Abstract
Six storage trials were conducted for preservation of Nile tilapia (Oreochromis niloticus) in ice storage at 5 °C using moringa leaves powder. The study aims to prolong the storage life of tilapia fish using moringa leaves powder of different concentrations. The effect of the moringa leaves powder (MLP) on biochemical, microbiological and sensory properties of fresh Nile tilapia stored in ice at 5 °C was investigated. Total volatile basic nitrogen, pH and thiobarbituric acid reactive substances values in MLP treated tilapia fish were 18.18 mg/100 g, 6.57, 5.63 mg MDA/kg respectively, which were significantly lower compared to untreated fish. Bacterial count (total viable count) was delayed significantly by increasing MLP concentration. The treated sample remains acceptable during storage in ice at 5 °C up to 11 days, whereas shelf life of untreated sample of tilapia was not extended beyond 7 days. The corresponding microbiological assessment also supports the results of sensory assessment that increased in shelf-life. The results revealed that the MLP is natural preservatives for extending the shelf life of Nile tilapia during transportation and ice storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nile tilapia (Oreochromis niloticus) is the predominant cultured fish species worldwide. It is more tolerant to lower temperatures than most of other tilapia species. Fish are highly perishable food at ambient temperature and high quantity of fish spoils due to lack of good preservation methods. The fish quality is generally assessed by sensory tests, biochemical methods (e.g. volatile basic compounds, lipid oxidation) and bacterial counts (Raatikainen et al. 2005).
The frozen or cold storage does not entirely stop bacterial and biochemical reactions and leads to the spoilage of fish meat. The fish spoilage generally occurred either by lipid oxidation, enhanced enzymatic activities, lack of metabolic activities and/or microbial growth (Li et al. 2011). Microbial growth produces biogenic amines e.g. histamine, cadaverine and putrescine; organic acids, alcohols, aldehydes, sulphides and ketones with unacceptable and unpleasant odor and off-flavors (Dalgaard et al. 2006).
Many chemicals such as formalin, nitrites, sulphites, ethylenediaminetetraacetic acid (EDTA), lactic acid, benzoic acid, ascorbic acid etc. are commonly used as antimicrobial or as antioxidants to delay the spoilage of fish (Ghaly et al. 2010). In developing countries, food and food constituents are possibly contaminated with various types of hazardous substances including dyes, formalin, insecticides, banned antibiotics etc. while passing through supply chain from farm to fork level (Yeasmin et al. 2010). The chemical substances, such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) have widely been used as preservatives during frozen fish storage. In order to evade the use of artificial preservatives, at present many studies are engaged in using organic ingredients to enhance the shelf life and quality of fish.
The use of organic antioxidants and antimicrobial especially plant materials, are appropriate for the development and preservation of food products (Sadiq et al. 2017a). Plant extracts are recently proposed to preserve the frozen minced and filleted fish products storage quality (Lugasi et al. 2007). Moringa oleifera universally known as Drumstick is believed to be the most useful tree in the world. The leaves of M. oleifera are used as food components for humans as well as animals (Dongmeza et al. 2006). The moringa leaves have antimicrobial and antioxidants activities (Sánchez-Machado et al. 2010) and recently have been described to increase the shelf life of chicken sausages (Jayawardana et al. 2015) and goat meat patties (Das et al. 2012).
None of the study has been reported for extension of the shelf life of fresh fish using moringa leaves in supply chain and storage. Thus, the present study was conducted to investigate the application of moringa powder to enhance the shelf life and quality attributes of Nile tilapia (O. niloticus) during storage and transportation. The quality attributes were analyzed in terms of sensory, microbiological (total viable count, TVC), biochemical [total volatile basic nitrogen (TVB-N), peroxide value (PV) and thiobarbituric acid reactive substances (TBARS)] and physical changes (color and texture).
Materials and methods
Preparation of moringa leaves powder ice
Moringa oleifera leaves powder (MLP, particle size 425 µm) was procured from ‘Organicthailand’, Lopburi province, Thailand. Different concentrations (1, 2, 3 and 4%, w/v) were prepared separately by completely dispersing (10, 20, 30 and 40 g) the powder in 1000 ml of filtered potable water (Adeyemi et al. 2013). The MLP-water mixtures were stored in freezer in cups and crushed ice was prepared for further experiments. Crushed ice from filtered potable water without MLP was used as control.
Preparation of fish samples
Live tilapia fish with a weight of 0.60 ± 0.10 kg each were obtained from the tilapia fish farm of Asian Institute of Technology, Thailand. Fishes were immediately stored in styrofoam boxes containing ice to kill by ice-shocking and transported to the laboratory immediately.
Whole fish were washed with potable water and used for the experiment. Four treatments of MLP ice were used as preservatives. The fish were stored in crushed ice prepared from moringa leaves powder (fish to ice ratio of 1:1.5, w/w) by keeping the fish in between two layers of MLP treated ice in insulated styrofoam ice boxes. Four batches of treated samples were stored in cold room (5 ± 1 °C) for further study. The batch of control samples were also kept in cold storage having 5 °C temperature. The fish samples stored in MLP treated ice and control one in cold storage were randomly taken every 24 h from each batch stored. The flesh from dorsal portion of the fish was uniformly cut with a sterile knife and used for sensory, biochemical and microbiological assessments.
Preparation of crude extracts from M. oleifera leaves powder
The MLP extracts were prepared as prescribed by Sadiq et al. (2017b) by slight modification. The leaves powder (30 g) in conical flasks filled with ethanol (80% v/v, 200 ml) were placed in shaking incubator (Gallenkamp, UK) at 200 rpm for 48 h. Whatman No.2 filter paper (GE Healthcare, UK) was used to filter the extracts and concentrated using rotary evaporator (Büchi Rotavapor R-144, Switzerland) and kept in a freezer at 5 ± 1 °C for 24 h. The frozen concentrates were then subjected to freeze drying (Scanvac Cool Safe 55-4, Denmark) for 24 h. The extracts were stored at 4 °C till further use and reconstituted in methanol (80% v/v, analytical grade) to make stock solutions (1 mg/ml) for DPPH assay and total phenolic content.
Phenolic content and antioxidant activity
Folin–Ciocalteu reagent (Sigma-Aldrich, Switzerland) was used to determine the total phenolic content (TPC) of moringa leaves extract as described by Hiranrangsee et al. (2016) with slight modification. The stock solution (1 mg/ml) of moringa leaves extract was prepared by diluting it in deionized water. The stock solution further diluted in deionized water to prepare 100 μg/ml. Folin–Ciocalteu reagent was prepared by diluting with de-ionized water (1:10). For the extract, 0.5 ml (100 μg/ml) was mixed with 2 ml of the freshly prepared reagent and added sodium carbonate (4 ml; 7.5% w/v) to neutralize the reaction mixture. After neutralization, the mixture was stored at 25 °C for 30 min with intermittent shaking. The absorbance was measured at 765 nm by UV-spectrophotometer (UV-1800, Shimadzu UV spectrophotometer, Japan.). The results were expressed as gallic acid equivalents (GAE) per g of sample.
Determination of diphenylpicrylhydrazyl (DPPH1:1 diphenyl-2-picryl hydrazyl) radical scavenging activity of moringa leaves extract was determined as described by Sadiq et al. (2015) with slight modification. Different concentrations of the extract (400, 200, 100, 50 and 20 μg/ml) were prepared from stock solution for the assay. Ascorbic acid was used as a positive control. Fifty microliters from each concentration of samples was then mixed with methanol solution of DPPH (5 ml, 40 ppm; Sigma-Aldrich, USA). The mixture was incubated at 25 °C for 30 min in a dark place. UV spectrophotometer (UV-1800, Shimadzu UV spectrophotometer, Japan.) was used to read the absorbance against blank at 517 nm. The methanol and DPPH solution without leaves extract was used as blank and control respectively. The DPPH assay was performed in triplicate and the inhibition (%) was calculated by following formula:
where, AC indicates absorbance of control and AS indicates absorbance of test sample.
The IC50 values representing the concentration of sample required to scavenge 50% of the DPPH free radical (Chen et al. 2013). The IC50 values were found out by plotting the percent inhibition with the concentration of moringa leave extract.
Microbial estimation
Fish sample (10 g) was shifted aseptically into a stomacher bag containing sterile saline water (NaCl 90 ml, 8.5 g/l) and homogenized using a stomacher (Bagmixer 400, Interscience, France) for 1 min. From the resulting slurry, decimal dilutions (10−1–10−6) were made and spread on the surface of sterile plate count agar (PCA, HiMedia Laboratories, India) plates. The plates were further incubated at 30 °C for 72 h (Li et al. 2011). Total viable counts (TVC) were estimated by counting the number of colony forming unit (cfu) and expressed as cfu g−1 of fish sample.
Chemical analysis
Fish muscle was taken and ground using grinder. A proximate composition (moisture content, lipid content, crude protein and ash content) analyses was carried out by AOAC standard procedures (AOAC 2002). Ground fish sample (10 g) was mixed with 100 ml of distilled water. The pH of the mixture was determined using a digital pH meter (Cyberscan PC 510 UK). The total volatile basic nitrogen (TVB-N) content of fish samples was determined after extracting fish muscle in 0.6 M perchloric acid solution according to the method described by Anderson (2008) with slight modification. Lipid oxidation in fish muscle was assessed by measuring thiobarbituric acid reactive substances (TBARS) spectrophotometrically by slightly modifying the method described by Yarnpakdee et al. (2012). TBARS were expressed as mg of malonaldehyde (MDA) equivalents/kg sample. Peroxide value (PV) was measured by spectrophotometric method as described by Anal et al. (2014) with slight modifications.
Color measurement
The tilapia meat color was determined by colorimeter (ColourFlex, Hunter Lab Reston, VA, USA) method as describe by Gao et al. (2014) with slightly modifications. The colorimeter was calibrated to a white standard (L* = 93.33, a* = − 0.91, b* = 1.46) and black standard. The color was measured at three locations; anterior, middle and posterior, as the fish fillet color was not uniform over the whole surface area. The color of untreated and treated samples was measured six times.The whiteness was calculated using the following equation:
where, L* = 100 indicates ‘white’, L* = 0 indicates ‘black’, a* (positive) values indicate ‘redness’, a* (negative) indicate ‘greenness’ and b* (positive) values indicate ‘yellowness’ while b* (negative) values indicate ‘blueness’.
Texture analysis
A hardness of preserved samples was carried out by texture profile analysis (TPA) method ‘Texture Analyzer (TA-XT plus, Stable Microsystems, UK)’ as suggested by Gao et al. (2014) with slight modification by compressing the sample twice. The TPA was determined by a ‘Texture Analyzer (TA-XT plus, Stable Microsystems, UK)’ with a 30 mm diameter cylindrical probe to compress the fish meat. The texture analyzer was calibrated with 50 kg load and the instrument was set with test speed (3.0 mm/s), pre-test speed (1.0 mm/s), post-test speed (5.0 mm/s), the distance (3 mm) and trigger force (5 g). The mean values (± SE) of the TPA parameters were calculated with force by time data from every test and expressed as gram force (gf). The hardness values were determined at 24 ± 1 °C.
Organoleptic evaluation of preserved tilapia
Sensory analysis and overall acceptability of preserved tilapia were assessed with the quality index method (QIM) by a panel of ten trained panelists (Nielsen and Hyldig 2004). Samples were observed physically for appearance, flesh consistency, odor, gills, eyes, and scored as zero, one, two and three. Higher the scores values indicate the poorer of fish quality. The scores of different features were summed to make an overall sensory evaluation. Panelists were requested to state whether the given fish samples were acceptable or rejected.
Statistical analysis
Experiments were run with six replications of three samples analyzed for all treatments. The data were conducted to descriptive statistics and one-way analysis of variance to test the effect of MLP levels and time intervals. The values were expressed as arithmetic mean with the standard errors of means. The significance level of P < 0.05 was used for the significant differences among means using a post hoc test (Tukey’s test). All analyses of statistics were run with SPSS program (IBM SPSS Statistics 2016, Version 24).
Results and discussion
Total phenolic content and antioxidant activity in moringa leaves
The TPC of moringa leaves was 37.64 ± 0.61 mg GAE/g dry extract. The percentage of DPPH radical scavenging activity of moringa leaves extract with the concentration of 400, 200, 100, 50 and 20 μg/ml was exhibited as 72.10 ± 0.43, 64.64 ± 0.27, 49.87 ± 0.36, 38.79 ± 0.22 and 26.09 ± 0.19% respectively and a DPPH radical scavenging activity with IC50 of 0.15 ± 0.001 mg/ml. Similarly Sreelatha and Padma (2009) reported the significant reduction in DPPH radicals in moringa leaves extract.
Proximate composition
Proximate compositions analyses of fresh tilapia fish meat showed 16.30 ± 0.14% protein, indicates good table fish. Lipid content of the tilapia fish meat was found to be 1.74 ± 0.10% which gives delicious taste. Moisture and ash (w/w) contents were determined as 79.09 ± 0.23 and 1.74 ± 0.10%, respectively. The various studies have been reported the tilapia fish proximate composition at various locations (El-Hanafy et al. 2006), stated some variations, mainly in the lipid and protein content. Those variations are closely related to the variation in fish size, different nutrition, gender, season of catch and catching methods (Alasalvara et al. 2002). The variations in proximate compositions may affect the organoleptic attributes and microbial growth.
Microbial estimation by TVC
The changes in bacterial load in flesh (without skin) of tilapia fish during ice storage with 1, 2, 3 and 4% MLP at 5 °C are shown in Fig. 1. The initial total viable count in control sample at 5 °C storage was 3.26 log10 cfu g−1. The TVC in untreated sample increased significantly during ice storage, which reached 7.11 log10 cfu g−1 at the end of 6 days storage. The total bacterial count of 7 log10 cfu g−1 is suggested as the maximum limit of acceptability for marine and fresh water fish Ozyurt et al. (2009). The initial microbial quantity and quality are closely related to the fish storage life. Nile tilapia treated with different concentrations of MLP had no significant effect (P > 0.05) on the initial bacterial count wherein the results were 3.21, 3.32, 3.29 and 3.30 log10 cfu g−1 for the samples treated with MLP with levels of 1, 2, 3 and 4% at time zero stored at 5 °C. Whereas, the TVC were significantly increased (P < 0.05) reaching 7.41, 6.66, 7.51 and 7.37 log10 cfu g−1 during the samples stored with MLP treatment with levels of 1% (after 7 days), 2% (after 9 days), 3% (after 11 days) and 4% (after 11 days), respectively. The samples stored at 5 °C showed that 3 and 4% MLP treated samples retained acceptable microbial count till 11 days which is higher than that of other treatments. Similarly, the continuous increased of TVC in pompano fillets was reported by Gao et al. (2014) and in crucian carp, treated with tea polyphenol and rosemary extract during storage at 4 ± 1 °C temperature was documented by Li et al. (2012). The results of this study showed that bacteria grew more quickly in control samples than MLP treated samples during the storage periods. The results of the treatment also indicated that 3 and 4% MLP treatments were efficient in slower the growth rate of spoilage bacteria and exceeded the shelf life of tilapia in cold storage to 11 days, compared to 6 days of control sample. The conclusion suggests that the bacteriological analyses considerably correlated with sensory analyses of the fish which is discussed further.
Chemical analysis
pH value and total volatile basic nitrogen (TVB-N)
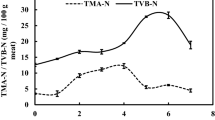
Variations in the pH values of Nile tilapia meat during ice storage are presented in Fig. 2. Throughout the entire storage period at 5 °C, the pH of untreated samples increased from 6.49 at zero day to 7.43 at the end of 7 days. The MLP treatments with the level of 2, 3 and 4% maintained the pH to 6.92, 6.62 and 6.57, respectively at the end of 7 days which is significantly lower (P < 0.05) pH was observed than that of the untreated and 1% MLP treated samples during the subsequent cold storage at the end of 7 days (Fig. 2). The increase in pH with storage time was also observed in treated samples during the entire storage period. The initial pH of the samples observed was slightly lower than the initial pH reported by Li et al. (2012) which was 6.61 for crucian carp. Variations in the initial pH values are generally caused by the diet, fish species, seasonal conditions and stress during the catch and muscle type Li et al. (2012). El-Hanafy et al. (2011) reported that the pH of untreated sample was slightly increased from initial pH 6.51 to 6.83 after 6 days of storage during frozen storage of tilapia fish. Similarly increase in pH has been reported by Ozyurt et al. (2009) for red mullet and goat fish during ice storage. The volatile compounds (such as ammonia, trimethylamine) produced from bacterial spoilage and the addition of alkaline compounds produced from the enzymatic degradation are mainly responsible for the increase in pH (Manat et al. 2005). The pH may use as fish freshness indicator because it starts with low value with the good nutritional state of fish at initial storage and increased with certain storage period (Abbas et al. 2008). The reduction in bacterial growth and increase in the shelf life of MLP treated fish samples was observed.
Total volatile basic nitrogen (TVB-N) consists of the nitrogenous components (such as ammonia and amines) produced from the degradation of protein and non-protein nitrogenous compounds due to enzymatic hydrolysis, caused by spoilage microorganisms (Manat et al. 2005). The volatile basic nitrogen value is one of the most commonly used quality index for fish foods (Kostaki et al. 2009). Various authors have reported that different species with different acceptability limit for TVB-N value e.g. 35–40 mg/100 g (Kostaki et al. 2009); 30 mg/100 g (Ocaño-Higuera et al. 2011); 25 mg/100 g (Li et al. 2012).
The initial TVB-N values increased gradually in all ice stored samples at 5 °C (Fig. 2). The TVB-N value of the untreated fish sample increased from the starting values (mg N/100 g of fish) of 4.57 to 35.65 at the end of day 7, at the same time it was lower in all MLP treated samples. The TVB-N value for 3 and 4% MLP treated samples were 19.56 ± 0.82 and 18.18 ± 0.67 respectively which were the lowest values among all the samples at the end of day 7 and those were further increased to 35.14 ± 0.85 and 34.39 ± 0.96 at the end of the day 11 of cold storage, respectively. The 3 and 4% MLP treated groups successfully maintained a considerably lower (P < 0.05) TVB-N values than the untreated samples. As total volatile basic nitrogen is accumulated due to bacterial spoilage of fish meat, the initial values were associated with the comparatively lower TVC values, and the higher TVB-N values for untreated samples could interpret the higher TVC values in untreated samples as compared to treated samples. The tea polyphenols and rosemary extract were conducive to decrease the efficiency of microorganisms for oxidative degradation of non-protein nitrogenous compounds, when storage of whole crucian carp fish at 4 ± 1 °C reported by Li et al. (2012). Similarly, the TVB-N values level was significantly lower for the MLP treated samples than for the untreated samples.
Peroxide value and thiobarbituric acid-reactive substances
Peroxide value was used to determine the development of first stage oxidation products of tilapia fish during storage. The TBARS are formed over the second stage of oxidation when hydrogen peroxides are oxidized to ketones and aldehydes. Both PV and TBARS are widely used as important indicators to determine the level of lipid oxidation in the food storage (Barbosa-Pereira et al. 2013). In this study, the PV and TBARS values were increased in all samples throughout the storage at 5 °C (Fig. 3a, b). All samples started with low PV and TBARS values of 1.91–1.94 meq/kg and 0.16–0.24 mg MDA/kg sample, respectively. The initial value of PV suggests that lipid oxidation was started during postharvest handling. A gradual increase of PV was observed in the untreated fish samples and the 1% MLP treated samples, reached above 5 meq/kg sample after day 7. The significantly lower PV was observed in the samples treated with 3 and 4% MLP than that of the untreated group, 1 and 2% MLP treated group during the storage. The sample treated with 3 and 4% MLP greatly inhibited the lipid oxidation of tilapia meat. Similarly, continuously increased in the initial PV of 1.67 meq/kg of fresh tilapia meat during storage period of 18 days in ice was observed by Yarnpakdee et al. (2012). The initial TBARS values were increased to the highest 5.63 ± 0.10 mg MDA/kg in untreated samples whereas lowest 0.88 ± 0.12 mg MDA/kg in 4% MLP treated samples, after 7 days. During the whole storage period, PV and TBARS values of 1% MLP treated fish were similar to the control, indicating 1% MLP has almost no role in reduction of the lipid oxidation whereas considerably lower values (P < 0.05) in 2, 3 and 4% MLP treated group were observed. Similarly, the initial TBARS values 0.16–0.19 mg MDA/kg samples were observed in all pompano fish fillets which were increased gradually during 15 days chilled storage (Gao et al. 2014). The increased values of TBARS in Nile tilapia fish even stored at ice were reported by Yarnpakdee et al. (2012). The lipid oxidation slowly increased in stored tilapia fish throughout the storage. However, the PV and TBARS values in control samples was increased with higher rate (P < 0.05) than the MLP treated samples throughout the storage period at 5 °C storage. The results suggested that the MLP can be used to delay the lipid oxidation successfully and increase the storage period of fish. Similarly, El-Hanafy et al. (2011) reported that the green tea extract can work as preservative by inhibiting lipid oxidation during fish storage at refrigerated temperature. The inhibition of lipid oxidation might be occurred as a result of enzyme inhibition and precipitation of protein by TPC of moringa leaves.
Color measurement
The color of fresh meat products is also one of the important aspect from a consumer point of view. Hence color stability is important to fish processing industries and retailers during storage and retail display. The color changes in tilapia fish in the ice stored samples at 5 °C are shown in Table 1. During storage all the samples passed through progressive decreased (P < 0.05) in whiteness and lightness (L*), while yellowness (b*) gradually increased (P < 0.05) but greenness (−a*) of control samples reduced, while increased (P < 0.05) in greenness was observed in all MLP treated fish samples. It is a well-known that, a red color develops in the flesh of white meat fish during ice storage. On the last days of the storage period (Table 1), 3 and 4% MLP treated samples had higher values of lightness and whiteness than untreated samples, 1 and 2% MLP treated samples (P < 0.05). Mørkøre et al. (2010) suggested that both pigment concentrations and muscle structure characteristics influenced the color of fish meat. The oxidation of protein is also responsible to color loss in fish muscle (Li et al. 2012). The results showed that 3 and 4% MLP was effective on protecting whiteness and lightness of Nile tilapia fish meat during storage at 5 °C.
Texture measurements
Fish meat texture depends on the various intrinsic biological aspects, such as collagen and fat content, and bacteriological and autolytic processes leads to myofibrillar protein degradation and finally to soften the meat (Cardoso et al. 2009). The decrease in hardness value was observed in all stored samples (Table 2). The moringa leaves powder treatments to fish proved the efficiency of delaying the softening of meat during storage at 5 °C. The MLP treated fish samples showed the higher (P < 0.05) hardness values than the untreated fish samples on the last day (day 7) of storage. Similarly, Li et al. (2012) reported that hardness of crucian carp meat slowly decreased as increasing the storage time, the hardness of the crucian carp meat control sample was lower than that of the fish meat treated with tea polyphenols and rosemary extract during chilled storage.
Organoleptic quality assessment of tilapia fish
The total demerit points of tilapia fish during storage in ice with moringa leaves powder (MLP) of 1, 2, 3 and 4% in an insulated box kept at 5 ± 1 °C temperature presented in Table 3. All zero-day fish samples with the score points ‘0’ were in excellent quality as fresh state. Fish quality of all samples significantly lost with the increased demerit points as storage time increased. Rejection of raw fish by the test panelists was mainly characterized by discolored skin, strong fishy to off odors and soft texture. The samples were found unacceptable quality at the end of 7 days in control batch and 1% MLP treated fish samples. The samples were found unacceptable in 2, 3 and 4% MLP treated fish samples at the end of 9, 11 and 12 days respectively.
Sensory evaluation scores showed a similar trend of increasing the deterioration of fish quality for the untreated and the MLP treated group. The fish treated with moringa leaves powder provided lower sensory assessment scores with better attributes of physical characteristics, color, texture compared to the untreated samples. Panelist preferred the MLP treated fish of both groups over the untreated as those smelled less fishy and maintained firm texture. Spoilage in fish produces strongly fishy smell and sour odor. Treatment of moringa leaves powder could maintain preferable quality attributes and prolong the shelf life of Nile tilapia by 5 days as compared to untreated fish samples in storage at 5 ± 1 °C temperature. These results are consistent with the results of bacteriological and biochemical quality analyses.
Conclusion
The present study has shown that a treatment with 3% (w/w) moringa leaves powder could effectively delay chemical deterioration, lower microbial growth, prolong the shelf life of Nile tilapia for 5 days during storage at 5 °C temperature. Hence, moringa leaves powder can be used safely and commercially as an organic preservative to prolong the storage life of fish. The application of moringa leaves powder in preservation of fish will helps to minimize fish post-harvest losses. In addition, as a good quality of fish was maintained, an appropriate storage technique presented in this paper is highly applicable.
References
Abbas KA, Mohamed A, Jamilah B, Ebrahimian M (2008) A review on correlations between fish freshness and pH during cold storage. Am J Biochem Biotechnol 4(4):416–421
Adeyemi KD, Ahmed El-Imam AM, Olorunsanya AO, Sola-ojo FE, Okukpe KM, Dosunmu OO, Shittu RM, Idris JT (2013) Effect of Moringa Oleifera marinade on proximate composition and sensory characteristics of smoke-dried African cat fish (Clarias gariepinus). Croat J Fish 71:11–18
Alasalvara C, Taylora KDA, Zubcovb E, Shahidic F, Alexisd M (2002) Differentiation of cultured and wild sea bass (Dicentrarchus labrax): total lipid content, fatty acid and trace mineral composition. Food Chem 79(2):145–150
Anal AK, Jaisanti S, Noomhorm A (2014) Enhanced yield of phenolic extracts from banana peels (Musa acuminata Colla AAA) and cinnamon barks (Cinnamomum varum) and their antioxidative potentials in fish oil. J Food Sci Technol 51(10):2632–2639
Anderson AK (2008) Biogenic and volatile amine-related qualities of three popular fish species sold at Kuwait fish markets. Food Chem 107:761–767
AOAC (2002) Official methods of analysis, 16th edn. Association of Official Analytical Chemists, Arlington
Barbosa-Pereira L, Cruz JM, Sendón R, Bernaldo de Quirós AR, Ares A, Castro-López M et al (2013) Development of antioxidant active films containing tocopherols to extend the shelf life of fish. Food Control 31(1):236–243
Cardoso CML, Mendes R, Nunes ML (2009) Instrumental texture and sensory characteristics of cod Frankfurter sausages. Int J Food Prop 12(3):625–643
Chen Z, Bertin R, Froldi G (2013) EC 50 estimation of antioxidant activity in DPPH assay using several statistical programs. Food Chem 138(1):414–420
Dalgaard P, Madsen HL, Samieian N, Emborg J (2006) Biogenic amine formation and microbial spoilage in chilled garfish (Belone belone belone)—effect of modified atmosphere packaging and previous frozen storage. J Appl Microbiol 101(1):80–95
Das AK, Rajkumar V, Verma AK, Swarup D (2012) Moringa oleifera leaves extract: a natural antioxidant for retarding lipid oxidation in cooked goat meat patties. Int J Food Sci Technol 47(3):585–591
Dongmeza E, Siddhuraju P, Francis G, Becker K (2006) Effects of dehydrated methanol extracts of moringa (Moringa oleifera Lam.) leaves and three of its fractions on growth performance and feed nutrient assimilation in Nile tilapia (Oreochromis niloticus L.). Aquaculture 261(1):407–422
El- Hanafy AEA, Ramadan MF, Ahmed MH, Showky HE (2006) Changes in fatty acid composition, cholesterol contents and quality attributes of bolti (Tilapia nilotica) fingerlings in relation to dietary lipid levels and sources in feeding regime. Dtsch Lebensmitt Rundsch 102:518–522
El-Hanafy AEA, Shawky HA, Ramadan MF (2011) Preservation of Oreochromis niloticus fish using green tea extract: impact on biochemical, microbiological and sensory characteristics. J Food Process Preserv 35(5):639–646
Gao M, Feng L, Jiang T, Zhu J, Fu L, Yuan D, Li J (2014) The use of rosemary extract in combination with nisin to extend the shelf life of pompano (Trachinotus ovatus) fillet during chilled storage. Food Control 37:1–8
Ghaly AE, Dave D, Budge S, Brooks MS (2010) Fish spoilage mechanism and preservation techniques review. Am J Appl Sci 7(7):859–877
Hiranrangsee L, Kumaree KK, Sadiq MB, Anal AK (2016) Extraction of anthocyanins from pericarp and lipids from seeds of mangosteen (Garcinia mangostana L.) by ultrasound-assisted extraction (UAE) and evaluation of pericarp extract enriched functional ice-cream. J Food Sci Technol 53(10):3806–3813
Jayawardana BC, Liyanage R, Lalantha N, Iddamalgoda S, Weththasinghe P (2015) Antioxidant and antimicrobial activity of drumstick (Moringa oleifera) leaves in herbal chicken sausages. LWT Food Sci Technol 64(2):1204–1208
Kostaki M, Giatrakou V, Savvaidis IN, Kontominas MG (2009) Combined effect of MAP and thyme essential oil on the microbiological, chemical and sensory attributes of organically aquacultured sea bass (Dicentrarchus labrax) fillets. Food Microbiol 26(5):475–482
Li X, Li J, Zhu J, Wang Y, Fu L, Xuan W (2011) Postmortem changes in yellow grouper (Epinephelus awoara) fillets stored under vacuum packaging at 0 °C. Food Chem 126(3):896–901
Li TT, Li JR, Hu WZ, Zhang XG, Li XP, Zhao J (2012) Shelf-life extension of crucian carp (Carassius auratus) using natural preservatives during chilled storage. Food Chem 135(1):140–145
Lugasi AV, Losad J, Hovari H, Lebovcs V, Jakoczi I, Aubourg S (2007) Effect of pre-soaking whole pelagic fish in a plant extract on sensory and biochemical changes during subsequent frozen storage. LWT Food Sci. Technol 40(5):930–936
Manat C, Soottawat B, Wonnop V, Cameron F (2005) Changes of pigments and color in sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) muscle during iced storage. Food Chem 93(4):607–617
Mørkøre T, Rødbotten M, Vogt G, Fjæra SO, Kristiansen IØ, Manseth E (2010) Relevance of season and nucleotide catabolism on changes in fillet quality during chilled storage of raw Atlantic salmon (Salmo salar L.). Food Chem 119(4):1417–1425
Nielsen D, Hyldig G (2004) Influence of handling procedures and biological factors on the QIM evaluation of whole herring (Clupea harengus L.). Food Res Int 37(10):975–983
Ocaño-Higuera VM, Maeda-Martínez AN, Marquez-Ríos E, Canizales-Rodríguez DF, Castillo-Yáñez FJ, Ruíz-Bustos E et al (2011) Freshness assessment of ray fish stored in ice by biochemical, chemical and physical methods. Food Chem 125(1):49–54
Ozyurt G, Kuley E, Ozkutuk S, Ozogul F (2009) Sensory, microbiological and chemical assessment of the freshness of red mullet (Mullus barbatus) and goldband goatfish (Upeneus moluccensis) during storage in ice. Food Chem 114(2):505–510
Raatikainen O, Reinikainen V, Minkkinen P, Ritvanen T, Muje P, Pursiainen J, Hiltunen T, Hyvönen P, Wright AV, Reinikainen SP (2005) Multivariate modelling of fish freshness index based on ion mobility spectrometry measurements. Anal Chim Acta 544(1–2):128–134
Sadiq MB, Hanpithakpong W, Tarning J, Anal AK (2015) Screening of phytochemicals and in vitro evaluation of antibacterial and antioxidant activities of leaves, pods and bark extracts of Acacianilotica (L.) Del. Ind Crops Prod 77:873–882
Sadiq MB, Tarning J, Aye Cho TZ, Anal AK (2017a) Antibacterial activities and possible modes of action of Acacia nilotica (L.) del. against multidrug-resistant Escherichia coli and Salmonella. Molecules 22(1):47
Sadiq MB, Tharaphan P, Chotivanich K, Tarning J, Anal AK (2017b) In vitro antioxidant and antimalarial activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. BMC Complement Altern Med 17(1):372
Sánchez-Machado DI, Núnez-Gastélum JA, Reyes-Moreno C, Ramírez-Wong B, López-Cervantes J (2010) Nutritional quality of edible parts of Moringa oleifera. Food Anal Methods 3(3):175–180
Sreelatha S, Padma PR (2009) Antioxidant activity and total polyphenol content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum Nutr 64(4):303–311
Yarnpakdee S, Benjakul S, Nalinanon S, Kristinsson H (2012) Lipid oxidation and fishy odor development in protein hydrolysate from Nile tilapia (Oreochromis niloticus) muscle as affected by freshness and antioxidants. Food Chem 132(4):1781–1788
Yeasmin T, Reza MS, Khan MNA, Shikha FH, Kamal M (2010) Present status of marketing of formalin treated fishes in domestic markets at Mymensingh district in Bangladesh. Int J Biores 1(4):21–24
Acknowledgements
The author acknowledges, Commissionrate of Social Welfare, Maharashtra State, India and Asian Institute of Technology, Thailand for providing financial support to one of the authors to pursue Ph.D.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jadhav, R., Anal, A.K. Experimental investigation on biochemical, microbial and sensory properties of Nile tilapia (Oreochromis niloticus) treated with moringa (Moringa oleifera) leaves powder. J Food Sci Technol 55, 3647–3656 (2018). https://doi.org/10.1007/s13197-018-3293-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3293-9