of treatment yielded triterpenoids and adenosine concentrations of 410.41 mg/100 mL and 0.47 mg/100 mL, respectively, which did not differ significantly from those with the two other treatments—shake extraction at 180 rpm for 8 h and ultrasonic extraction at 50 Hz for 60 min. The HPE extracts significantly attenuated reactive oxygen species, nitric oxide and prostaglandin E2 production in lipopolysaccharide-stimulated RAW 264.7 cells than shake extracts did. SEM micrographs revealed that high-pressure caused physical morphological damage to the mycelium of fruiting bodies, such as distortion and disruption of mycelial cells, and increased the mass-transfer effectiveness of the solvent and solute. HPE can be employed as an efficient extraction technique for production of bioactive ingredients that might have a potential application in food and related industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antrodia cinnamomea is a valuable medicinal fungus that only grows on the Cinnamomum kanehirai tree at an altitude of 600–1800 m, which is native to Taiwan. The morphological features of the fruiting bodies of A. cinnamomea are very diverse, and the fruiting bodies have reddish brown, light brown, or light yellowish-brown surfaces. (Chang and Chou 1995). A. cinnamomea contains a variety of useful compounds that can be used for medicinal purposes, including polysaccharides, triterpenes, diterpenes, maleic and succinic acid derivatives, and ubiquinone. A novel polysaccharide extraction from mycelia of A. cinnamomea displayed anti-proliferative effect against several tumor cell lines (Zhang et al. 2018). Wu and Chen (2016) reported that A. cinnamomea contains active component, triterpenes, which reduce the inflammation to promote the wound healing via the streptozotocin-inducing hyperglycemia-diabetes mice model. Many studies have confirmed the fungus’s anticancer, hepatoprotective, and anti-inflammatory effects, as well as its ability to increase immunity and prevent cardiovascular diseases (Cheng et al. 2009, 2018; Geethangili and Tzeng 2011). Recent studies have explored different extraction technologies to improve the yield of the active ingredients of A. cinnamomea, such as ultrasonic extraction and supercritical extraction (Zhao and Leung 2010; Liang et al. 2014), both of which have been commercialized. Ma et al. (2014) added citrus peel extract to the extraction process of A. cinnamomea and found that the triterpenoid extraction rate increased tenfold. Tu (2008) compared the extraction yields of A. cinnamomea components among heat reflux extraction, microwave extraction, and ultrasonic extraction, and the results indicated that the extraction of polysaccharides, adenosine, and crude triterpenoids with microwave-assisted extraction required only half of the extraction time to achieve the same extraction yield as heat reflux and ultrasonic extractions.
In recent years, many new technologies have been developed to improve the extraction yields of active ingredients. For example, ultrasonic, microwave, and high-pressure-assisted extraction (HPE) technologies can improve the extraction rate of active ingredients from food or medicinal plants (Wang and Weller 2006). During extraction, when the pressure increases, the volume of the mixture is compressed, and the pressure will physically damage the cell structure of the mixture, which changes the permeability of the cell wall or the membrane and in turn causes the solvent to rapidly enter into cells, increasing the mass-transfer effectiveness of the solvent and solute (Xi 2013). Because HPE is carried out at room temperature, it avoids the adverse effects caused by heat sensitivity and results in shorter extraction times, lower costs, and higher extraction yields (Huang et al. 2013). This technique has been successfully used in the extraction of ginsenosides from ginseng, anthocyanin from grape skins, catechins and caffeine from tea, pectin from lime peel, corilagin from longan fruit pericarp, and flavonoids from propolis (Chen et al. 2009; Corrales et al. 2009; Xi 2009; Naghshineh et al. 2013; Prasada et al. 2009; Zhang et al. 2005). However, there are no reports on the use of high hydrostatic pressure for extracting active ingredients from the fruiting bodies. In this study, the optimum extraction conditions for triterpenoids, adenosine, and polysaccharides from A. cinnamomea mycelia were identified by evaluating different variables, such as the solvent (water, 70% ethanol, and methanol), high hydrostatic pressure (100–700 MPa), dwell time (1–9 min), and liquid/solid ratio (10:1–50:1). In addition, the extraction efficiencies for the active ingredients of A. cinnamomea were compared among the HPE, traditional shake extraction, and ultrasonic extraction methods. The potential mechanisms of the reactive oxygen species (ROS) inhibition and anti-inflammatory action of extracts were investigated in lipopolysaccharide (LPS)-induced macrophage cells (RAW 264.7). The morphological changes in A. cinnamomea mycelia after the three extraction procedures were also compared by scanning electron microscopy (SEM).
Materials and methods
Antrodia cinnamomea fruiting bodies
Fresh fruiting bodies of A. cinnamomea were obtained from a local agricultural supplier and dried for 4 h at 80 °C in a hot air dryer until the water content was approximately 9%. The fruiting bodies were then ground into a powder, filtered through a 40-mesh filter, placed in vacuum-sealed bags, and stored in an electronic damp-proof cabinet until use.
High-pressure treatments
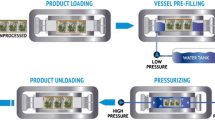
For high-pressure treatment, 2.5 g of A. cinnamomea fruiting body powder was mixed with 25, 50, 75, 100, or 125 mL of solvent (water:ethanol = 30:70) to determine the optimal liquid/solid ratio. The dissolution of bioactive components into the solvent is a physical process. When the amount of solvent increases, the chance of the bioactive components coming into contact with the solvent increases, which leads to higher leaching-out rates. The mixture was then placed in a sterile polyethylene bag, which was vacuum-sealed and subjected to high-pressure treatment. The high-pressure machine used in this study has a chamber volume of 0.3 L and water was used as the pressure-transmitting medium. The packed mixture was placed in the high-pressure chamber and subjected to 100–700 MPa at 25 °C (initial temperature). After the high-pressure treatment, the extraction mixture was immediately centrifuged at 4000 rpm for 10 min, the supernatant was collected and stored at 4 °C for subsequent component analyses, and the remaining pellet was used for SEM analysis.
Conventional shake extraction
For shake extraction, 2.5 g of A. cinnamomea fruiting body powder was mixed with 25, 50, 75, 100, or 125 mL of solvent (water:ethanol = 30:70) in a 200-mL serum bottle. The mixture was then placed in a shaker at 150 rpm at 25 °C (Lien et al. 2014). After extracting for 8 h, the mixture was centrifuged at 4000 rpm for 10 min. Then, extracts were collected and stored at 4 °C for subsequent component analyses, and the remaining pellet was used for SEM analysis.
Ultrasonic extraction
For ultrasonic extraction, 2.5 g of the A. cinnamomea fruiting body powder was mixed with 25, 50, 75, 100, or 125 mL of solvent (water:ethanol = 30:70) in a 200-mL beaker. After 60 min of ultrasound-assisted extraction (frequency 20 kHz, power 125 W) at 25 °C (Q125 Sonicator, Qsonica), the extracts were collected by centrifugation at a speed of 4000 rpm for 10 min. The supernatants were stored at 4 °C for subsequent component analyses, and the remaining pellet was used for SEM analysis.
Analysis of bioactive ingredients
Triterpenoids
Triterpenoids were evaluated using the method of Lu et al. (2011). Briefly, 0.2 mL of sample solution was placed in a 10-mL volumetric flask and heated to evaporation in a water-bath. Then, 1 mL of freshly mixed 5% (W/V) vanillin-acetic solution and 1.8 mL of sulfuric acid were added, followed by mixing and incubation at 70 °C for 30 min. The mixed solution was then cooled and diluted to 10 mL with acetic acid. The absorbance was measured at 573 nm against a blank using a spectrophotometer. The blank consisted of all reagents and solvents without the sample solution. The triterpenoid content was determined using a standard ursolic acid calibration curve. The calibration equation for ursolic acid was Y = 0.0605X − 0.0122 (R2 = 0.9991), where Y is the absorbance value and X is the concentration of ursolic acid (mg/mL).
Adenosine
The adenosine content of the samples was determined using the HPLC determination method described by Chang et al. (2005). The separations were performed using a HPLC system (Hitachi Ltd., Tokyo, Japan), with 15-μL loop and a reversed-phase column (Merck LiChrospher 100 RP-18, 5 μm, 4.0 × 250 mm I.D.; Darmstadt, Germany) followed by linear gradient elution using eluents A, B, C, and D [A: H2O; B: CH3CN/MeOH (1:1, v/v); C: 0.1 N HCl; D: 0.1 N NH4H2PO4 (adjusted to pH 4.0 with H3PO4)] according to the following A-D profile: 0–15 min, 60–30% A, 0–30% B, 20% C, and 20% D; 15–20 min, 30–60% A, 30–0% B, 20% C, and 20% D. The flow rate was 1.0 mL/min. Detection was performed at 260 nm. To prepare standards for the calibration curve and assessment of validation, a stock solution of adenosine was prepared at a concentration of 0.05 mg/mL. This solution was diluted with distilled water to yield 0.5, 0.25, 5.0, and 50 mg/mL working standard solutions for preparation of the calibration curve.
Polysaccharides
The polysaccharide content of the fruiting bodies of A. cinnamomea was measured using the phenol–sulfuric acid colorimetric method (Dubois et al. 1956). The polysaccharide content was determined colorimetrically at 280 nm. First, 0.1 mg of polysaccharide was hydrolyzed at 100 °C for 48 h with 5 mL of 2.5 M H2SO4 in a sealed test tube. The residue was neutralized with BaCO3 and Ba(OH)2 followed by filtration. The liquid portion was subjected to ion exchange chromatography using Amberlite IR-120 (H-form) and Amberlite IRA-400 (Cl-form) resins and then neutralized in sugar solution. The sugar solution was dried and resolved in 0.5 mL of distilled water for further analysis. The polysaccharide content of the mixture was determined using an HPLC (Hitachi Ltd., Tokyo, Japan) and a sphereclone-CHO column (Phenomenex Inc., Torrance, CA, USA). The results are expressed as mg per 100 mL of extract (mg/100 mL).
ROS, NO and PGE2 inhibition
The RAW 264.7 cells were cultured in triplicate at a density of 1 × 105 cells/well in a 96-well flat-bottomed plate, incubated for 24 h, treated with various concentrations of extracts, and then incubated for 4 h at 37 °C in an atmosphere of 5% CO2. The intracellular ROS levels were measured by detecting the fluorescence intensity of the oxidant-sensitive probe, 2′,7′-dichlorofluorescein-diacetate (DCFH-DA). The process involves the conversion of DCFH-DA to DCFH by deacetylase in the cells, which is then oxidized by various intracellular ROS to yield 2′,7′-dichlorofluorescein (DCF), a highly fluorescent compound. The cells were incubated with 50, 100 and 150 μg/mL of extracts in the presence or absence of LPS (1 μg/mL) for 24 h. Then, the cells were stained with 20 μM DCFH-DA for 15 min at room temperature and the intracellular ROS production was determined by using an ELISA microplate reader.
Nitrite constitutes the end product of the NO generated by activated macrophages, RAW 264.7 cells were incubated with 50, 100 and 150 μg/mL of extracts in the absence or presence of LPS (1 μg/mL) for 24 h. Then, 100 μL of the Griess reagent (1:1 mixture of 1% sulfanilamide and 0.1% naphthylethylenediamine in 5% phosphoric acid solution) was added to 100 μL of the cellular supernatant samples in 96-well plates, and the mixture was automatically agitated for 10 min at room temperature. The absorbance at 550 nm was measured using an ELISA microplate reader (Molecular Devices Co., USA). The reduction or increase in the nitrite level was estimated as the percentage absorbance of the sample relative to that of the respective control. Standard calibration curves were prepared using sodium nitrite serial dilutions. For PGE2 assay, the RAW 264.7 cells were cultured with 50, 100 and 150 μg/mL of extracts in the absence or presence of LPS (1 μg/mL) for 24 h. Then, 100 µL of the culture medium supernatant was collected, and the PGE2 concentration was measured by using a PGE2 ELISA kit (Cayman Chemical Company, Ann Arbor, MI, USA). The concentration of PGE2 was photometrically determined by using a microplate reader at 405 nm. The standard calibration curves were prepared by using PGE2 serial dilutions.
SEM analysis
The cell pellets from extracted and unextracted A. cinnamomea fruiting bodies were centrifuged at 10,000×g for 10 min after washing twice in 0.1 M phosphate buffer (pH 7.4). The cell pellets were then resuspended in 1 mL of 0.1 M phosphate buffer. The suspended cells were filtered (0.22-μm MF-Millipore, GSWP; Millipore Corp., Billerica, MA, USA) and fixed on a membrane using 10 mL of 1.0% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). The fixatives were left in contact with the cells overnight at 4 °C. The membranes were subsequently transferred to glass vials, subjected to three 10-min washes in buffer, and post-fixed for 1 h in 1% osmium tetroxide. Next, the membranes were rinsed twice in buffer for 10 min each time. The membranes were dehydrated using a series of 10-mL ethanol solutions (10–90% ethanol in 10%-steps and 90–100% ethanol in 5%-steps); the dehydration treatment consisted of 15 min in each solution. The samples were then soaked in isopentyl acetate before critical point drying in CO2 medium, which was performed using a critical point dryer (Hitachi HCP-2). Filters were then attached to large SEM stubs with double-sided tape and coated with gold palladium. The samples were analyzed with an SEM (Hitachi S4700) at 15 kV, and photomicrographs were obtained.
Statistical analysis
The experimental results represent the means from triplicate experiments. The data are presented as the mean ± standard deviation (SD), and they were analyzed using a statistical analysis system (SAS Inc., NC, USA). One-way ANOVA analysis was conducted. Significant differences between the means were determined by Duncan’s multiple range tests. The results were regarded as statistically significant at p < 0.05.
Results and discussion
The effect of solvents on extraction yields
Figure 1 shows the extraction yields of the active components from A. cinnamomea fruiting bodies using three different solvents: water, 70% ethanol, and methanol. The results indicate that the highest extraction yield of triterpenoids was obtained when using 70% ethanol, which resulted in significantly higher amounts than with methanol and water. For adenosine, the extraction yields with 70% ethanol and methanol were not significantly different; however, both were higher than that with water. Regarding the comparison of extraction yields for polysaccharides, 70% ethanol resulted in a higher extraction yield than water and methanol. Zhang et al. (2007) used HPE techniques to extract ginsenosides from Panax ginseng and compared the extraction yields when using four different solvents: water, 50% ethanol, methanol, and n-butanol. Their results indicated that 50% ethanol resulted in the best extraction yield.
The effect of pressure on extraction yields
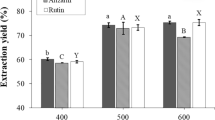
As shown in Fig. 2, the concentrations of active ingredients in the extracts of A. cinnamomea fruiting body powder increased as the pressure increased. At a processing pressure of 600 MPa, the concentrations of triterpenoids, adenosine, and polysaccharides increased to 410.41, 0.47, and 190.52 mg/100 mL, respectively. When the processing pressure was increased to 700 MPa, the concentrations of these three active ingredients did not increase significantly when compared to values at 600 MPa (p ≤ 0.05). Thus, a 600 MPa pressure was used for the following experiments. These results are consistent with a previous study in which Xi (2006) used HPE technology to extract lycopene from tomato paste waste and found that the extraction rate increased as the pressure increased up to a point. The extraction rate of lycopene was the highest at 500 MPa, and the extraction rate no longer increased above a pressure of 600 MPa. Similarly, Zhu et al. (2012) used HPE to extract lignan compounds from Dysosma versipellis and found that the maximum extraction rate was achieved at a pressure of 200 MPa and that the yield did not increase above that pressure, even at 500 MPa. According to the mass-transfer theory, the mass-transfer rate = pressure/resistance of mass transfer, and based on phase behavior theory, dissolution occurs faster at higher pressures. Under high-pressure conditions, the differential pressure between the inside and the exterior of the cell is very large, which causes the solvent to permeate very quickly through the disrupted cell wall, and the mass-transfer rate of the solute or the rate of dissolution is very high. This phenomenon could explain the shorter time needed to achieve results with HPE (Xi 2015).
The effect of pressure holding time on extraction yields
Table 1 shows the yields of the active ingredients of A. cinnamomea extracted under 600 MPa pressure with different extraction times. When holding times under 3 min, the extraction yields and concentrations of extracts increased as the extraction time increased. While extraction time reached 1 min, the concentrations of triterpenoids, adenosine, and polysaccharides were 351.47, 0.32, and 143.16 mg/100 mL, respectively; when it reached 3 min, the concentrations of triterpenoids, adenosine, and polysaccharides increased by 16.8%, 46.9%, and 33.1%, respectively, compared with the 1-min extraction. When the extraction time was over 3 min, no significant differences were observed. These results indicated that the optimal extraction yield with the greatest dissolution of solutes can be achieved with 600 MPa pressure and a 3-min extraction time. Prasada et al. (2009) found that when using high-pressure to extract corilagin from longan fruit pericarp, the extraction time did not affect extraction yields at 500 MPa, i.e., the concentrations of corilagin were not significantly different for extraction times of 2.5, 5, 15, and 30 min. Zhang et al. (2005) also found that the high-pressure holding time had no effect on the extraction yields of flavonoids from propolis. Xi et al. (2015) found that the extraction yield of polyphenols from green tea at a pressure of 300–500 MPa significantly decreased when the extraction time exceeded 2 min, and even longer extraction times did not result in increased extract concentrations. The extraction time should be long enough to ensure contact between the active ingredients and the solvent. Exposure to the solvent for a long time allows the matrix to swell, thus enhancing the penetration of solvent into the sample interstices and allowing contact between the solvent and target compounds. Among the various experimental conditions used for HPE, the holding time is usually between 1 and 10 min, as reported for the extraction of target compounds from plant materials (Huang et al. 2013).
The effect of the liquid/solid ratio on the extraction yields
Table 1 shows that the extraction yields of triterpenoids and polysaccharides were affected by the liquid/solid ratio, whereas the adenosine yield was not affected. When the liquid/solid ratio increased from 10:1 to 40:1 mL/g, the extraction yields of triterpenoids and polysaccharides increased from 297.54 and 133.71 mg/100 mL to 410.41 and 190.52 mg/100 mL, respectively, indicating that as the solvent amount increases, the rate of dissolution of biologically active ingredients increases. However, the extraction yield with a liquid/solid ratio of 50:1 mL/g was not significantly different than with a ratio 40:1 (p < 0.05), indicating that 40:1 was the optimal ratio for dissolving triterpenoids and polysaccharides in the solvent. Prasada et al. (2009) also found that a 50:1 liquid/solid ratio was the optimum ratio for dissolving the total phenolic contents in longan fruit pericarp, i.e., increasing the solvent further did not increase the extraction yield of the total phenolic compounds. A similar phenomenon was reported in a study by Zhang et al. (2005) who found that during the HPE of flavonoids from propolis, extraction yields increased as the liquid/solid ratio increased and reached a maximum at a liquid/solid ratio of 35:1 or more. When the liquid/solid ratio increased from 5:1 to 45:1 at 500 MPa for 1 min, the extraction yield of flavonoids increased from 4.19 to 5.25%. Xi (2009) reported that highest extraction yields (4.0%) were obtained at a liquid/solid ratio of 20:1 (mL/g) with 500 MPa pressure applied for 1 min. When the liquid/solid ratio increased from 10:1 to 25:1 (mL/g), the yield of caffeine increased from 3% to 4.11%. Prasad et al. (2009a, b) found that the HPE of the phenolic contents from litchi fruit pericarp was not affected by the solid/liquid ratio, as the extraction yields at solid/liquid ratios of 1:25, 1:50, 1:75, and 1:100 were not different (p < 0.05). However, Hu et al. (2015) demonstrated that the extraction yields of chlorogenic acid from Lonicera japonica flower buds using 70% alcohol and HPE were not affected by the liquid/solid ratio, whereas the extraction yield of cynaroside increased when the proportion of liquid increased, indicating that the dissolution rates of chemical components in solvents varied significantly.
Comparison of extraction yields among conventional, ultrasonic, and high-pressure-assisted methods
Table 2 shows the extraction yields obtained with the HPE, ultrasonic, and the traditional shake extraction methods. Extraction at 600 MPa for only 3 min resulted in the same similar concentrations of triterpenoids and adenosine as ultrasonic extraction for 60 min or shake extraction for 8 h. The concentrations of polysaccharides in extracts obtained with high-pressure or ultrasonic extraction were significantly higher than those obtained with shake extraction. These results confirmed that both the high-pressure and ultrasonic methods are effective in increasing the extraction yield and that the HPE method is the most efficient among the three extraction methods studied here. As indicated by the electron microscopy results (Fig. 4), both the ultrasonic and high-pressure methods can destroy the structure of A. cinnamomea mycelia. After the disruption of the mycelia, the extraction solvent permeates the cells rapidly and contacts the cellular contents, dissolving the active components in a short period of time. This result is consistent with the findings of Briones-Labarca et al. (2015), who compared the extraction yields of active ingredients from Chilean papaya seeds among high hydrostatic pressure, ultrasound, and conventional extraction methods. The results demonstrated that concentrations of total phenol and flavonoids in the extract after 5 min of extraction at 500 MPa were significantly higher than those in the extracts after 15 min of ultrasonic extraction (42 kHz, 130 W) and 30 min of conventional extraction (at 70 °C). In addition, the high-pressure extracts had a greater antioxidant capacity. Lee et al. (2014) utilized response surface methodology to identify the best conditions for HPE of saponins from ginseng. The optimum conditions for maximizing either extraction yield or crude saponin content were determined to be 446.46 MPa for 14.84 min and 450 MPa for 15 min. Corrales et al. (2009) also utilized high-pressure and ultrasonic methods to extract anthocyanin from grape byproducts and compared the two methods with conventional extraction. After 1 h of extraction, the extraction yields with the high-pressure and ultrasonic methods were three times and two times greater, respectively, than with conventional 70 °C extraction. However, Corrales et al. (2008) found that for the same extraction time, the yield of total phenolic compounds from grape byproducts with ultrasonic extraction (35 kHz) was significantly higher than those with HPE (600 MPa) and heat extraction (at 70 °C). However, the antioxidant capacity of the extracts from HPE was stronger than in the extracts from ultrasonic and thermal methods.
Inhibitory effects of extracts on ROS, NO, and PGE2 production
The inhibitory effects of A. cinnamomea extracts on the production of ROS and pro-inflammatory mediators such as NO and PGE2 were detected in the presence of LPS (1 μg/mL) for 24 h. During stimulation, a large increase in oxygen uptake resulted in the massive release of intracellular ROS in the cells treated with LPS (1 μg/mL), compared with untreated control cells. In Fig. 3a, the ROS inhibitory activity of the HPE and ultrasonic extracts at a concentration of 150 μg/mL were stronger than that of shake extracts. The HPE extracts at 50 μg/mL showed the same concentrations of ROS with shake extracts. However, ultrasonic extracts at 150 μg/mL induced a significant (p > 0.05) lower concentration of ROS than all treated group. The LPS-induced RAW 264.7 macrophages exhibited increased NO and PGE2 production when compared with the control group (Fig. 3a, b). The HPE and ultrasonic extracts exhibited an excellent inhibitory activity on the NO and PGE2 production compared with that of the shake extracts. However, the concentrations of NO and PGE2 treated by HPE extracts were lower than those in the cells treated with LPS only but showed no significant difference with the ultrasonic extracts. At a concentration of 150 μg/mL, the HPE extracts reduced the concentration of NO and PGE2 released from RAW 264.7 cells by 38.3% and 41.2%, respectively compared with the LPS group. Folk medicinal mushrooms are good resources for the development of therapeutic agents with antioxidant and anti-inflammatory potential that possess no or lower toxic effects. Inflammatory disorders are often characterized by the production of significant amounts of free radicals, nitrogen reactive species, and cytokines such as PGE2. Wu et al. (2011) reported that the extracts and metabolites isolated from A. cinnamomea exhibits free radical scavenging and superoxide radical scavenging activities. Wen et al. (2011) also found that methanol extract of liquid cultured mycelia of A. cinnamomea had significant anti-inflammatory activity by reducing the edema volume in carrageenan-induced paw edema in mice. In this study, the HPE extracts showed an increased polysaccharide content recovery with shorter extraction time that was approximately twice that of the shake extracts. The results indicate that exposure of RAW 264.7 cells to LPS apparently stimulated the accumulation of ROS, NO and PGE2 while the pretreatment of cells with HPE extracts significantly attenuated this effect. The HPE method promoted the antioxidant and anti-inflammatory activity of the extracts by increasing the amount of bioactive compounds extracted with shorter extraction time.
Observation by SEM
To understand the mechanism through which high-pressure and ultrasonic extraction affects extraction yields, the morphologic changes of A. cinnamomea after different methods of extraction were studied using SEM. Figure 4a shows the morphology of A. cinnamomea mycelia in the control group, with intact tissues. Figure 4c shows mycelia after 60 min of ultrasonic extraction, and Fig. 4d shows mycelia after 3 min of HPE at 600 MPa. Figure 4b shows the morphology of A. cinnamomea mycelia treated by shake extraction, which is similar to the morphology of the control group. Ultrasonic energy exerts strong shearing forces that can damage extraction samples. Figure 4c shows mycelia that are crushed and have fractures on the surface after ultrasonic extraction, whereas in Fig. 4d, mycelia exhibit irregular distortions, flattening, folding, and surface ruptures after high-pressure treatments, indicating that both ultrasonic and high-pressure treatments can damage the structure of mycelia. Physical damage to the morphology of extraction samples allows better penetration of the extraction solvent into cells, and the improved interaction between solute and solvent can in turn promote the dissolution of active ingredients and shorten the extraction time (Xi 2015). Chen et al. (2009) used an SEM to observe the morphology of ginseng samples after 5 min of pressure treatment at 200 MPa and found that the high pressure ruptured the ginseng cell wall, which led to the increased dissolution of intracellular active components by the solvent and an increased extraction yield of saponins from ginsenosides that was equal to the yield obtained by heat extraction at 90 °C for 6 h. Xi et al. (2011) used transmission electron microscopy to observe the morphological changes in green tea samples after 400 MPa of pressure treatment for 15 min. They found that the high pressure damaged the structure of cells and caused twisting, distortion, and breakage, which promoted the dissolution of the active components in the solvent.
Conclusion
In this study, the SEM micrograph data indicated that the HPE technique could cause physical damage to the cell structures of mycelia, resulting in better solvent penetration into cells and increased contact between solvent and solute, which is the main mechanism for increasing extraction yields. Compared with other techniques such as ultrasound extraction or shake extraction, extraction at 600 MPa of pressure takes only 3 min to achieve the extraction concentration obtained by ultrasonic extraction for 1 h or shake extraction for 8 h. The HPE technique, with its shortened extraction time and reduced energy consumption, is the most efficient extraction method. Thus, HPE could be an alternative extraction technique for the fast extraction of active compounds from A. cinnamomea. Hence, HPE is a potential technology that can be used in the food and biotechnology industries for the extraction of active components.
References
Briones-Labarca V, Plaza-Morales M, Giovagnoli-Vicuña C, Jamett F (2015) High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from Chilean papaya (Vasconcellea pubescens) seeds: effects of extraction conditions and methods. LWT Food Sci Technol 60:525–534
Chang TT, Chou WN (1995) Antrodia cinnamomea sp. nov. on Cinnamomum kanehirai in Taiwan. Mycol Res 99:756–758
Chang CY, Leu MY, Pan TM (2005) Determination of adenosine, cordycepin and ergosterol contents in cultivated Antrodia camphorata by HPLC method. J Food Drug Anal 13:338–342
Chang CJ, Lu CC, Lin CS, Martel J, Ko YF, Ojcius DM, Wu TR, Tsai YH, Yeh TS, Lu JJ, Lai HC, Young JD (2018) Antrodia cinnamomea reduces obesity and modulates the gut microbiota in high-fat diet-fed mice. Int J Obes 42:231–243
Chen R, Meng F, Zhang S, Liu Z (2009) Effects of ultrahigh pressure extraction conditions on yields and antioxidant activity of ginsenoside from ginseng. Sep Purif Technol 66:340–346
Cheng JJ, Huang NK, Lur HS, Kuo CI, Lu MK (2009) Characterization and biological functions of sulfated polysaccharides from sulfated-salt treatment of Antrodia cinnamomea. Process Biochem 44:453–459
Corrales M, Toepfl S, Butz P, Knorr D, Tauscher B (2008) Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: a comparison. Innov Food Sci Emerg Technol 9:85–91
Corrales M, García AF, Butz P, Tauscher B (2009) Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J Food Eng 90:415–421
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Geethangili M, Tzeng YM (2011) Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid Based Complement Altern Med 2011:1–17
Hu W, Guo T, Jiang WJ, Dong GL, Chen DW, Yang SL, Li HR (2015) Effects of ultrahigh pressure extraction on yield and antioxidant activity of chlorogenic acid and cynaroside extracted from flower buds of Lonicera japonica. Chin J Nat Med 13:445–453
Huang HW, Hsu CP, Yang BB, Wang CY (2013) Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci Technol 33:54–62
Lee AR, Choi SH, Choi HW, Ko JH, Kim W, Kim DO, Kim BY, Baik MY (2014) Optimization of ultra high pressure extraction (UHPE) condition for puffed ginseng using response surface methodology. Food Sci Biotechnol 23:1151–1157
Liang MT, Liang RC, Huang LR, Liang KY, Chien YL, Liao JY (2014) Supercritical fluids as the desorbent for simulated moving bed-application to the concentration of triterpenoids from Taiwanofugus camphorate. J Taiwan Inst Chem Eng 45:1225–1232
Lien HM, Chiu CH, Chen CC, Chang WL, Chyau CC, Peng RY (2014) Comparison of the apoptotic effects of supercritical fluid extracts of Antrodia cinnamomea mycelia on hepatocellular carcinoma cells. Molecules 19:9033–9050
Lu ZM, Gong JS, He Z, Xu HY, Dou WF, Shi JS (2011) Optimization of extraction of total triterpenoids from submergedly cultured Antrodia camphorata using response surface methodology. Nat Prod Res 23:946–951
Ma TW, Lai Y, Yang FC (2014) Enhanced production of triterpenoid in submerged cultures of Antrodia cinnamomea with the addition of citrus peel extract. Bioprocess Biosyst Eng 37:2251–2261
Naghshineh M, Olsen K, Georgiou CA (2013) Sustainable production of pectin from lime peel by high hydrostatic pressure treatment. Food Chem 136:472–478
Prasad KN, Hao J, Shi J, Liu T, Li J, Wei X, Qiu S, Xue S, Jiang Y (2009a) Antioxidant and anticancer activities of high pressure-assisted extract of longan (Dimocarpus longan Lour.) fruit pericarp. Innov Food Sci Emerg Technol 10:413–419
Prasad KN, Yang B, Zhao M, Wang BS, Chen F, Jiang Y (2009b) Effects of high-pressure treatment on the extraction yield, phenolic content and antioxidant activity of litchi (Litchi chinensis Sonn.) fruit pericarp. Int J Food Sci Technol 44:960–966
Prasada KN, Yang B, Zhao M, Wei X, Jiang Y, Chen F (2009) High pressure extraction of corilagin from longan (Dimocarpus longan Lour.) fruit pericarp. Sep Purif Technol 70:41–45
Tu WC (2008) Comparison of techniques for the extraction of active components from timber cultivated or media cultivated Antrodia camphorate. Master thesis, Department of Bioindustry Technology, Dayeh University, Taiwan
Wang L, Weller C (2006) Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol 17:300–312
Wen CL, Chang CC, Huang SS, Kuo CL, Hsu SL, Deng JS, Huang GJ (2011) Anti-inflammatory effects of methanol extract of Antrodia cinnamomea mycelia both in vitro and in vivo. J Ethnopharmacol 137:575–584
Wu YS, Chen SN (2016) Extracted triterpenes from Antrodia cinnamomea reduce the inflammation to promote the wound healing via the STZ inducing hyperglycemia-diabetes mice model. Front Pharmacol 7:154–164
Wu MD, Cheng MJ, Wang WY, Huang HC, Yuan GF, Chen JJ, Chen IS, Wang BC (2011) Antioxidant activities of extracts and metabolites isolated from the fungus Antrodia cinnamomea. Nat Prod Res 25:1488–1496
Xi J (2006) Effect of high pressure processing on the extraction of lycopene in tomato pastewaste. Chem Eng Technol 29:736–739
Xi J (2009) Caffeine extraction from green tea leaves assisted by high pressure processing. J Food Eng 94:105–109
Xi J (2013) High-pressure processing as emergent technology for the extraction of bioactive ingredients from plant materials. Crit Rev Food Sci Nutr 53:837–852
Xi J (2015) Ultrahigh pressure extraction of bioactive compounds from plants—a review. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2013.874327
Xi J, Shen D, Li Y, Zhang R (2011) Micromechanism of ultrahigh pressure extraction of active ingredients from green tea leaves. Food Control 22:1473–1476
Xi J, He L, Yan L (2015) Kinetic modeling of pressure-assisted solvent extraction of polyphenols from green tea in comparison with the conventional extraction. Food Chem 166:287–291
Zhang S, Xi J, Wang C (2005) High hydrostatic pressure extraction of flavonoids from propolis. J Chem Technol Biotechnol 80:50–54
Zhang S, Chen R, Wang C (2007) Experiment study on ultrahigh pressure extraction of Ginsenosides. J Food Eng 79:1–5
Zhang Y, Wang Z, Li D, Zang W, Zhu H, Wu P, Mei Y, Liang Y (2018) A polysaccharide from Antrodia cinnamomea mycelia exerts antitumor activity through blocking of TOP1/TDP1-mediated DNA repair pathway. Int J Biol Macromol 120:1551–1560
Zhao SS, Leung SY (2010) Quality evaluation of mycelial Antrodia camphorata using high-performance liquid chromatography (HPLC) coupled with diode array detector and mass spectrometry (DAD–MS). Chin Med 5:4–9
Zhu Q, Liu F, Xu M, Lin X, Wang X (2012) Ultrahigh pressure extraction of lignan compounds from Dysosma versipellis and purification by high-speed counter-current chromatography. J Chromatogr B 905:145–149
Acknowledgements
This research work was supported by the Ministry of Science and Technology, MOST 107-2221-E-002-110-MY2, Taiwan, Republic of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, HW., Chen, BY. & Wang, CY. Extraction of bioactive ingredients from fruiting bodies of Antrodia cinnamomea assisted by high hydrostatic pressure. J Food Sci Technol 56, 3988–3997 (2019). https://doi.org/10.1007/s13197-019-03867-7
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03867-7