Abstract
Peanut oil is widely used in food but is susceptible to oxidation. This study investigated the antioxidant stability of high oleic (HO: 78.85 g/100 g oleic acid) and regular (C: 43.85 g/100 g oleic acid) peanut oils with oregano essential oil (OEO) added as a natural antioxidant. OEO contained γ-terpinene (25.71%), carvacrol (16.73%) and terpinen-4-ol (16.17%) as the principal compounds. Thermal processing (60 °C for 28 days) of OEO increased the carvacrol and o-cymene contents and decreased the terpinen-4-ol, linalool and γ-terpinene levels. Thus, carvacrol was the major compound with high oxidative stability. Thermal processing of the peanut oils showed that HO peanut oil developed less oxidation than C peanut oil. OEO provided antioxidant activity, which increased as its concentration increased (at 0.02 and 0.10% p/p of OEO, the peroxide value decreased by 18 and 46%, respectively). OEO displayed 54.7% free radical scavenging activity and 9.2 mg/g total phenolic content, explaining its antioxidant activity. Sensory analysis showed that OEO was detected in all samples, but consumer acceptance was greater when OEO was present (hedonic values of 7.4 and 6.8 for OEO at 0.02 and 0.10 g/100 g, respectively) compared to the peanut oil only control (hedonic value of 6.0).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Peanuts (Arachis hypogaea L.) are a major crop worldwide, with a production of around 29 million metric tonnes per year (Wang et al. 2017). Some of the peanuts produced are intended for manufacturing vegetable oil. In Argentina, conventional peanuts (normal Runner-type) are being replaced with high oleic (HO) peanut cultivars, due to an increase in the agricultural production of HO peanuts since 2010 (Nepote et al. 2009). These HO cultivars present more oleic fatty acid (around 80 g/100 g) and less linolenic acid than conventional peanuts (about 45 g/100 g oleic acid). As already known, oleic acid is highly stable against lipid oxidation compared to linolenic acid (Roginsky and Lissi 2005). Nonetheless, fatty acids are susceptible to oxidation processes, generating a decrease in nutritional and chemical quality. The lipid oxidation products can modify the colour, flavour, texture and safety of food. Furthermore, chemical compounds formed by lipid oxidation, such as free radicals, can contribute to the formation of diabetes, cardiovascular diseases and cancer (Kalyanaraman 2013).
The oxidation process is primarily generated during storage of raw peanuts and continues in processed foods, and it is markedly enhanced by thermal processing. Antioxidants are used to reduce this oxidation process in food. Synthetic antioxidants, like butylated hydroxytoluene (BHT), tert-butylhydroquinone and butylated hydroxyanisole, are principally used by the food industry. However, concern over the use of synthetic additives is growing, due to their potential toxic health hazards (Cömert and Gökmen 2018).
A possible solution to replace synthetic antioxidants is naturally sourced antioxidants, such as essential oils (EOs) from aromatic plants. Based on the oxidative stability index, Bendini et al. (2002) showed that extracts of oregano leaves had an antioxidant effect on peanut oil, as an oxidation lipid model. The authors used ethanol, diethyl ether, 4:1 n-hexane:2-propanol (v/v) and n-pentane as extraction solvents and found that the extracts showed antioxidant activity in a range from 0.02 to 5% w/w. According to the extraction method, different kinds of compounds were obtained, such as flavonols, flavanones, dihydroflavonols (ethanolic extract), polyphenols and components from EOs. When EOs were obtained by hydrodistillation using the Clevenger-type apparatus, there were no other kinds of compounds besides flavonols and phenolic compounds, among others, with high molecular weight. Other authors have highlighted the antioxidant properties of EOs extracted by hydrodistillation of aromatic species, like oregano (Origanum vulgare L.), rosemary (Rosmarinus officinalis) and laurel (Laurus nobilis) (Quiroga et al. 2011; Olmedo et al. 2015). In addition, oregano essential oil (OEO) and olive oil protected against lipid oxidation of fried peanuts (Olmedo et al. 2009). The above-described studies performed by Bendini et al. (2002) and Olmedo et al. (2009) were made on normal peanuts (non-HO). However, the potential benefit of combining HO oil and OEO as an antioxidant agent against lipid oxidation has not yet been investigated.
Natural compounds in aromatic plants have some advantages over their synthetic counterparts, as they are accepted by and considered safe for consumers, do not require safety testing by legislation and are derived from natural resources with a well-established culinary use (Raut and Karuppayil 2014).
Diverse systems and tests exist to evaluate the antioxidant properties of natural compounds (Nikolic et al. 2014). Consequently, the result of one simple test may provide limited or inaccurate information about the antioxidant activity, particularly, for complex matrices, such as food. EOs possess a complex mixture of chemical compounds with diverse functional groups, chemical characteristics and polarity. Hence, antioxidant results should be interpreted with caution, and multiple tests and food matrices should be considered (Amorati et al. 2013). Indirect methods for estimating the antioxidant activity, such as free radical scavenging activity (FRSA) and total phenolic content (TPC), are based on the ability of the compound to donate electrons or hydrogen atoms (H-donor capacity). Conversely, direct methods are based on measuring the formation of chemical and volatile oxidation compounds (Roginsky and Lissi 2005). Other technologies, such as thermogravimetric analysis, can also be used to determine the deterioration of food systems. These analyses are based on the change of mass from food exposed to different time/temperature combinations and allow quantifying the weight loss caused by the deterioration of the food (Chen et al. 2017). However, this technology exceeded the purpose of the current research that was focused on analysing the oxidation process, by measuring chemical indicators related to the oxidative deterioration of HO peanut oil and the influence of OEO, added as a natural antioxidant.
Materials and methods
Peanut oil extraction and fatty acid profile
Kernels of blanched peanuts (Runner-type, HO) and regular/commercial (C) peanuts, size 38/42 kernels per ounce (2016 crop) were provided by Lorenzati, Ruetsch and Cia SA (Ticino, Córdoba, Argentina). Peanuts were inspected, and any damaged and bruised kernels were manually removed before oil extraction. The peanut oil was obtained by cold pressing seed peanut using a 20-tonne press (HE-DU, Hermes I. Dupraz e Hija SRL, Córdoba, Argentina). Fatty acid methyl esters (FAMEs) were produced by transmethylation with a methanolic solution of sulphuric acid. The FAMEs from both seeds were analysed using a Perkin-Elmer® Clarus 600 gas chromatograph (Shelton, CT, USA) equipped with a flame ionisation detector and an Elite Wax capillary column (60 m × 0.25 mm × 0.25 µm). The conditions for chromatographic separation and the identification and quantification of the FAMEs were performed according to Grosso et al. (2000). Iodine value (IV) was calculated using the following formula: IV = (%C16:1 × 0.9983) + (%C18:1 × 0.8601) + (%C18:2 × 1.7321) + (%C18:3 × 2.7410) + (%C20:1 × 0.7854). The oleic/linoleic ratio (O/L) and saturated/unsaturated ratio were also calculated.
Oregano essential oil (OEO): extraction and chemical composition
Leaves of oregano (O. vulgare spp.) were collected from the experimental area of Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba (FCA-UNC), Argentina (April 2016), and an exemplar was deposited in the ACOR herbarium (FCA-UNC). OEO was obtained by hydrodistillation and stored with sodium sulphate in a glass vial at − 18 °C until use (Olmedo et al. 2015).
OEO composition was determined using a Perkin-Elmer® Clarus 600 gas chromatography-mass spectrometry (GC–MS) instrument equipped with a DB-5 capillary column (30 m × 0.25 µm). The following conditions were used: helium as carrier gas with a 0.9 mL/min flow rate; temperature program: 40 °C for 5 min, then ramped at 10 °C/min until 100 °C, and finally, ramped at 15 °C/min until 245 °C; detector and injector temperatures set at 280 and 260 °C, respectively; electron impact ionisation at 70 eV; mass spectral data acquired in scan mode (mass interval m/z 35–450). The compounds were identified by comparing the retention time (Adams 1995) and mass spectra of the main components available in NIST libraries with the injection of standards (Sigma®, St. Louis, MO, USA). Results were expressed as g/100 g, from triplicate analyses (Olmedo et al. 2014).
Oregano essential oil (OEO) thermal stability
For determining the thermal stability of OEO, a 10 µL aliquot was placed in a glass flask (capacity 10 mL), sealed with a rubber lid, stored at 60 °C and samples removed at 0, 14 and 28 days. Volatiles were captured using a solid-phase microextraction fibre (SPME) of polydimethylsiloxane/divinylbenzene (PDMS/DVB) introduced in the flask, heated at 70 °C for 20 min and then injected into the GC–MS. The chromatographic conditions and compound identification and quantification were undertaken, according to Olmedo et al. (2015).
Indirect antioxidant activity: total phenolic content (TPC) and free radical scavenging activity (FRSA)
The TPC was determined by spectrophotometry using gallic acid as the standard, according to the Folin–Ciocalteu method (Olmedo et al. 2014). Dry oregano leaves (200 mg) were extracted with 5 mL deionised water (40 mg of dry weight/mL) at room temperature for 24 h, and then, filtrated and collected. The results were expressed as mg gallic acid equivalents (GAE)/g dry leaf weight.
The FRSA was calculated as described by Choi et al. (2000) with modifications. OEO was mixed with 1,1-diphenyl-2-picrylhydrazyl (DPPH) in ethanol and the spectrophotometric absorbance at 517 nm measured after 30 min. The result was expressed as DPPH percentage inhibition (Olmedo et al. 2014).
Oxidation test: thermal acceleration
OEO was mixed with HO and regular oleic (C) peanut oils at 0.02 and 0.10 g/100 g for the thermal oxidation process. BHT (0.02 g/100 g) was used as the reference antioxidant. The samples obtained were termed HO (HO peanut oil, control), HO.10 (HO peanut oil with 0.10 g OEO/100 g), HO.02 (HO peanut oil with 0.02 g OEO/100 g), HB (HO peanut oil with 0.02 g BHT/100 g), C (commercial/regular peanut oil, control), C.10 (commercial-regular peanut oil with 0.10 g OEO/100 g), C.02 (commercial/regular peanut oil with 0.02 g OEO/100 g), and CB (commercial/regular peanut oil with 0.02 g BHT/100 g). All samples were stored in an oven at 60 °C for 28 days, along with replacement samples at 0, 7, 14, 21 and 28 days, as detailed by Olmedo et al. (2015). Oxidation was evaluated by measurement of several chemical and volatile indicators.
Chemical indicators: The peroxide value (PV), anisidine value (AV), conjugated dienes (CD), and total oxidation (totox) value (TV: 2 × PV [+ AV]) were measured as the chemical oxidation indicators (Olmedo et al. 2014).
Volatile indicators: A SPME fibre of PDMS/DVB was introduced into a glass flask (50 mL) containing 10 g of sample and the flask heated at 130 °C for 20 min (Olmedo et al. 2015). The fibre was injected into the GC–MS for 1 min, under the same chromatographic conditions described in Sect. 2.2. Hexanal and 2-heptenal were identified with standards (Sigma®). Acetaldehyde (Sigma®) was used as the internal standard. The volatile compounds were expressed as µg/g (ppm).
Sensory analysis: discriminative and affective evaluations
Discriminative evaluation
A panel of 10 semi-trained panellists (8 females and 2 males) was prepared to identify samples with OEO in vegetable oil by using the duo–trio test. Samples were presented in three rounds for all panellists, and the combination between samples was modified in each round. A comparative duo–trio test was carried out between C–HO, C–C.0.02, and C.02–C.10 samples. BHT did not modify the sensory properties of the vegetable oil, and therefore, it was not necessary to test these samples. A slice of bread (size 2 × 2 cm) was presented to all panellists with 0.5 mL of the samples. Samples were coded with a random three-digit number, and the reference sample was identified with the letter “R”, according to Asensio et al. (2013).
Affective evaluation
The affective test was made using a 9-point hedonic scale, ranging from dislike extremely (1) to like extremely (9) (Asensio et al. 2013). Each treatment was assigned a random three-digit number. Non-trained consumers (n = 77; age: 18–54 years) were invited to participate in the analysis. Samples evaluated were C, HO, C.02 and C.10.
Statistical analysis
All experiments were carried out in three repetitions. The results were analysed using Infostat software, version 1.1 (FCA-UNC). Means and standard deviations were calculated. Analysis of variance was used to detect significant differences in the chemical data between sampling days, and Fisher’s LSD test (α = 0.05) was performed to identify significant differences between means. Principal component analysis (PCA) was done to evaluate the association between chemical and volatile oxidation variables, with respect to all treatments.
Results and discussion
Fatty acid composition of peanut oils
Table 1 presents the fatty acid compositions of HO and C peanut oils. The main difference between the oils was the oleic and linoleic acid percentages. As expected, the HO peanut oil possessed 80% more oleic acid (78.85 g/100 g) than C peanut oil (43.84 g/100 g) and, consequently, a greater O/L ratio (13.1) than C peanut oil (1.2). This marked difference in O/L ratio indicates that HO peanut oil is more resistant against lipid oxidation in comparison with C peanut oil. Nepote et al. (2009) found similar values in comparative cultivars (Tegua and Granoleico). Another study found 44.78–82.17 g oleic acid/100 g and 2.85–33.92 g linoleic acid/100 g in 10 commercial Runner peanut cultivars in the USA (Shin et al. 2010), and classified HO peanut oils as having 69.98–82.17 g oleic acid/100 g and 2.85–11.47 g linolenic acid/100 g. Shin et al. (2010) defined C, mid-oleic and HO peanut oils as having O/L ratios of 1.0–1.5, 1.5–9.0 and > 9.0, respectively. In the current study, besides having a lower O/L ratio, C oil had a higher IV and saturated/unsaturated fatty acid ratio than HO oil, further suggesting that the C oil is more susceptible to lipid oxidation processes than those with HO composition. Accordingly, Nepote et al. (2009) recorded higher chemical oxidation indicator values in Tegua roasted peanuts (C peanuts) compared to Granoleico (HO cultivar), due to their different fatty acid profiles.
Chemical composition of oregano essential oil (OEO)
Table 2 shows OEO contained γ-terpinene (25.71 g/100 g), carvacrol (16.73 g/100 g), and terpinen-4-ol (16.17 g/100 g) as the principal compounds. These three components represented 58.6% of the total EO and, probably, contributed most to the chemical action observed, given they are antioxidant molecules that provide an antioxidant effect in oil samples (Olmedo et al. 2014). The chemical composition of OEO is highly variable because it depends on many factors associated with the plant from which it is derived, such as geographic location, genetics, environment and stress. Dambolena et al. (2010) documented differences in the EO composition of oregano populations cultivated in a similar location. The major compounds were trans-sabinene hydrate (27.7–36.7 g/100 g) and thymol (17.7–30.8 g/100 g). In four different oregano varieties from Argentina (Medocino, Compacto, Criollo and Cordobés), Quiroga et al. (2011) found that the main EO compounds were trans-sabinene hydrate, thymol and γ-terpinene, with variations between 6.3–38.2, 14.9–29.7 and 5.1–18.2 g/100 g, respectively. These findings evidence the variability among oregano samples, even those from similar locations, and the environmental influences.
Oregano essential oil (OEO) thermal stability
Based on the SPME analysis, the main volatiles present in OEO were terpinen-4-ol (27.62 g/100 g), carvacrol (21.91 g/100 g) and linalool (12.81 g/100 g), which together represented 62% of the volatiles detected by SPME. Terpinel-4-ol and carvacrol are the main molecules responsible for the oregano taste, explaining their predominance in OEO (Quiroga et al. 2011). However, the chemical composition was influenced by the methods of determination. Compared to SPME, simple injection presented lower values for terpinen-4-ol and carvacrol, and the inverse order of magnitude of these two compounds (Table 2).
Regarding thermal stability after oven storage, the principal changes occurred in five compounds. Carvacrol (31.4%) and o-cymene (609%) showed the greatest increase, rising from 21.91 to 28.79 and 1.82–12.91 g/100 g, at storage day 0 and 28, respectively). Terpinen-4-ol (− 20.3%), linalool (− 120.5%) and γ-terpinene (− 239.6%) had the highest decreases (from 27.62 to 22.95, 12.81− 5.81 and 11.58− 3.41 g/100 g, at storage day 0 and 28, respectively). These changes can be attributed to the boiling point of the individual components. Those with a relatively low boiling point are less thermostable and thereby undergo more thermal decomposition than those with a high boiling point (Olmedo et al. 2014). In addition, compositional changes in component proportions verified that carvacrol is the main responsible for the antioxidant effect because carvacrol presented the greatest concentration at the end of storage (day 28). Similar results were observed in previous research (Olmedo et al. 2015), where terpinen-4-ol and linalool decreased from 29.5 to 26.0 and 13.1–5.7 g/100 g, after 28 storage days, respectively. Additionally, carvacrol and o-cymene increased from 20.4 to 26.4 and 1.94–13.13 g/100 g, respectively (Olmedo et al. 2015). γ-Terpinene demonstrated a similar behaviour to that reported by Olmedo et al. (2015) (decreased from 1.3 to 0.5 g/100 g at 0 and 28 storage days, respectively), but the decrease in its proportion was higher in the current research (8.2 vs. 0.8 g/100 g). This trend was observed in other research during 14 storage days (60 °C and similar conditions), confirming the changes caused by the temperature (Olmedo et al. 2014).
Indirect antioxidant activity: TPC and FRSA
As mentioned in Sect. 1, EOs from different kinds of culinary, aromatic plants have shown antioxidant activity (Bajalan et al. 2017; Olmedo et al. 2015; Quiroga et al. 2011). Table 2 documents the TPC and FRSA values found in the current study. OEO had 54.7% FRSA and a TPC of 9.2 mg GAE/g. Both indicators explain the antioxidant activity of OEO because the antiradical activity (FRSA) is mediated by the capacity of the compounds to donate electrons or hydrogen atoms. Phenols are molecules with H-donor ability, and the TPC is often correlated to the FRSA. Skotti et al. (2014) found a correlation coefficient of 0.979 for TPC and FRSA (DPPH assay). The high TPC concentration recorded for the oregano was comparable to values reported in the literature. For instance, Dambolena et al. (2010) analysed the EOs from 40 different oregano species and recorded 8.84–19.36 mg GAE/g for the TPC and 17.5–75.3% for the FRSA. Olmedo et al. (2014) documented a higher FRSA (84.1% inhibition) than the values obtained in the current research while the TPC (11.1 mg GAE/g) was similar. These values indicated that OEO had H-donor activity and this contributed to its antioxidant activity.
Chemical oxidation indicators
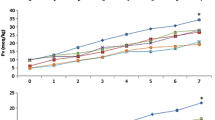
Figure 1a–d, respectively provides the PV, AV, CD and TV. For all chemical oxidation indicators, HO peanut oil showed a better resistant against oxidation relative to C peanut oil. Generally, the samples with OEO had less oxidation than the control samples. C had the highest PV, AV, CD and TV (82.97, 76.31, 17.25 and 242.26, respectively) while HO.0.10 possessed the lowest PV and TV (36.90 and 113.21 meqO2/kg, respectively) and HB the lowest CD value (6.41) (p < 0.05). All chemical oxidation indicators increased during storage. However, the protective effect of the OEO and the improved stability of HO versus C peanut oils were evident, as also demonstrated in earlier research (Olmedo et al. 2015). Talcott et al. (2005) determined PVs of 24, 10 and 4 meqO2/kg in dry roasted C, mid-oleic and HO peanuts, respectively, stored at 35 °C for 4 months, evidencing the oxidation resistance of HO peanuts, as supported by the present investigation. Likewise, Nepote et al. (2009) noted a positive correlation between the oxidative stability of peanuts and their O/L ratio. The chemical indicators show that the fatty acid composition plays an important role in chemical oxidation, and the use of natural compounds with antioxidant activity can increase the stability of the vegetable oils. Natural antioxidants with H-donor or electron-donating capacities, such as OEO, scavenge radicals and break the free radical chain oxidation, generating a lower PV than the control sample. Donor-H compounds stabilise lipid radicals by contributing an H atom or electron, transforming the radical into a more stable form, while the donor-H compounds themselves become stable radicals (Roginsky and Lissi 2005).
Volatile oxidation compounds
Odour-active monocarbonyl compounds are generated when unsaturated fatty acids undergo auto-oxidation. In peanuts, the main oxidation compounds are produced from the oxidation of linolenic acid. Belitz et al. (2009) evidenced an uptake of 0.5 mol O2/mole fatty acid during autoxidation of sunflower oil (1 g at 20 °C) and detected hexanal as the most dominant compound in the volatile fraction, followed by 2-octenal and 2-heptenal (5100, 990 and 450 µg/g, respectively). Some chemical oxidation indicators reached a higher concentration in C than in the HO peanut oils (Fig. 2). C had the highest hexanal (158.76 µg/g) and 2-heptenal (30.38 µg/g) contents, which were significantly different to the other samples (Fig. 2a, b). Both compounds were the most representative in the volatile fraction; other compounds in the sample had a low concentration or were not quantifiable. HB showed the best protection against the formation of volatile oxidation compounds in the current study, being significantly different to the other samples (83.83 µg hexanal/g and 16.06 µg 2-heptenal/g). The presence of OEO decreased the volatile oxidation compounds formed in the accelerated oxidation. The OEO was most effective against lipid oxidation when used in combination with HO peanut oil. However, the EO provided better protection to peanut oil C.10 than HO, but similar to HO.02 (hexanal, Fig. 2a). Olmedo et al. (2015) noticed a similar behaviour between sunflower oil samples with and without (oil only) OEO. In that study, the control (sunflower oil only) and sunflower oil with 0.02% OEO reached 81 and 35 µg hexanal/g and 25 and 15 µg 2-heptenal/g, respectively (Olmedo et al. 2015).
Multivariate analysis: PCA
When analysing data from an experiment with multiple variables and treatments, it is often challenging to visualise which of the treatments are the best. Thus, PCA was performed, presenting the chemical oxidation variables (PV, AV, CD and TV) and volatile oxidation variables (hexanal and 2-heptenal) for all treatments, in a biplot with two axes (Fig. 3). The sum of both axes explained 97.9% data variation, which is beyond the minimum 70% defined as satisfactory. The x-axis was the most important, explaining 87.1% variability, where all oxidation variables were located on the positive x-axis. Sample HB was opposite to the oxidation variables (negative side of the x-axis) and showed the best protection against oxidation. OEO displayed a protective oxidation activity, which was augmented by the increase in EO concentration. In contrast, sample C was strongly associated with the oxidation variables. Construction of a minimum path tree indicated the HO, HB, HO.01 and HO.02 were highly related to each other but less so with C. Thus, the use of antioxidants (natural or synthetic) is relevant to lipid oxidation, but the matrix in which the antioxidant is used is also fundamental (fatty acid profile).
Amorati et al. (2013) explained that terpenoids (components in EOs) co-oxidised with food lipids and the antioxidant mechanism did not involve a chain breaking effect, but instead the oxidation of the terpenoids increased the rate of the termination step in the oxidation process. This increase was called “termination-enhancing”.
Discriminative and affective sensory analysis
Discriminative analysis using the duo-trio method for the combination C–HO, C–C.02 and C.02–C.10 revealed all the panellists found a significant difference between the samples with OEO (i.e., C–C.02 and C.02–C.10) but not between C and HO peanut oils. OEO endowed an odour to the oil samples, which was detected by the semi-trained judges, particularly as the OEO concentration was increased. Although the odour properties of the peanut oils were affected by the added OEO, they were not influenced by the differences in the fatty acid profiles of the peanut oils.
The discriminative analysis showed that OEO modified the odour properties of the peanut oils, yet, it did not inform whether the modification affected the consumer acceptance value assigned to peanut oil. Therefore, hedonic acceptance of the samples C, HO, C.02 and C.10 was measured, with scores of 6.0 ± 0.4, 5.9 ± 0.5, 7.4 ± 0.3 and 6.8 ± 0.2, respectively. Samples C and HO were not significantly different because the fatty acid profile did not affect consumer acceptance. The O/L ratio, altered in HO and C, did not impact on the odour properties. Samples with OEO were more accepted by the consumers than samples without OEO, but as the concentration of EO increased, the acceptance decreased (p < 0.05). Samples with 0.10% OEO scored less for acceptance compared to C.02, with 0.02% OEO. Asensio et al. (2013) observed that olive oil samples incorporated with OEO had higher acceptance values (hedonic values of 6.1) than the control (hedonic value of 5.6). These acceptance values were lower relative to those found in the current research, but the difference can be explained by the vegetable oil used. In the present work, the sensory analysis showed that OEO modified the odour profile of peanut oil (discriminative test), but this change was positive for the consumer acceptability.
Conclusion
The fatty acid profile of the vegetable oil and the addition of OEO influence the resistance of peanut oil to lipid oxidation and increase its shelf life. HO peanut oil has a fatty acid profile with a strong resistance to lipid oxidation, while the OEO affords antioxidant properties. OEO modifies the odour properties of peanut oil, but the resultant samples show good consumer acceptance. The use of OEO in peanut oil decreases lipid oxidation in both HO and C peanut oils. Considering the results of this research, OEO can be used to replace synthetic antioxidants, like BHT.
References
Adams RP (1995) Identification of essential oils by ion trap mass spectroscopy. Allured Press, Carol Stream
Amorati R, Foti MC, Valgimigli L (2013) Antioxidant activity of essential oils: review. J Agric Food Chem 61:10835–10847
Asensio CM, Nepote V, Grosso NR (2013) Consumers’ acceptance and quality stability of olive oil flavoured with essential oils of different oregano species. Int J Food Sci Technol 48:2417–2428
Bajalan I, Rouzbahani R, Pirbalouti AG, Maggi F (2017) Antioxidant and antibacterial activities of the essential oils obtained from seven Iranian populations of Rosmarinus officinalis. Ind Crops Prod 107:305–311
Belitz HD, Grosch W, Schielberle P (2009) Food chemistry. Springer, Berlin
Bendini A, Toschi TG, Lercker G (2002) Antioxidant activity of oregano (Origanum vulgare L.) leaves. Ital J Food Sci 14(1):17–24
Chen J, Wang Y, Lang X, Ren X, Fan S (2017) Comparative evaluation of thermal oxidative decomposition for oil-plan residues via thermogravimetric analysis: thermal conversion characteristics, kinetics, and thermodynamics. Bioresour Technol 243:37–46
Choi HS, Song HS, Ukeda H, Sawamura M (2000) Radical scavenging activities of citrus essential oils and their compoents. Detection using 1,1 diphenyl-2-picrylhydrazyl. J Agric Food Chem 48:4156–4161
Cömert ED, Gökmen V (2018) Evolution of food antioxidants as a core topic of food science for a century. Food Res Int 105:76–93
Dambolena J, Zunino MA, Lucini EI, Olmedo RH, Banchio E, Bima P, Zygadlo JA (2010) Total phenolic content, radical scavenging properties and essential oil composition of origanum species from different populations. J Agric Food Chem 58:1115–1120
Grosso NR, Nepote V, Guzman CA (2000) Chemical composition of some wild peanut species (Arachis L.) seeds. J Agric Food Chem 48:806–809
Kalyanaraman B (2013) Teaching the basics of redox biology to medical and graduate students: oxidants, antioxidants and disease mechanisms. Redox Biol 1:244–257
Nepote V, Olmedo RH, Mestrallet MG, Grosso NR (2009) A study of the relationships among consumer acceptance, oxidation chemical indicators, and sensory attributes in High-Oleic and Normal Peanuts. J Food Sci 74(1):1–8
Nikolic M, Glamoclija J, Ferreira ICFR, Calhelha RC, Fernandes A, Markovic T, Markovic D, Giweli A (2014) Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L. Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind Crops Prod 52:183–190
Olmedo RH, Asensio C, Nepote V, Mestrallet MG, Grosso NR (2009) Chemical and sensory stability of fried-salted peanuts flavored with oregano essential oil and olive oil. J Sci Food Agric 89(12):2128–2136
Olmedo RH, Nepote V, Grosso NR (2014) Antioxidant activity of fraction from oregano essential oils obtained by molecular distillation. Food Chem 156:216–219
Olmedo RH, Asensio CM, Grosso NR (2015) Thermal stability and antioxidant activity of essential oils from aromatic plants farmed in Argentina. Ind Crops Prod 69:21–28
Quiroga PR, Riveros CG, Zygadlo JA, Grosso NR, Nepote V (2011) Antioxidant activity of essential oil of oregano species from Argentina in relation to their chemical composition. Int J Food Sci Technol 46(12):2648–2655
Raut JS, Karuppayil SM (2014) A status review on the medicinal properties of essential oils. Ind Crops Prod 62:250–264
Roginsky V, Lissi E (2005) Review of methods to determine chain-breaking antioxidant activity in food. Food Chem 92:235–254
Shin EC, Craf BD, Pegg RB, Phillips RD, Eitenmiller RR (2010) Chemometric approach to fatty acid profiles in Runner-type peanut cultivars by principal component analysis (PCA). Food Chem 119:1262–1270
Skotti E, Anastasaki E, Kanellou G, Polissiou M, Tarantilis PA (2014) Total phenolic content: antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatics plants. Ind Crops Prod 53:46–54
Talcott ST, Duncan CE, Del Pozo-Insfran D, Gorbet DW (2005) Polyphenolic and antioxidant changes during storage of normal, mid, and high oleic acid peanuts. Food Chem 89:77–84
Wang S, Adhikari K, Hung Y (2017) Effects of short storage on consumer acceptability and volatile compound profile of roasted peanuts. Food Pack Shelf Life 13:27–34
Acknowledgements
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Secretaria de Ciencia y Tecnología de la Universidad Nacional de Córdoba (SECYT-UNC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Olmedo, R., Ribotta, P. & Grosso, N.R. Oxidative stability, affective and discriminative sensory test of high oleic and regular peanut oil with addition of oregano essential oil. J Food Sci Technol 55, 5133–5141 (2018). https://doi.org/10.1007/s13197-018-3459-5
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3459-5