Abstract
This study aimed to evaluate different pre-treatments and solvent ratios on the total phenolic content and antioxidant activity of Citrus aurantium (sour orange) peel extracted by ultrasound. A two-factor (2 × 3) factorial design was implemented, with fresh and dry peels as pre-treatment conditions, and water (100%), 50% aqueous ethanol (v/v) and 96% aqueous ethanol (v/v) as the solvents. The phenolic compounds were identified and quantified by ultra-performance liquid chromatography–photodiode array and electrospray ionisation–mass spectrometry, respectively. The compounds were partially purified by advanced automated flash purification. The results indicated that the maximal phenolic content (40.95 ± 3.44 mg gallic acid equivalents/g dry weight) was obtained when fresh sour orange peels were extracted with 50% (v/v) aqueous ethanol while the maximal antioxidant activity (730.04 ± 28.60 μmol Trolox equivalents/g dry weight) was achieved from aqueous extraction of dry sour orange peels. Nine phenolic compounds were identified and quantified. Naringin and neohesperidin predominated in sour peel extracts, whereas, caffeic and chlorogenic acids were the least abundant. Evaluation of the antioxidant activity in the fractions suggested that this activity might be attributed to the synergistic effect of the nine phenolic compounds present in the crude extract. Accordingly, sour orange peel is a potential source of phenolic compounds with antioxidant activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mexico is ranked as the fifth highest citrus producer worldwide, with an average of 4.2 million tonnes per year. Citrus fruits alone comprise 22.5% of the total volume of fruit produced in the country. Approximately 90% of Mexico’s citrus production is dedicated to fresh consumption, and 10% is utilised in the commercial juice and concentrate industry (SAGARPA 2012). Around 45–60% of the product becomes waste, including peels, seeds and membranes. Among the varieties of citrus fruit, sour orange (Citrus aurantium) is rarely used for fresh consumption, due to its natural sourness (Hosseini et al. 2016). Instead, the sour orange juice is often used as an ingredient in meat marinades or salad dressings. Previous studies have shown that sour orange peel contains a variety of phenolic compounds, such as flavonoids, like naringin, neohesperidin, hesperidin, neoeriocitrin, narirutin, didymin, and chlorogenic and coumaric acids (Jayaprakasha et al. 2008; Safdar et al. 2017; Sawalha et al. 2009). Most of the Citrus phenolic compounds have been linked to biological activities that promote health, attributed to the presence of various reactive groups, which favour antioxidant activity (Ferreira et al. 2018). Antioxidants are capable of delaying or inhibiting oxidation, by disrupting the formation and propagation of free radicals (Ferreira et al. 2018). Ferreira et al. (2018) observed that phenolic compounds obtained by aqueous ethanolic, aqueous and solid phase extraction of mandarin (Citrus reticulata) presented a strong 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical scavenging activity.
The interest in phenolic compounds present in fruit by-products has been increasing, given their potential use as biologically active additives or supplements in food products. Additionally, studies of extraction conditions to increase recovery yields while retaining the inherent bioactivities of components have also increased (Papoutsis et al. 2018). Conventional extraction methods, such as Soxhlet (Heleno et al. 2016) and maceration (Castro-López et al. 2017; Xu et al. 2017) are common approaches to obtaining citrus phenolic compounds. Nevertheless, a reduction in the bioactivity of the resulting products occurs (Jovanović et al. 2017). Ultrasound-assisted extraction (UAE) is a non-conventional method that has demonstrated advantages over conventional methods, because of the reduction in both time and solvent, easy handling and high reproducibility (Picó 2013). Factors, like sample pre-treatment, and solvent type and concentration, influence the yield of phenolics recovered (Molina-Calle et al. 2015). During UAE of mandarin peels by ethanol and methanol, respectively, at 50%, 80% and 100% (v/v), the 80% solvents led to the highest recovery of phenolic compounds (Safdar et al. 2017).
Identification and quantification of phenolic compounds from citrus residues are commonly performed by high-performance liquid chromatography (HPLC) (Díaz-García et al. 2013; Sawalha et al. 2009). However, improved chromatography technologies coupled with mass spectroscopy techniques, such as ultra-performance liquid chromatography–photodiode detector electrospray ionisation mass spectrometry (UPLC–PDA ESI–MS), have provided valuable information while allowing the reduction in time and solvent. As far as we know, few reports have investigated the UAE of phenolic compounds, and the phenolic profile or antioxidant activity of such constituents in sour orange peel extracts. This study determined the effect of pre-treatment and solvent concentration on the total phenolic content (TPC) and antioxidant activity of ethanolic and aqueous extracts obtained by UAE from sour orange (C. aurantium) peels and characterized the phenolic compounds by UPLC–PDA ESI–MS.
Materials and methods
Plant material and reagents
Orange fruits (C. aurantium), commonly known as “sour orange”, were purchased from a local market in Yucatan, Mexico. The fruits were peeled, and the fresh peels were shredded and divided into two groups: one was immediately processed and the second group was dried in a steam dehydrator (Jersa 148-09, Mexico) with air circulation at 45 °C for 24 h. The sour orange peels were finely ground (Pulvex 200 grinder, Mexico) and passed through a < 0.500 mm metal sieve. The following reagent and standards were purchased from Sigma–Aldrich, USA: Folin–Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), caffeic acid, p-coumaric acid, sinapic, naringin, neohesperidin, ellagic acid, morin, chlorogenic acid, and HPLC grade methanol and acetonitrile. Ultra-pure water was prepared in a Milli-Q water filtration system (Millipore, Bedford, MA, USA).
Physicochemical characterisation
The total soluble solids in fresh and dry sour orange peel samples was measured using an Abbe bench-top refractometer (Atago, Japan). Total titratable acidity (TTA) was measured according to the standard Association of Official Analytical Chemists method (AOAC 1990) and expressed as a citric acid percentage. The pH was recorded using a potentiometer (Oaklon pH 700, Singapore) (AOAC 1990). Moisture content was calculated (AOAC 1990), by employing a thermobalance (Ohaus MB-45, Switzerland). Colour was characterised using a Mini Scan EZ colourimeter (MSEZ 1505, VA, USA) and expressed as the L* (black/white), a* (green/red) and b* (blue/yellow) coordinates.
UAE evaluation
A two-factor (2 × 3) factorial design was used to identify the major factors influencing the extraction process. Fresh and dry sour orange peels were used as two levels of the factor sample pre-treatment, and 50% aqueous ethanol (v/v) (E50D), 96% aqueous ethanol (v/v) (E90D) and 100% water (WD) were used as the three solvent levels. During the UAE process, 4 g of dry ground peels or 9 g of fresh peels (to maintain the same amount of peel material on a dry basis, in both samples) was immersed separately in 200 mL of each solvent. The samples were then sonicated by an ultrasound probe (frequency 20 kHz, power 130 W, maximum capacity of 200 mL (Ultrasonic Processor, Model GEX130PB, Newtown, CT, USA) for 12.5 min at 80% radiation, concordant with previous work performed at our laboratory (Covarrubias-Cárdenas et al. 2018). A cold bath was used to maintain the temperature below 50 °C. A control sample was prepared by maceration of the dry peel material with 50% ethanol (v/v) (Castro-López et al. 2017). After sonication, samples were vacuumed-filtered (Whatman filter paper no. 1) and stored at 4 °C for further analysis.
Determination of TPC and antioxidant activity
The TPC of the sour orange peel extracts was measured by using the modified Folin and Ciocalteu (1927) method, and the results expressed as milligrams of gallic acid equivalents (GAE) per gram of dry weight (dw) based on a gallic acid calibration curve. The DPPH radical scavenging effect was expressed as μmol of Trolox equivalents (TE) per gram of dried fruit, based on a Trolox calibration curve.
UPLC–PDA and UPLC–PDA ESI–MS analysis
The chromatographic profiles of phenolic compounds in dry sour orange peel extracts were analysed using a Waters UPLC Acquity H Class (Milford, MA, USA, 2010) instrument equipped with a quaternary pump (UPQSM), autosampler injector (UPPDALTC) and eλ PDA (UPPDALTC). Waters Empower 3 software was used for data acquisition and processing. The phenolic compounds were chromatographically separated on a Waters Acquity UPLC BEH C18 column (1.7 μm, 100 × 2.1 mm i.d) at room temperature, flow rate 0.2 mL min−1, and injection volume programmed to 1.56 at 2 μL. The mobile phase comprised 0.1% formic acid in ultra-pure water (A) and 0.1% formic acid in acetonitrile (B). The gradient was programmed as follows: 100% A (0.0–2.0 min), 90.0% A (2.0–4.0 min), 77.0% A (4.0–6.0 min), 77.0% A (6.0–7.0 min), 76.5% A (7.0–17.5 min), 0.0% A (17.5–18.0 min), 50% A (18.0–24.0 min), and 100% A (24.0–30 min). Quantification of the phenolic compounds was performed using standard curves constructed at concentrations from 1 to 100 ppm with a limit of quantification of 1 ppm (caffeic acid, p-coumaric acid, sinapic, naringin, neohesperidin, ellagic acid, morin and chlorogenic acid). The TPC was expressed as the sum of all compounds obtained by UPLC.
The UPLC–PDA ESI–MS chromatographic analysis was performed with the same instrument described above, linked to a Waters Xevo TQ-S micro mass spectrometer detector. Waters MassLynx V4.1 software was used for data acquisition and processing. The mass spectrometer detector was operated in negative ESI mode, with the capillary voltage at 4.0 kV, 50 V cone voltage, 350 °C desolvation temperature, 150 °C source temperature, and 1 V collision energy. Ultra-high-purity argon was used as collision gas. Nitrogen, generated by a Peak Scientific NM32LA nitrogen generator (Inchinnan, Scotland, UK), was used as both the cone and desolvation gas, with flow rates of 50 and 650 L h−1, respectively. The mass spectra were recorded in full-scan mode over the range m/z 20–700. Chromatographic separation of the phenolic compounds was achieved under the same conditions as those used in the quantitative analysis by UPLC–PDA.
Partial purification of phenolic compounds
The phenolic compounds were purified by using an advanced automated flash chromatography device (Isolera One, Biotage, Sweden) equipped with a SNAP C18 30 g Biotage cartridge. Four millilitres of the sample was reduced to 50% volume by rotary evaporation (Buchi R-215, Switzerland). Samples were eluted at a flow rate of 25 mL min−1 using a gradient of 0.1% formic acid in ultrapure water (A) and 0.1% formic acid in acetonitrile (B), programmed as follows: 3 column volumes (CVs) of 0% isocratic B; linear gradient from 0 to 10% B in 2 CVs; linear gradient from 10 to 23% B in 2 CVs; 1 CV of isocratic 23% B; linear gradient from 23 to 24% B in 10.5 CVs; linear gradient from 24 to 100% in 2 CVs; 100% to 50% B in 6 CVs. Peak monitoring was by UV detection at 290 nm wavelength. Fractions were evaluated for antioxidant activity and TPC determinations, as explained above. To obtain comparable results, the TPC of fractions was fixed to 4 mg GAE/g dw for antioxidant activity determination.
Statistical analysis
All experiments were conducted in triplicate, and the data are reported as mean ± standard deviation. The two-factor factorial design was analysed by a multifactorial analysis of variance (ANOVA), followed by least square design (LSD) using Statgraphics Centurion version XVI, (Manugistic, Inc., Rockville, MD, USA software), and p < 0.05 was considered as significant.
Results and discussion
Physicochemical analysis of fresh and dry sour orange peels
The physicochemical parameters of the fresh and dry sour orange peels (Table 1) revealed pH values of 4.12 and 4.23, and TTA values of 0.75% and 1.43%, respectively. Rekha et al. (2012) and Moufida and Marzouk (2003) agree that the pH and TTA values are related to the high content of ascorbic and citric acids present in the sour orange peels. The TTA obtained for fresh sour orange peels was similar to that documented by Ersus and Cam (2007), of 0.7%. Acidity can inform about the conservation of a food product, and increased acidity can be associated with decreased microbial growth (Jönsson et al. 2013). Dry sour orange peels exhibited a higher °Brix value (2.0 ± 0.0) than fresh samples (1.5 ± 0.0), which may be attributed to the hydrolysis of sugars, formation of products and the concentration of solids, during the drying pre-treatment. Moisture contents of 69.33 ± 0.69% and 10.67 ± 0.65% were observed for fresh and dry sour orange peels, respectively. A low moisture content promotes the preservation of the biological material since it reduces the microbiological degradation. Fresh sour orange peels had colour coordinates (L*, a*, b*) of 65.50 ± 0.67, − 1.12 ± 0.13 and 43.99 ± 0.13, respectively. After drying pre-treatment, the L* and a* values increased, indicating that the sour orange peel appeared brighter with an increased reddish tone, whereas there was a decrease in b*, implying a reduction in yellowness.
TPC and antioxidant activity
An ANOVA performed on the two-factor (2 × 3) factorial design indicated a significant effect (p < 0.05) on the two responses evaluated (TPC and antioxidant activity) (Online Resource 1) of both factors (sample pre-treatment and solvent concentration) individually and their interaction. The TPC from fresh and dry treatments varied from 22.65 ± 0.94 to 40.95 ± 3.44 mg GAE/g dw (Table 2). Dry sour orange peels presented the lowest phenolic content while the highest TPC was observed for fresh sour orange peels. The varied results may be attributed to the drying process, during which, the cell membranes and organelles adhere, impeding the extraction of phenolic compounds by the solvent, and thereby reducing the yields. Similarly, Casquete et al. (2015) noted a higher phenolic content (2.84 mg GAE/g dw) in fresh rather than dry sweet orange peels. Another study mentioned that pre-treatment of biological plant materials using high temperatures and prolonged times could decrease the phenol content (Li et al. 2006). In contrast, Jeong et al. (2004) described a significant increase in TPC from sour orange peel extracts treated at temperatures above 50 °C. Likewise, Que et al. (2008) indicated the possible formation of phenolic compounds during thermal drying at > 70 °C. Interestingly, the 50% ethanol extracts had the most phenolic contents. The interaction analysis, indicated by ANOVA, showed that the combination of fresh sour orange peel and 50% ethanol presented the greatest recovery of phenolic compounds (40.95 ± 3.44 mg GAE/g dw). Likewise, results from dry sour orange peel extract also demonstrated a relatively higher phenol content when 50% ethanol (32.68 ± 0.55 mg GAE/g dw) rather than 100% water or 96% aqueous ethanol was used (Table 2). Ethanol causes cell dehydration, which could decrease the phenolic content when a high concentration is used, which corroborates the findings of Safdar et al. (2017), in which a higher phenolic content was found in samples extracted with 50% ethanol compared to 80% and 100% ethanol solutions. The polarity of the solvent plays an important role in the extraction process (Safdar et al. 2017). In ethanol–water mixtures, water can create a more polar medium, which favours the extraction of compounds with similar polarity (Nayak et al. 2015). Additionally, ethanol is a useful solvent with Generally-Recognised-As-Safe (GRAS) status, meaning it can be used in the food industry.
The antioxidant activity responses varied from 253.9 ± 3.65 to 730.04 ± 28.60 μmol TE/g dw, also expressed as DPPH inhibition percentage (Table 2). An increased antioxidant activity was observed when dry sour orange peels were used for the extraction. It suggests some phytochemical compounds react during the drying process, potentially forming other antioxidant compounds by complexation of phenols with proteins (Martín-Cabrejas et al. 2009). It has been noticed that drying pre-treatment influences the antioxidant activity of phenolic extracts. Jeong et al. (2004) found the antiradical activity increased significantly when a thermal treatment was applied to citrus peel, due to the liberation of low molecular weight phenolic compounds that possess antioxidant activity. Other authors have similarly documented the thermal-induced formation of such phenolic compounds (Tomaino et al. 2005; Xu and Chang 2007). Here, the water extracts exhibited the highest antioxidant activity. In contrast, Xu et al. (2017) found an increase in the antioxidant activity in the citrus Limonium sinuatum flower extracts when the ethanol concentration was increased because the polarity more closely matched to the polarity of the antioxidants.
Given the high antioxidant activity of the dry peel samples, these samples were further tested. A conventional (maceration) extraction was conducted using 50% aqueous ethanol because this solvent was most effective at extracting the phenolic compounds during UAE. The maceration extraction led to a significantly lower TPC (28.1 ± 0.55 mg GAE/g dw) than that achieved by the UAE method (p < 0.05). Additionally, the antioxidant activity observed for the macerated sample was lower than that exhibited by all the dry peel samples extracted by ultrasound, implying the UAE method enhances the preservation of the antioxidant activity, as suggested by Jovanović et al. (2017).
Analysis of phenolic compounds composition in dry sour orange peel extracts
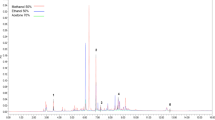
Considering that the drying pre-treatment of sour orange peel resulted in the highest antioxidant activity, the UAE treatments corresponding to the three different solvent concentrations were selected for profiling the phenolic compounds by UPLC–PDA. The phenolic compounds were identified based on their retention times and quantified according to their respective standard calibration curves. Reverse-phase chromatography analysis of the three extracts showed similar chromatographs, with two major peaks attributed to naringin and neohesperidin, respectively (Fig. 1). Minor chromatographic peaks were also detected, indicating the presence of coumaric acid, morin, caffeic acid, chlorogenic acid, diosmin and ellagic acid (Fig. 1). The phenolic content was also calculated by summation of the concentrations of all compounds identified in the chromatogram, which indicated the maximum TPC for the E50D (39.41 ± 5.22 mg/g dw). Similar behaviour was established using the Folin–Ciocalteu reagent, although the value was slightly higher. The WD presented the lowest concentration (11.21 ± 1.39 mg/g dw) (Table 3). This value was 50% lower than that determined by the spectrophotometric procedure. Naringin contents of 14.06, 12.31 and 3.11 mg/g dw, and neohesperidin amounts of 13.32, 3.33 and 12.87 mg/g dw were quantified in the E50D, E96D and WD extracts, respectively. Among the other compounds observed, ellagic acid was not detected in WD extract, which may be attributed to the aromatic ring, which confers it lipophilic properties and makes it insoluble in water (Li et al. 2013a). Phenolic compounds, such as naringin, narirutin and didymin, have been previously identified in sour orange fruit extracts by Jayaprakasha et al. (2008). Also, Safdar et al. (2017) and Karimi et al. (2012) reported naringin and neohesperidin as the predominant flavanone glycoside flavonoids in kinnow citrus peel and sour orange bloom, respectively. Nevertheless, no prior studies have identified and quantified the phenolic compounds assessed in the current paper. Sawalha et al. (2009) recorded naringin at 5.1 ± 0.2 mg/g dw of sour citrus peel. This principal phenolic compound present in citrus peels has shown health-promoting effects, such as antioxidant, antimicrobial and anticancer activities (Bacanlı et al. 2015; Li et al. 2013b).
UPLC–PDA ESI–MS analysis
The UPLC–PDA ESI–MS spectroscopy and spectrometry data (Table 4). The parameters of the mass spectrometer were adjusted until the quasi-molecular ions [M-H]−, as these were the most intense and, in several cases, represented the base peak with additional phenolic compound fragmentations observed. The mass spectra of hydroxycinnamic acid derivatives, such as caffeic, p-coumaric and sinapic acids, displayed fragments of [M-H-44]−, representing 2-phenylethen-2-ide anion moiety derivatives, produced by the loss of hydrogen and carbon dioxide from the chemical structure (Fang et al. 2002). Furthermore, sinapic acid manifested a fragment of [M-H-16]−, attributed to the hydroxyl group while chlorogenic acid showed a fragment corresponding to the loss of [M-H-163]− (caffeic acid), releasing the (−)-quinic acid anion (detected). Diosmin exhibited a fragment of [M-H-308]−, associated with the loss of rutinoside, liberating the aglycone (Barreca et al. 2011). It is notable that the loss of rutinoside in both molecules differs by one unit that may be explained by the ionisation of rutin that occurs in the aglycone, which is subsequently fragmented, generating the rhamnose–glucose disaccharide [309]. Diosmin is potentially ionised in the disaccharide [309-1] after which, a fragmentation occurs, with a probable charge transfer, transforming it into the aglycone. Morin displayed the fragment [M-H-16]−, which represents the loss of a hydroxide.
Analysis of the antioxidant activity of partially purified phenolic compounds
The phenolic compounds were partially purified by using an advanced automated flash purification system, and the antioxidant activity, identification and quantification of the fraction compositions were determined. A modified report from the automated flash chromatography system using the 290 nm wavelength is provided (Fig. 2a). It evidences four peaks, producing 11 fractions of purified phenolics, which formed each of the peaks and were combined to obtain four final fractions (Fig. 2a), termed F1, F2, F3 and F4, respectively. Identification and quantification of phenolic compounds obtained in each fraction were determined by UPLC. Figure 2b shows the main phenolic compounds present in F1, F2, F3 and the crude extract. It was seen that chlorogenic acid was the most prevalent compound (38.31%) in F1, followed by an unknown compound coded NC-1 (31.50%), and caffeic acid (30.19%), and with 11.4 μg/mL TPC. Coumaric acid predominated (98.12%) in F2, followed by ellagic acid (1.88%), resulting in the most purified fraction, with a TPC of 5.97 μg/mL. F3 presented the largest number of compounds, but mainly comprised naringin (48.64%) and neohesperidin (48.17%), with small amounts of hesperidin (2.08%) and diosmin (1.98%), resulting in a final fraction with 43.4 μg/mL TPC. Due to the lower concentration of TPC in F4 than the others, identification was difficult. The phenolic compounds present in the crude extract may have different antioxidant activities to the fractions, so the crude extract and the purified fractions were fixed at a concentration of 4 μg/mL, to evaluate the individual antioxidant activities. The data were compared, to ascertain the influence of the different phenolic compounds on the antioxidant activity. The crude extract had the highest antioxidant activity (88 µmol TE/g dw), followed by F2, which showed the highest antioxidant activity among the fractions (68 µmol TE/g dw). These results suggest that a synergistic behaviour may occur in the crude extract, due to the combination of the nine compounds present in the sample, as mentioned by Shiraishi et al. (2018), who evaluated the phenolic compounds in table grapes.
Modified chromatogram at 290 nm of fractions (F1, F2 and F3) obtained by separation using an Isolera One system (a). Phenolic profiles of fractions obtained by ultra-high-performance liquid chromatography, and (b) antioxidant activity expressed as micromoles of Trolox equivalents per gram of dry weight (μmol TE/g dw) of each fraction fixed at 4 μg/mL total phenolic content (TPC). Caf Caffeic acid, Clo Chlorogenic acid, Cum Coumaric acid, Dios Diosmin, Ela Ellagic acid, Hes Hesperidin, Mor Morin, Neo Neohesperidin, Nar Naringin, NC-1 not identified compound
Conclusion
A rapid and efficient method for extracting phenolic compounds from C. aurantium peels using UAE was successfully developed. The highest TPC and antioxidant activity were identified in fresh peels extracted with 50% aqueous ethanol and the dry peel aqueous extracts, respectively. Nine phenolic compounds, including four phenolic acids and five flavonoids, were identified and quantified by UPLC–PDA and confirmed by ESI–MS. Naringin and neohesperidin were the most abundant compounds. Determinations of the antioxidant activity of four fractions obtained by advanced automated flash purification of the peels extract indicated a potential synergistic effect of all the phenolic compounds identified. Sour orange peel is a potential source of phenolic compounds with antioxidant activity that can be used in the production of functional food.
References
AOAC (1990) Official methods of analysis of the Association of Official Analytical Chemists. In: Horwitz W, Latimer G (eds) 2015th edn. Maryland, USA, p 10
Bacanlı M, Başaran AA, Başaran N (2015) The antioxidant and antigenotoxic properties of citrus phenolics limonene and naringin. Food Chem Toxicol 81:160–170. https://doi.org/10.1016/j.fct.2015.04.0150278-6915
Barreca D, Bellocco E, Caristi C, Leuzzi U, Gattuso G (2011) Flavonoid profile and radical-scavenging activity of Mediterranean sweet lemon (Citrus limetta Risso) juice. Food Chem 129:417–422. https://doi.org/10.1016/j.foodchem.2011.04.093
Casquete R, Castro SM, Martín A, Ruiz-Moyano S, Saraiva JA, Córdoba MG, Teixeira P (2015) Evaluation of the effect of high pressure on total phenolic content, antioxidant and antimicrobial activity of citrus peels. Innov Food Sci Emerg Technol 31:37–44. https://doi.org/10.1016/j.ifset.2015.07.005
Castro-López C, Ventura-Sobrevilla JM, González-Hernández MD, Rojas R, Ascacio-Valdés JA, Aguilar CN, Martínez-Ávila GCG (2017) Impact of extraction techniques on antioxidant capacities and phytochemical composition of polyphenol-rich extracts. Food Chem 237:1139–1148. https://doi.org/10.1016/j.foodchem.2017.06.032
Covarrubias-Cárdenas AG, Martinez-Castillo JI, Medina-Torres N, Ayora-Talavera T, Espinosa-Andrews H, García-Cruz NU, Pacheco N (2018) Antioxidant capacity and UPLC–PDA ESI–MS phenolic profile of Stevia rebaudiana dry powders extracts obtained by ultrasound assisted extraction. Agronomy 8:170. https://doi.org/10.3390/agronomy8090170
Díaz-García MC, Obón JM, Castellar MR, Collado J, Alacid M (2013) Quantification by UHPLC of total individual polyphenols in fruit juices. Food Chem 138:938–949. https://doi.org/10.1016/j.foodchem.2012.11.061
Ersus S, Cam M (2007) Determination of organic acids, total phenolic content, and antioxidant capacity of sour Citrus aurantium fruits. Chem Nat Compd 43:607–609. https://doi.org/10.1007/s10600-007-0203-1
Fang N, Yu S, Prior RL (2002) LC/MS/MS characterization of phenolic constituents in dried plums. J Agric Food Chem 50:3579–3585. https://doi.org/10.1021/jf0201327
Ferreira SS, Silva AM, Nunes FM (2018) Citrus reticulata Blanco peels as a source of antioxidant and anti-proliferative phenolic compounds. Ind Crop Prod 111:141–148. https://doi.org/10.1016/j.indcrop.2017.10.009
Folin O, Ciocalteu V (1927) On tyrosine and tryptophane determinations in proteins. J Biol Chem 73:627–650
Heleno SA, Diz P, Prieto MA, Barros L, Rodrigues A, Barreiro MF, Ferreira ICFR (2016) Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Food Chem 197:1054–1063. https://doi.org/10.1016/j.foodchem.2015.11.108
Hosseini SS, Khodaiyan F, Yarmand MS (2016) Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr Polym 140:59–65. https://doi.org/10.1016/j.carbpol.2015.12.051
Jayaprakasha GK, Girennavar B, Patil BS (2008) Radical scavenging activities of Rio Red grapefruits and sour orange fruit extracts in different in vitro model systems. Bioresour Technol 99:4484–4494. https://doi.org/10.1016/j.biortech.2007.07.067
Jeong SM, Kim SY, Kim DR, Jo SC, Nam KC, Ahn DU, Lee SC (2004) Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J Agric Food Chem 52:3389–3393. https://doi.org/10.1021/jf049899k
Jönsson JL, Alriksson B, Nils-Olof N (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:16. https://doi.org/10.1186/1754-6834-6-16
Jovanović AA, Đorđević VB, Zdunić GM, Pljevljakušić DS, Šavikin KP, Gođevac DM, Bugarski BM (2017) Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep Purif Technol 179:369–380. https://doi.org/10.1016/j.seppur.2017.01.055
Karimi E, Oskoueian E, Hendra R, Oskoueian A, Jaafar HZE (2012) Phenolic compounds characterization and biological activities of Citrus aurantium Bloom. Molecules 17:1203–1218. https://doi.org/10.3390/molecules17021203
Li Y, Guo C, Yang J, Wei J, Xu J, Cheng S (2006) Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem 96:254–260. https://doi.org/10.1016/j.foodchem.2005.02.033
Li B, Harich K, Wegiel L, Taylor LS, Edgar KJ (2013a) Stability and solubility enhancement of ellagic acid in cellulose ester solid dispersions. Carbohydr Polym 92:1443–1450. https://doi.org/10.1016/j.carbpol.2012.10.051
Li H, Yang B, Huang J, Xiang T, Yin X, Wan J, Luo F, Zhang L, Li H, Ren G (2013b) Naringin inhibits growth potential of human triple-negative breast cancer cells by targeting β-catenin signaling pathway. Toxicol Lett 220:219–228. https://doi.org/10.1016/j.toxlet.2013.05.006
Martín-Cabrejas MA, Aguilera Y, Pedrosa MM, Cuadrado C, Hernández T, Díaz S, Esteban RM (2009) The impact of dehydration process on antinutrients and protein digestibility of some legume flours. Food Chem 114:1063–1068. https://doi.org/10.1016/j.foodchem.2008.10.070
Molina-Calle M, Priego-Capote F, Luque de Castro MD (2015) Development and application of a quantitative method for determination of flavonoids in orange peel: influence of sample pretreatment on composition. Talanta 144:349–355. https://doi.org/10.1016/j.talanta.2015.05.054
Moufida S, Marzouk B (2003) Biochemical characterization of blood orange, sweet orange, lemon, bergamot and bitter orange. Phytochemistry 62:1283–1289. https://doi.org/10.1016/S0031-9422(02)00631-3
Nayak B, Dahmoune F, Moussi K, Remini H, Dairi S, Aoun O, Khodir M (2015) Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem 187:507–516. https://doi.org/10.1016/j.foodchem.2015.04.081
Papoutsis K, Pristijono P, Golding JB, Stathopoulos CE, Bowyer MC, Scarlett CJ, Vuong QV (2018) Screening the effect of four ultrasound-assisted extraction parameters on hesperidin and phenolic acid content of aqueous citrus pomace extracts. Food Biosci 21:20–26. https://doi.org/10.1016/j.fbio.2017.11.001
Picó Y (2013) Ultrasound-assisted extraction for food and environmental samples. Trends Anal Chem 43:84–99. https://doi.org/10.1016/j.trac.2012.12.005
Que F, Mao L, Fang X, Wu T (2008) Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int J Food Sci Technol 43:1195–1201. https://doi.org/10.1111/j.1365-2621.2007.01590.x
Rekha C, Poornima G, Manasa M, Abhipsa V, Devi JP, Kumar VH, Kekuda TR (2012) Ascorbic acid, total phenol content and antioxidant activity of fresh juices of four ripe and unripe citrus fruits. Chem Sci Trans 1:303–310. https://doi.org/10.7598/cst2012.182
Safdar MN, Kausar T, Jabbar S, Mumtaz A, Ahad K, Saddozai AA (2017) Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J Food Drug Anal 25:488–500. https://doi.org/10.1016/j.jfda.2016.07.010
SAGARPA (2012) Mexico, quinto productor mundial de citricos. http://www.sagarpa.gob.mx/Delegaciones/yucatan/Boletines/Paginas/201209B068.aspx. Accessed 21 Dec 2017
Sawalha SMS, Arráez-Román D, Segura-Carretero A, Fernández-Gutiérrez A (2009) Quantification of main phenolic compounds in sweet and bitter orange peel using CE–MS/MS. Food Chem 116:567–574. https://doi.org/10.1016/j.foodchem.2009.03.003
Shiraishi M, Shinomiya R, Chijiwa H (2018) Varietal differences in polyphenol contents, antioxidant activities and their correlations in table grape cultivars bred in Japan. Sci Hortic 227:272–277. https://doi.org/10.1016/j.scienta.2017.09.032
Tomaino A, Cimino F, Zimbalatti V, Venuti V, Sulfaro V, De Pasquale A, Saija A (2005) Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem 89:549–554. https://doi.org/10.1016/j.foodchem.2004.03.011
Xu BJ, Chang SKC (2007) A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci 72:S159–S166. https://doi.org/10.1111/j.1750-3841.2006.00260.x
Xu DP, Zheng J, Zhou Y, Li Y, Li S, Li HB (2017) Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: optimization and comparison with conventional methods. Food Chem 217:552–559. https://doi.org/10.1016/j.foodchem.2016.09.013
Acknowledgements
The authors gratefully acknowledge CONACYT to the grant of Ana Covarrubias and the financial support thought the Project SEP-CONACYT CB2015-01. To ASPELAB® to provide the equipment (Advanced Automated Purification Biotage System Model Isolera) for polyphenol partial purification performed in this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ana, CC., Jesús, PV., Hugo, EA. et al. Antioxidant capacity and UPLC–PDA ESI–MS polyphenolic profile of Citrus aurantium extracts obtained by ultrasound assisted extraction. J Food Sci Technol 55, 5106–5114 (2018). https://doi.org/10.1007/s13197-018-3451-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3451-0