Abstract
Natural and de-novo biosynthesized phyto-compounds have gained much significance because of their non-controversial nutritional, health and safety benefits as compared with chemically synthesized commercially rivalry antioxidants. However, none of natural de-novo biosynthesized phyto-compounds has been commercially available and used in customary food business and processing. In this study, efficacy of sesame seed extracts (SSEs) in stabilizing sunflower oil during storage has been studied. Fine powder of sesame seed was extracted in different solvents. The results showed that significant differences in extractability of different solvents and maximum extraction yield (29.48%) were achieved with methanol. The antioxidant components and capability of different extracts were further investigated and evaluated via total phenolic contents, DPPH radical scavenging activity and β-carotene/linoleic acid calorimetric assays respectively. Being highest in yield and antioxidant potential, methanolic extract was used; three different concentrations of SSE (500, 750, and 1000 μL) were added in 100 mL of sunflower oil to further evaluate its oxidative stability. Sensory and oxidative analysis of baked product from these groups was also evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sunflower (Helianthus annuus) is one of the most important oil seed crops in the world (Stefansson 2009). Besides palm, soy and rapeseed oil, sunflower oil ranks fourth with a worldwide production of about 16.67 million metric tons in 2016 (FAO 2016). This edible oil is rich in mono and polyunsaturated fatty acids i.e. oleic acid (14–40%) and linoleic acid (48–74%), these fatty acids have beneficial effects on health (Hashemi et al. 2015). However, due to relatively higher proportion of unsaturated fatty acids, sunflower oil is more prone to oxidation or/and degradation while processing and storage, the auto-oxidation occurring during the processing or/and storage or/and consumption of sunflower oil has raised serious concerns about the quality and safety of sunflower oil itself but also for the products manufactured using this oil (Asnaashari et al. 2014). Furthermore, the level of degradation of these oxidation prone unsaturated fatty acids increases with their exposure to light, heat, high temperature, trace metals and oxygen (Farahmandfar et al. 2015). To address this issue, the application of antioxidants is the easiest and preferred way to inhibit the lipid oxidation and degradation (Farhoosh et al. 2016). For this purpose, two kinds of antioxidant (i.e. synthetic antioxidants and natural antioxidants) have been enormously employed at industrial scale in past. The application of synthetic antioxidants at industrial scale i.e. Butylated hydroxyanisole (BHA) and Butylated hydroxytoluene (BHT) started from the beginning of twentieth century to prevent the lipid oxidation (Eshghi et al. 2014). However, recent reports in literature questioned the safety of these synthetic antioxidants and revealed that the continuous consumption of these synthetic antioxidants may lead to many serious health constraints such as cancer and carcinogenesis and thus, being excluded from the Generally Recognized As Safe list of compounds (GRAS) (Prior 2004). Due to the safety concerns, there is an increasing trend to replace these synthetic antioxidants with the natural antioxidants which are supposed to be safe. The effectiveness of different natural antioxidants in terms of their chelating ability, reducing power and scavenging activity has been reported previously which can effectively replace the synthetic antioxidants (Hameed et al. 2017).
As previously reported, many natural antioxidants like ginger extract, raspberry leaves, pussy willow extract, olive leaf juice have been used to protect oxidizable constituents of essential oils from oxidation and known as shelf life enhancers (Iqbal and Bhanger 2007; Farag et al. 2006). However, natural antioxidants have not been used on large commercial scale owing to its high input cost, seasonal availability of their sources and a lack of scientific and technological data in favors of their safe, nutritious, and sensory parameters. So a common, worldwide available and cheap source of phyto-compounds is the prime interest of the researchers nowadays (Hameed et al. 2017). In this context, sesame seeds could be a good candidate with respect to get the phyto-chemical of interests with the safety, nutritious, and sensory perspective as sesame seeds categorically fall in the above mentioned pre-requisites.
Sesame seed oil (Sesamum indicum L.) is highly unsaturated edible oil rich in linoleic and oleic acids and has been used in herbal remedies in many countries of the world (Anilakumar et al. 2010). It also contains many bioactive compounds including phytosterols, tocopherols and lignans such as sesamin, sesamolin and sesaminol which are considered useful for stabilizing the lipids (Lee et al. 2007). Beside as an antioxidant, many authors claimed that the sesame extracts possess the antimicrobial, antimutagenic properties with prospective applications in the food preservative and nutraceutical industries (Reshma et al. 2010). It has also been reported that the sesame seeds and sesame seeds oil exhibit a preventive role against the onset of many disorders like atherosclerosis, low density lipoprotein oxidation, cancer, platelet aggregation and various cardiovascular diseases (Saleem et al. 2011).
Further, sesame seed oil exhibited higher oxidative stability than soybean, corn and other vegetable oils (Miniotia and Georgioua 2010). The higher oxidative stability of this oil reveals its possession of a high amount of antioxidants. Zeb et al. (2017) revealed that there were 16 polyphenolic compounds in sesame seed oil i.e. hydroxybenzoic acid, Sesamol, Quinic acid, Protocatechuic acid, Catechin, Syringic acid, Rosmarinic acid hexoside, 3-O-p-Coumaroylquinic Acid, Ellagic acid pentoside, Kaemferol-3-feruloylsophoroside-7-glucoside, Kaempferol-3-(p-coumaroyldiglucoside)-7-glucoside, Quercetin-3-(sinapoyldiglucoside)-7-glucoside, Quercetin-3-O-d-galactopyranoside, Quercetin- 3-O-triglucoside, Epicatechin, Quercetin-3,4-diglucoside-3-(6-feruloy-glucoside). Many authors also cited that the antioxidative activity of sesame oil was higher than that of apple, pomegranate, banana and citrus peels (Bopitiya and Madhujit 2012; Opara et al. 2009; Li et al. 2005). Therefore, in this study, sesame seed extract (SSE) was used to protect the rancidity of sunflower oil for the first time.
In this investigation, we evaluated the ethanolic, methanolic and aqueous sesame seed extract (SSE) as a source of natural antioxidant that could increase the shelf life of sunflower oil. The total phenolic content, DPPH free radical scavenging activity and β-carotene linoleic acid assays were determined to assess the antioxidative activity of the sesame seed extracts. Moreover, the antioxidative activity off SSE in the sunflower oil was evaluated during storage and after baking and was compared with antioxidative activity of commercially available antioxidant BHT.
Materials and methods
Chemicals and reagents
Refined sunflower oil without the addition of any synthetic antioxidant was kindly provided by Nishat oil mills industries Pvt. Ltd Faisalabad-Pakistan. Sesame seeds (brown cultivar) were purchased from local market of Faisalabad-Pakistan and respective characteristics of sesame seeds were authenticated by Prof. Dr. Muhammad Jafar Jaskani Institute of Horticulture, University of Agriculture Faisalabad-Pakistan. Butylated hydroxytoulene (BHT), 1, 1-diphenyl-2-picrylhydrazyl (DPPH) and β-carotene were obtained from Sigma-Aldrich (St Louis, MO, USA). All other chemicals used were of analytical grade or higher were appropriate and purchased from Merck (Darmstadt, Germany). Flour for baking was obtained from the baking section of National Institute of Food Science & Technology, University of Agriculture Faisalabad-Pakistan.
Sesame seed oil extraction
Sesame seeds were grounded to fine powder with the warren electric grinder and aliquot of 5 g sesame seeds powder was extracted with 50 mL of semi-polar to polar solvent systems individually (i.e. ethanol, methanol and water) at 28–30 °C in orbital shaker with a rotation of 200 rpm for 16–20 h. The extracts were then filtered through Whatman paper No. 1 (× 2) by vacuum filtration. The residue was then resuspended in another 50 mL of respective solvents (i.e. ethanol, methanol and water individually). The process was repeated twice. The combined filtrate was transferred to a pre-weighted steriletubeand the solvent was evaporated by rotary vacuum evaporator at 40 °C (EYELA, N–N series, Japan). Residual ethanol/methanol was removed by vacuum at 30 °C overnight in an oven and the dry weight was recorded.
Antioxidant components
Total phenolic content (TPC)
The total phenolic content of SSEs was determined according to the method described by Hameed et al. (2017) with minor alterations, and results were expressed as mg/g of Gallic acid equivalents (GAE). At a concentration of 1–10 mg/mL SSEs were prepared in each solvent (methanol, ethanol and water) separately and 0.5 mL of each sample was mixed with 2.5 mLof 10%Folin-Ciocalteu reagent and 2 mL of 7.5% sodium carbonate solution. After the samples were kept for 30 min at room temperature for incubation, the absorbance was measured at 760 nm in a UV/visible spectrophotometer.
Colorimetric assays
DPPH measurement
The 1, 1-diphenyl-2-picrylhydrazyl (DPPH) assay (Ramadan et al. 2007) was performed with some modifications. The stock reagent (1 × 10−3 M) was prepared by dissolving 22 mg of DPPH in 50 mL ethanol and store at − 20 °C until use. The working solution (6 × 10−5 M) was obtained by mixing the 6 mL of stock solution with 100 mL of ethanol. The SSE obtained with different solvents and the synthetic antioxidant BHT (0.1 mL of each) was allowed to react with 3.9 mL of DPPH solution and absorbance was measured at 515 nm after 30 min. The DPPH scavenging percentage was calculated according to the following formula.
A = absorbance at 515 nm
Β-carotene/linoleic acid bleaching assay
The ability of the extract to prevent the bleaching of β-carotene was determined as described by Keyvan et al. (2007). 3.34 mg of β-carotene 1 mL of chloroform, 40 mg of linoleic acid and 400 mg of Tween 20 were added in a round bottom flask. Once the chloroform was removed by vacuum evaporator, 100 mL of distilled water was added and the resulting mixture was stirred vigorously. 5 μL of emulsion were transferred to the tubes containing 0.2 mL SSE obtained from each solvent (i.e. methanol, ethanol and water) or standard BHT. After mixing the absorbance (A0) at 470 nm was recorded. The remaining samples were put in the water bath at 40 °C for 60 min and then absorbance was measured (A60) and antioxidant activity % (AA%) values were calculated by using the following equation.
Oxidative stability of sunflower oil with SSE
Sample preparation
Methanolic extracts of sesame seeds were added to the 100 mL sunflower oil at concentrations of 500 μL (T2), 750 μL (T3) and 1000 μL (T4). Synthetic antioxidant BHT was employed at their legal limit of 200 ppm in sunflower oil (T5) (Duh and Yen 1997). Refined sunflower oil without any antioxidant was used as a negative control (T1).
Analytical procedures
Measurement of Peroxide value (PV), free fatty acid (FFA), specific gravity value, saponification value, Iodine value and thiobarbituric acid reactive substances (TBARS) value were made after a regular interval of 10 days, following the AOCS official methods (AOAC 2003). Storage temperature was 25 °C ± 5 and relative humidity 30–35%.
Oxidative stability of products developed by sunflower oil with SSE
Muffins were made by using the sunflower oil stabilized by different concentrations of SSE as described earlier. Sunflower oil was again extracted from bakery products for the analysis (i.e. peroxide value, free fatty acids and TBARS value) by the Soxhlet extraction method as described by (Castro and Capote 2010) with minor modifications. Briefly muffins were minced and homogenized and stored in hermetic sealing at − 4 °C until used. Five grams (5.0 g) of sample was placed in a cellulose thimble which was capped on with cotton wool. The thimble was then placed in the Soxhlet chamber, which was fitted to a distillation flask containing 80 mL hexane and a boiling glass regulator. After extraction for 16 h, the solvent was released by a rotary-evaporator and the last traces were removed by placing the flask with the extracts in a water bath at 40 °C for 12–16 h. The next day, the flask was cooled in a desiccator and weighed. This heating-weighing step was repeated until the difference between two consecutive weighing was smaller than 10 mg. The analysis of the extracted oil was carried out after predefined storage periods of muffins after which the oil was extracted and subjected to analysis same day. The sensory evaluation of stored muffins was carried out by nine point hedonic scale method as described by Lim (2011). Forty panelists were chosen for the sensory evaluation of the baked muffins. Among forty panelists, twenty trained panelists were chosen who were regularly involved in the baking operation of muffins at baking section of National Institute of Food Science & Technology and twenty panelists were regular buyers of muffins from institutional operated outlet.
Statistical analysis
All experiments were performed in triplicate and data is reported as mean ± standard deviation defined by Steel et al. (1997). One-way ANOVA with posthoc Turkey’s test was conducted by using GraphPadsoftware (GraphPad Prism 7.0 USA).
Results and discussion
Sesame seed extraction yield
Table 1 showed the yield of ethanolic, methanolic and aqueous sesame seed extracts. Range of extraction yield was 13.37–29.48%. The yield obtained from different solvents found to be significantly different (p ≤ 0.05) as highest yield (29.48%) was obtained in methanol and lowest yield was detected in water extracts (13.37%). These differences in extraction yield may be attributed to the nature (miscibility and polarity) of antioxidants. Methanolic and ethanolic extracts showed higher yields than absolute aqueous extracts which synonymous to existence of less to medium polar antioxidant compounds in sesame with their preferred higher miscibility or/and solubility and hence higher extraction in like solvent types i.e. methanol and ethanol. Conversely, the low yield of water extracts is an indicator of less polysaccharides as (hot) aqueous extraction is a standard procedure for polysaccharides extraction in clinical and nutritional studies (Hameed et al. 2017). Additionally, various pro antioxidant compounds such as water soluble bioactive peptides, amines, lactones etc. might be the contributor towards the yield of aqueous extracts. So, higher extraction yield from methanolic extracts credited to higher solubility of antioxidants of sesame in the semi-polar solvent systems (i.e. methanol). Amazingly, methanolic extract also exhibited highest total phenolic contents (TPC) which is symbolic of higher solubility of TPC in semi-polar solvent systems. Besides, many authors also demonstrated the methanolic extracts of apple peels, banana peel, citrus peel (Opara et al. 2009), rice bran (Iqbal et al. 2005) and garlic exhibited the highest antioxidant activity (Iqbal and Bhanger 2007) and were used in diverse industrial and pharmaceutical applications. Thus, methnanolic extract was highly recommended for the extraction of less to medium polar antioxidant compounds (Iqbal et al. 2005). Therefore, in this study, we used methanolic extract of sesame seed for further analysis.
Total phenolic content (TPC)
TPC of all sesame extracts were calculated as gallic acid equivalent (GAE) and represented as mg GAE/g of extracts (Table 1). Significant differences (p < 0.05) were found among the TPC of different solvent systems. TPC found to constitute the main class of antioxidants from sesame seed extracts with substantial contribution towards antioxidant activity. Table 1 represented the TPC of the SSEs. SSE with methanol solvent (3.56 ± 0.120 mg GAE/g sample) was higher in TPC than its counterpart solvent type i.e. ethanolic and aqueous extracts. So the nature and polarity of solvent systems markedly influenced the phenolic contents of SSE. Furthermore, a linear correlation between the TPC and antioxidant activity of methanolic SSE was also observed and these results further confirmed that TPC as the main antioxidant constituent of SSE. Our results also established that the TPC of methanolic SSE was much higher than the previously reported TPC from sesame seed cake extract (1.94 mg GAE/g), potato peels, sugar beet (2.91 mg GAE/g) (Mohdaly et al. 2010), banana (2.32 mg GAE/g) (Balasundram et al. 2006), carrot (1.52 mg GAE/g) and wheat bran (1.0 mg GAE/g) (Zhou and Liangli 2006).
DPPH radical scavenging activity
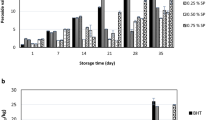
Two calorimetric assays were selected to evaluate the antioxidant bioactivity of SSEs. The first assay was DPPH and second was β-carotene bleaching assay. The reason behind this selection was that each assay evaluates different kind of antioxidant with difference in their mechanism of actions. DPPH is the most simple and effective method for antioxidant ability evaluation for the antioxidant with scavenging activities only. DPPH free radical scavenging activity of the sesame seed extracts along with the reference BHT are shown in Fig. 1a. The methanolic extract represented the highest radical scavenging activity (p < 0.05) as compared to ethanolic and water extracts and satisfied the aforementioned findings that extract with more phenolic contents exhibited the highest DPPH radical scavenging activity (Heim et al. 2002). Scavenging activity of methanolic extract was less significant (p < 0.05) than ethanol and water extracts and lower than the reference BHT. Despite of higher TPC contents of methanolic and ethanolic extracts, aqueous extracts exhibited relatively less DPPH radical scavenging activity than the methanolic and ethanolic extracts. However, in the β-carotene bleaching assay (see below), antioxidant bioactivity of aqueous extract was found to be strongly significantly (p ≤ 0.001) less than that of ethanolic and methanolic extracts. The reason of this contrast is may be attributed to higher scavenging action from the water soluble pro antioxidant compounds of water extract. DPPH radical scavenging activity and presence of less reductant antioxidants in water extracts consequently resulted in gravely less antioxidant capacity in β-carotene bleaching assay.
Antioxidant activity of the BHT and sesame seed oil extracts (ethanol, methanol and aqueous extracts) evaluated by DPPH radical scavenging test (a) and β-carotene/linoleic acid bleaching assay (b). Asterisks indicate that the differences between the means of activities are significantly different (*p < 0.05; **p < 0.01; ***p < 0.001)
The results of the DPPH free radical scavenging assay suggested that the components in the sesame seed extract are capable of scavenging free radicals via electron and hydrogen donating mechanisms and capable of preventing/slowing the initiation of free radical mediated chain reaction. These results showed the capability of sesame seed extract to scavenge the free radicals and act as an antioxidant.
β-carotene/linoleic acid bleaching assay
Synthetic free radical scavenging assays (i.e. DPPH) is a valuable tool to indicate the potential antioxidant activity of the plant extract. However, this system does not use food and its biological relevant oxidizable substrates. Therefore, no direct information of an extract’s scavenging/chelating/reducing actions can be determined in biological or/food systems (Dorman et al. 2003). To circumvent the aforesaid shortcoming, it was essential to check scavenging activity of methanolic SSE in some food/biological systems. Therefore, we carried out the β-carotene/lionleic acid lipid:water emulsion assay. The presence of reductant antioxidants in the extracts will minimize the oxidation of β-caroteneduring incubation at designated temperature for predefined time intervals. Thus the variations in the degradation rate of β-carotene would largely depend upon the (potential) antioxidants of the extract in samples and positives controls. The effect of sesame seed extract on oxidation of β-carotene was shown in Fig. 1b. The data suggested that sesame seed extract with methanol solvent (92.21%) was significantly higher (p < 0.05) in reducing the oxidation of β-carotene than other extracts. Ethanoilc extracts were next to methanoilc extracts in their antioxidative activity against the oxidation of β-carotenes whereas the least scavenging activity was shown by the water extract. The least antioxidant activity of water extracts is may be due to fact that phenolic compounds are more soluble in the semi-polar organic solvents than in highly polar solvents systems like water.
Oxidative stability of sunflower oil with SSE
In order to prevent the oxidative deterioration of the sunflower oil and to increase its shelf-life, various treatments/blends of the BHT and the methanolic sesame seed extracts [(0 μL SSE (T1), 500 μL SSE (T2), 750 μL SSE (T3), 1000 μL (T4)] with 100 mL refined sunflower oil were prepared and further investigated for their potential stability at room temperature. Free fatty acids (FFA), Iodine value, Peroxide value (PV), specific gravity, saponification and TBAR values were measured in different time intervals to determine the indices of lipid oxidation.
Free fatty acids (FFA)
Oxidative deterioration of fats and oils resulted in the formation of free fatty acids. FFAs were formed due to hydrolytic degradation and considered an important marker to measure the rancidity of the food/lipids. The statistically analyzed data (Fig. 2a) indicated that the free fatty acids increased with the storage time. Highest FFA contents (0.8%) were observed in control group (T1) (p < 0.05) on the 30th day while T4 and T5 showed the lowest FFA contents (0.5% and 0.51% respectively) on the same day of storage (i.e. 30th day). These results signify anti-oxidation activity of the SSE. Initially, there was no substantial increment in FFA (i.e. ranging from 0.15 to 0.3% only) but after 7–8 days of storage; there was an increase in FFA contents in treated groups ranging from 0.15% to 0.55%. Results (Fig. 2a) revealed that T4 group had lower FFA contents (e.g. 0.16, 0.36 and 0.5% on day 10, 20 and 30) which were close to FFA values (0.16, 0.39 and 0.51%on day 10, 20 and 30) of the reference group (T5) respectively. So we deduced that SSE can be a proven “asset” in enhancing the shelf life of oils and lipids by offering an effective check against the FFA productionas our outcomes showing promising results in maintaining the quality attributes of sunflower oil. Previously, Iqbal and Bhanger (2007) also claimed that antioxidant activity of garlic extracts in the sunflower oil exhibited the lowest/equal FFA contents as compared with synthetic antioxidant BHT. So our results also suggested that the SSE can also be used as potential natural antioxidant source in the oil and fats with their antioxidative activity comparable to synthetic antioxidants.
Effect of different concentrations of sesame seed extracts on Free fatty acid value (a) iodine value (b) specific gravity (c) saponification value (d) peroxide value (e) and the TBARs value (f) of sunflower oil during storage. T1: refined sunflower oil T2: (500 μL of SSE/100 mL of refined sunflower oil), T3: (750 μL of SSE/100 mL of refined sunflower oil), T4: (1000 μL of SSE/100 mL of refined sunflower oil), T5: (BHT 200 ppm/100 mL of refined sunflower oil). Asterisks indicate that the differences (*p < 0.05; **p < 0.01; ***p < 0.001) between the means of different treatments are statistically significant as determined by one-way ANOVA with posthoc Tukey’s honestly significant difference test. Sunflower oil with sesame seed extracts exhibited the less oxidation value than the refined sunflower oil. Among all group, T4 was more able to reduce the lipid oxidation during storage after synthetic antioxidant BHT
Iodine value
The iodine value of control (T1), treated (T2 to T4) and referenced group (T5) are shown in Fig. 2b. The outcomes clearly indicated that iodine value of all groups varied significantly (p < 0.01) over the designated storage period. Marked variation in the iodine value depicted vast capability of oil to hinder the occurring of saturation. Obviously, the groups with added antioxidants (both SSEs and BHT) showed significant improvement in hampering the sensitive level of unsaturations, although, the capacity of hampering the unsaturation varied markedly from group to group depending upon the nature of added antioxidant. Generally, Fig. 2b revealed relatively less Iodine value of all groups within first ten (10) storage days (except control group). Then, an abrupt and flat decrease in iodine values were seen across all the treated groups (T2 to T5) till end of storage duration. Nonetheless, the reduction in iodine value was noticed higher during the last 10–20th days of storage. The iodine value of control group (T1) was found profoundly decrease from 130.4 g/100 g (day 0) to 79.9 g/100 (30th day) than that of all treated and referenced group. It is obvious that addition of SSE in all concentrations markedly prevent the iodine value of oil sample from decreasing throughout the storage period. Increment in the SSE concentration (T2 to T4) in oil samples correspondingly reduced the iodine value in treated groups. Furthermore, T4 showed decreased in iodine values (i.e. 130.5.6 g/100 g (day 0) to 124.2 g/100 (30th day)) as compared to other treated group. The iodine value of sunflower oil from group T4 were found significantly closer to iodine value of reference group (T5); (i.e. 130.6, (day 0) to 120.4 g/100 (30th day). this relation clearly symbolized its capability of replacing the synthetic antioxidant. Farag et al. (2006) also used the sunflower oil and checked its stability by utilizing the olive leaf juice after heating at 180 °C; they also documented the less iodine values of stored oil by using the olive leaf juice extracts. Similar results were found for stabilization of sunflower by SSE in this study, a lower iodine value was found in T4 group.
Specific gravity
Specific gravity is important to measure the incident of polymerization, which makes the oil denser. Previous studies on sunflower oil demonstrated that there was a gradual increment in specific gravity under storage condition (Anjum et al. 2006). Antioxidants are used to stabilize the oil polymerization and specific gravity but this event found to be increased with storage time. However, a slight decrease in specific gravity with sesame seed extracts ranging from 0.91 to 0.92 was observed and T4 demonstrated the least specific gravity value among all the extracts except the reference T5 (Fig. 2c), therefore it was considered as the best concentration of sesame seed extracts to stabilize the sunflower oil from getting denser.
Saponification value
Saponification value of sunflower oil increased with the increment of time, moisture and temperature (Anjum et al. 2006).The effect of natural antioxidants in stabilizing the oils represented by saponification value was less reported. The saponification value of sunflower oil with sesame seed extract groups was shown in Fig. 2d. The data showed a slight increase in saponification value while using sesame seed extract. Highest saponification value was obtained by control group (T1) and lowest in T4 group after reference group (T5) which is a good indication of using natural antioxidant in order to decrease the saponification value of sunflower oil.
Peroxide value (PV)
Peroxide value is a measure of peroxide and hydroperoxide formed in the initial stage of primary oxidation of the oil, act as an indicator for the primary oxidation and rancidity of the fats and oils (Asnaashari et al. 2015). By increasing the storage time, a gradual increase in peroxide value of all the samples was observed (Fig. 2e). At all stages, highest PV was observed for control sample followed by T2, T3, T4 and T5 respectively. Sesame seed extract at all concentrations decreased the peroxide value of the oil which concluded the good antioxidant ability in stabilizing the sunflower oil. Peroxide value for all the samples increase at all storage period. Such pattern was also observed by Iqbal and Bhangar who studied the effect of methanolic extract of garlic on the stability of sunflower oil. The garlic extract had lower peroxide value than control sample and comparable to BHT (Iqbal and Bhanger 2007). The present results revealed that the T5 group of SSE have better antioxidant activity than the previously reported garlic extract and can be considered as BHT alternative to stabilize the sunflower oil.
Thiobarbituric acid reactive substances (TBARS)
TBARS measures the secondary oxidation product of the oil (i.e. aldehydes or carbonyl) and important to check the rancidity of fats and oils. TBARS for all the samples were determined up to 30 days of storage (Fig. 2f). The results indicated the use of SSEs resulted in significant inhibition of TBARS. The control group (T1) exhibited the highest TBARS value (i.e. 0.11, 0.2767 and 0.351 μM/g of oil on 10, 20 and 30th day of storage) whereas least TBARS values (i.e. 0.1, 0.1867 and 0.23 μM/g of oil on 10, 20 and 30th day of storage) were observed with reference group T5. Comparing the antioxidant potential of the reference group (T5) with all treatments, T4 group represented the lowest TBARS value which was close to the T5 group. Thus, SSEs are considered as the best natural antioxidants to stabilize the sunflower oil. Results of our study were found in accordance with the previous studies, which were done on garlic extract and pussy willow (Iqbal and Bhanger 2007).
Oxidative stability of products developed by sunflower oil with SSE
Degradation, rancidity and autoxidation of lipids in food systems caused the changes in the quality of the cooked and baked product (Ngadi et al. 2009). The primary and secondary oxidation products not only demolish the sensory attributes, but also have serious health hazard issues. The oxidative stability of sunflower oil with added SSEs were further evaluated by baking the muffins, its oxidative and sensory evaluation after cooking as well as during storage were also determined.
Sensory evaluation
Color, taste, texture, flavor and total acceptability of muffin were evaluated at 0, 3, 6 and 9 days interval. A decreasing trend in all these factors was observed during storage as compared to control group (Fig. 3). The color, taste, texture and flavor of muffins baked with SSE added sunflower oil were improved or/maintained and was comparable with the standard BHT group. Appearance is first judgmental tool for the selection of any food item and color is an integral part of it. Figure 3a showed that SSE from T4 group not only enhance the shelf life of product, but also improved the color of muffins compared with control group. Regarding flavor, referenced group T5 got more scores (i.e. 7.9, 8.5 and 8 on day 3, 6 and 9) throughout the period followed by T4 group (i.e. 7.7, 8.2 and 8.5 on day 3, 6 and 9) (Fig. 3b). In addition, significant improvement was also observed in the taste of muffins baked with sunflower oil containing SSE. T4 group demonstrated the substantial score (i.e. 8.2, 8.2 and 7.8 on day 3, 6 and 9) than the referenced group (Fig. 3c). BHT is a white crystalline solid with a faint phenolic odor. Previously, some authors reported the addition of BHT in food causes no change in color, odor and flavor of the food (Adegoke et al. 1998). But in our case, we found that T4 group possesses the equivalent or/highest score (in some parameters) for all these factors after the reference group (T5) and considered as the best group among samples (Fig. 3). To best of our knowledge, the current investigation provides first evidence in regard to improvement in the sensory parameters of baked product by SSEs.
Sensory evaluation of muffins baked from sunflower oil groups. a Color of muffins, b flavor of muffins, c taste of muffins and d texture of muffins. T1: refined sunflower oil, T2: (500 μL of SSE/100 mL of refined sunflower oil), T3: (750 μL of SSE/100 mL of refined sunflower oil), T4: (1000 μL of SSE/100 mL of refined sunflower oil), T5: (BHT 200 ppm/100 mL of refined sunflower oil). Asterisks indicate that the differences (*p < 0.05; **p < 0.01; ***p < 0.001) between the means of different treatments are statistically significant as determined by one-way ANOVA with posthoc Tukey’s honestly significant difference test. The muffins baked from sunflower oil with 1000 μL sesame seed extract (T4) achieved the highest score than other groups and more effective than synthetic antioxidant BHT
The possible reasons behind this dilemma include the potential interactions of pigments or/and antioxidant or/and flavoring/coloring compounds from sesame extracts with the compounds of Millard reaction which occurred during the baking of sugary muffins leading to an overall improvement in stated parameters. In addition, the T5 treatment was solely composed of one phenolic compounds BHT with its own stated bitterness in literature and so there would be no diverse reactions among mallard reaction products and BHT which could improve the sensory parameters. Moreover, it is also noteworthy that sesame has also been a customary item in the production of various cereal based bakery products where it is added to enhance the flavor, taste, palatability, nutrition and physical appeal. Our current findings not only in line with these customary practices but also provide scientific based evidence about this subject. Sensory evaluation of cooked products by using the natural antioxidants is, so far, carried out by very few researchers. In 2008, the sensory evaluation of cooked sausage in linseed oil along with antioxidant (green tea catechins and green coffee) was reported. Flavor, odor, color and overall acceptability of the sausage remain unaffected and demonstrated the potential of antioxidant for the production of nutritionally enhanced sausages (Valencia et al. 2008). Our study also proved that the addition of natural antioxidants from sesame seed cannot only extend the shelf life of sunflower oil but also found to have least influence on the sensory attributes (taste, flavor, color, texture) of resulting products.
Oil oxidation evaluation
In Manral et al. (2008), reported that the quality of the fish fried in rancid sunflower oil was affected. Total polar compounds, free fatty acids, iodine value and peroxide value of the rancid sunflower oil was evaluated by drawing out the samples from the fryer at different intervals. Gradual elevation of oil quality parameters which caused the rancidity of the sunflower oil was observed. Previously, Naveena et al. (2008) reported that sesame seed extract at the level of 10 mg equivalent phenolics/100 g was sufficient to protect the chicken patties against oxidative rancidity. Similarly, after interventions with the storage and shelf life studies, we also measured the free fatty acids, peroxides and TBARS values of sunflower oil after baking the muffins to check the oxidation level of sunflower oil with sesame seed extracts (SSEs). The results were presented in Fig. 4. Control group (T1) showed maximum amount of oxidation products (Fig. 4a) ranged from 6.7 to 14.21 meq/mg while the treated group T4 showed minimum peroxide values (i.e. 1.15, 3.24 and 3.56 meq/mg for day 10, 20 and 30th respectively) which was less than referenced groups (T5) peroxide values (i.e. 1.77, 3.25 and 3.81 meq/mg for day 10, 20 and 30th respectively). In case of free fatty acid values, similar trend with a minor variation during the last 10 days of storage was observed (Fig. 4b). The T4 group showed the least free fatty acid values during the first 20 days of storage (i.e. 0.15 and 0.356% for 10 and 20th day of storage) after that, free fatty acid values of T4 slightly increased (i.e. 0.576%) than that of referenced group (i.e. 0.56%) on 30th day. The TBARS values clearly made a distinction among all groups. The treated groups (T2-T4) clearly showed less TBARS values than control and referenced group which proved that SSE even at low concentrations can be effective against the generation of secondary oxidation products (Fig. 4c). The lowest TBARS values were shown by T4 group (i.e. 0.14, 0.19 and 0.25 μM/g on 10, 20 and 30th day) followed by referenced group T5 (i.e. 0.18, 0.22 and 0.27 μM/g on 10, 20 and 30th day)(Fig. 4c).Thus, results revealed that T4 group reduced the lipid oxidation more effectively than other groups before and after baking, and act as a best natural antioxidant for sunflower oil. Despite the health and sensory benefits of sesame, its application in fat and oil industries was also found economical as price of 1 kg SSE ranging from 12 to 18 USD per kg). 1 kg of SSE was adequate to preserve 13.3 × 106 L oil (T4 data), whereas 1.0 kg BHT (21–28 USD per kg) can only be used to preserve 10,000 L oil at legal of 0.02% of weight of fat/oil. However, a comprehensive and long term research study would be require in this aspect to finally recommend the dose of sesame extracts for quality and enhancing the shelf life of solely fats and oils.
Effect of different storage time on peroxide value (a), free fatty acid (b), TBARs value (c). T1: represents refined sunflower oil, T2: (500 μL of SSE/100 mL of refined sunflower oil), T3: (750μL of SSE/100 mL of refined sunflower oil), T4: (1000 μL of SSE/100 mL of refined sunflower oil), T5: (BHT 200 ppm/100 mL of refined sunflower oil). Asterisks indicate that the differences (*p < 0.05; **p < 0.01; ***p < 0.001) between the means of different treatments are statistically significant as determined by one-way ANOVA with posthoc Tukey’s honestly significant difference test. Sunflower oil with sesame seed extracts exhibited the less oxidation value than the refined sunflower oil. Among all groups, T4 group was more able to reduce the lipid oxidation during storage after synthetic antioxidant (BHT)
Conclusion
From the present study, it was concluded that SSE can stabilize sunflower oil effectively at a concentration of 1000 μL/100 mL of sunflower oil. It inhibits the thermal deterioration of oil by improving its hydrolytic stability, inhibit the lipid oxidation and reduce the loss of polyunsaturated fatty acids (PUFAs). SSE at concentration of 1000 μL/100 mL of oil has stabilization efficacy comparable to the common synthetic antioxidant BHT at its legal limit. Therefore, SSE can be recommended as the potent source of natural antioxidant for the stabilization of food and food product, especially edible vegetable oils rich in unsaturated fatty acids.
References
Adegoke GO, Vijay KM, Gopala KAG, Varadaraj MC, Sambaiah K, Lokesh B (1998) Antioxidants and lipid oxidation in foods: a critical appraisal. J Food Sci and Technol 35:283–298
Anilakumar KR, Pal A, Khanum F, Bawa AS (2010) Nutritional, medicinal and industrial uses of sesame (Sesamum indicum L.) seeds – an overview. Agric Conspec Sci 75:159–168
Anjum F, Anwar F, Jamil A, Iqbal M (2006) Microwave roasting effect on the physiochemical composition and oxidative stability of sunflower seed oil. J Am Oil Chem Soc 83:777–784
AOAC (2003) AOCS official method CD 8–53. Official methods and recommended practises of the American Oil Chemists’ Society. Champaign, IL, USA
Asnaashari M, Faroosh R, Sharif A (2014) Antioxidant activity of gallic acid and methyl gallate in triacylglycerols of Kilka fish oil and its oil in water emulsion. Food Chem 159:439–444
Asnaashari M, Tajik R, Khodaparast MHH (2015) Antioxidant activity of raspberry (Rubus fruticosus) leaves extract and its effect on oxidative stability of sunflower oil. J Food Sci Technol 52:5180–5187
Balasundram N, Sundram K, Samman S (2006) Phenolic compounds in plants and agri-industrial byproducts: antioxidant activity, occurrence, and potential uses. Food Chem 99:191–203
Bopitiya D, Madhujith T (2012) Antioxidant potential of pomegranate (Punica Granatum L.) cultivars grown in Sri Lanka. Trop Agric Res 24(1):71–81
Castro MD, Capote FP (2010) Soxhlet extraction: past and present panacea. J choromo A 1217:2383–2389
Dorman HJD, Kosar M, Kahlos K, Holm Y, Hiltunen R (2003) Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties and cultivars. J Agric Food Chem 51:4563–4569
Duh PD, Yen GC (1997) Antioxidant efficacy of methanolic extracts of peanut hulls in soybean and peanut oils. J Am Oil Chem Soc 74:745–748
Eshghi N, Asnaashari M, Haddad KMH, Hosseini F (2014) Evaluating the potential of natural curcumin for oxidative stability of soybean oil. Nat Prod Res 28:1375–1378
FAO (2016) The Global Agriculture Perspectives System (GAPS): Version 1.0, by Aikaterini Kavallari, Piero Conforti and Dominique van der Mensbrugghe. ESA Working Paper No. 16-06. Rome, FAO
Farag RS, Mahmoud EA, Basuny AM (2006) Use crude olive leaf juice as a natural antioxidant for stability of sunflower oil during heating. Int J Food Sci Technol 42:107–115
Farahmandfar R, Asnaashari M, Sayyad R (2015) Comparison antioxidant activity of Tarom Mahali rice bran extracted from different extraction methods and its effect on canola oil stabilization. J Food Sci Technol 52:6385–6394
Farhoosh R, Johnny S, Asnaashari M, Molaahmadibahraseman N, Sharif A (2016) Structure antioxidant activity relationships of o-hydroxyl, o-methoxy, and alkyl ester derivatives of p-hydroxybenzoic acid. Food Chem 194:128–134
Hameed A, Hussain SA, Yang J, Ijaz MU, Liu Q, Suleria HAR, Song Y (2017) Antioxidants potential of the filamentous fungi (Mucor circinelloides). Nutrients 9:1101
Hashemi SMB, Khaneghah AM, Tavakolpour Y, Asnaashari M, Mehr HM (2015) Effects of ultrasound treatment, UV radiation and Avishan-e-Denaei essential oil on oxidative stability of sunflower oil. J Essent Oil Bear Plants 18:1083–1092
Heim KE, Taigliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationship. J Nutr Biochem 13:572–584
Iqbal S, Bhanger MI (2007) Stabilization of sunflower oil by garlic extract during accelerated storage. J Food Chem 100:246–254
Iqbal S, Bhanger MI, Anwar I (2005) Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chem 93:265–272
Keyvan DHJ, Damien D, Into L, Raimo H (2007) Chemical composition and antioxidative activity of Moldavian balm (Dracocephalum moldavica L.) extracts. LWT Food Sci Technol 40:1655–1663
Lee J, Kim M, Choe E (2007) Antioxidant activity of lignin compounds extracted from roasted sesame oil on the oxidation of sunflower oil. Food Sci Biotechnol 16:981–987
Li Y, Guo C, Yang J, Wei J, Xu J, Cheng S (2005) Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem 96:254–260
Lim J (2011) Hedonic scaling, a review of methods and theory. Food Qual Prefer 22:733–747
Manral M, Pandey MC, Jayathilakan K, Radhakrishna K, Bawa AS (2008) Effect of fish (Catlacatla) frying on the quality characteristics of sunflower oil. Food Chem 106:634–639
Miniotia KS, Georgioua CA (2010) Comparison of different tests used in mapping the Greek virgin olive oil production for the determination of its total antioxidant capacity. Grasa Y Aceites 61:45–51
Mohdaly AA, Sarhan MA, Smetanska I, Mahmoud A (2010) Antioxidant properties of various solvent extracts of potato peels, sugar beet pulp and sesame cake. J Sci Food Agric 90:218–226
Naveena BM, Sen AR, Vaithiyanathan S, Babji Y, Kondaiah N (2008) Comparative efficacy of pomegranate juice, pomegranate rind powder extract and BHT as antioxidants in cooked chicken patties. Meat Sci 80:1304–1308
Ngadi MO, Wang Y, Adedeji AA, Raghavan GSV (2009) Effect of microwave pretreatment on mass transfer during deep-fat baking of chicken nugget. LWT Food Sci Technol 42:438–440
Opara LU, Al-Ani MR, Al-Shuaibi YS (2009) Physico-chemical properties, vitamin C content, and antimicrobial properties of pomegranate fruit (Punica granatum L.). Food Bioprocess Technol 2:315–321
Prior RL (2004) Absorption and metabolism of anthocyanins: potential health effects. Phytochemical: mechanism of action, 1st edn. CRC Press, Boca Raton, pp 1–19
Ramadan MF, Zayed R, El-Shamy H (2007) Screening of bioactive lipids and radical scavenging potential of some solanaceae plants. Food Chem 103:885–890
Reshma MV, Balachandran C, Arumughan C, Sunderasan A, Sukumaran D, Thomas S, Saritha SS (2010) Extraction, separation and characterization of sesame oil lignan for nutraceutical applications. Food Chem 120:1041–1046
Saleem TM, Basha SD, Mahesh G, Rani PS, Kumar NS, Chetty CM (2011) Analgesic, anti-pyretic and anti-inflammatory activity of dietary sesame oil in experimental animal models. Pharmacologia 2:172–177
Steel RGD, Torrie JH, Dicky DA (1997) Principles and procedures of statistics. A biological approach, 3rd edn. McGraw Hill Book Co., Inc., New York
Stefansson BR (2009) Oilseed crops, the Canadian encyclopedia. Historica Foundation, Toronto
Valencia I, O’Grady MN, Ansorena D, Astiasarán I, Kerry JP (2008) Enhancement of the nutritional status and quality of fresh pork sausages following the addition of linseed oil, fish oil and natural antioxidants. Meat Sci 80:1046–1054
Zeb A, Muhammad B, Ullah F (2017) Characterization of sesame (Sesamum indicum L.) seed oil from Pakistan for phenolic composition, quality characteristics and potential beneficial properties. Food Meas. https://doi.org/10.1007/s11694-017-9514-5
Zhou K, Liangli Y (2006) Total phenolic contents and antioxidant properties of commonly consume vegetables grown in Colorado. LWT Food Sci Tech 39:1155–1162
Acknowledgements
This study is funded by National Natural Science foundation, PR. China (Grant Nos. 31670064 and 31271812) and TaiShan Industrial Experts Programme.
Author information
Authors and Affiliations
Contributions
Y.S. and H.A.R.S supervised this work. S.A.H. and A.H performed all the experimental work. S.A.H wrote this manuscript. I.A and S.N analyzed the data and helped in the statistical analysis. Y.S and H.A.R.S. edited and critically reviewed the whole manuscript and provided suggestions to main authors about overall research plan. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Hussain, S.A., Hameed, A., Ajmal, I. et al. Effects of sesame seed extract as a natural antioxidant on the oxidative stability of sunflower oil. J Food Sci Technol 55, 4099–4110 (2018). https://doi.org/10.1007/s13197-018-3336-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3336-2