Abstract
This paper deliberates the modelling and validation of bacteriocin-like inhibitory substance (BLIS) secretion by Pediococcus acidilactici Kp10 at different agitation speeds in a stirred tank bioreactor. A range of models namely the re-parameterised logistic, Luedeking–Piret and maintenance energy were assessed to predict the culture performance of the said bacterium. Growth of P. acidilactici Kp10 was enhanced with increased agitation speed up to 600 rpm while BLIS secretion was maximum at 400 rpm but decreased at higher agitation speed. Growth of P. acidilactici aptly subscribed to the re-parameterised logistic model while BLIS secretion and lactose consumption fitted well with the Luedeking–Piret model. The models revealed a relationship between growth of the bacterium and BLIS secretion. Bacterial growth and BLIS secretion were largely affected by the agitation speed of the stirred tank bioreactor which regulated the oxygen transfer to the culture. BLIS secretion by P. acidilactici Kp10 was however enhanced in oxygen-limited culture. The study also assessed BLIS from the perspective of its stability when subjected to factors such as temperature, pH and detergents. Results showed that BLIS produced by this strain was not affected by heat (at 25–100 °C for 20 min and at 121 °C for 15 min), surfactant (Tween 40, 60 and 80 and urea), detergents (up to 1% SDS), organic solvents (50% each of acetone, methanol and ethanol) and stable in a wide range of pH (2–10). The above information are pertinent with reference to commercial applications of this bacterial product in food manufacturing which invariably involve various sterilization processes and subjected to a wide pH range.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, bacteriocins have been recognised as natural food preservatives and antimicrobial agents. Bacteriocins produced by lactic acid bacteria (LAB) are biologically active against both gram-negative and gram-positive bacterial species (Line et al. 2008) and exhibit broad- or narrow-spectrum antibacterial activities. However, amongst the bacteriocins reported to date only nisin and pediocin have been approved as food preservatives (Guerra and Pastrana 2002). Nisin has several disadvantages including unstable at neutral to alkaline pHs, reduction in antimicrobial activity in complex foods, reduction in solubility within a wide pH range and only active against gram-positive bacteria. Thus, many researchers are prompted to focus on the use of pediocin as food preservatives. Pediocins produced by Pediococcus species are highly protective in terms of food spoilage and specifically effective against the pathogenic bacterium, Listeria monocytogenes (Abbasiliasi et al. 2017). Most pediocins are stable over a wide range of temperature and pH (Ray and Daeschel 1992; Guerra and Pastrana 2002) which single out this product being most ideal as a food preservative.
To be commercially and economically viable, pediocin production by LAB fermentation should be increased to a maximum yield (Guerra and Pastrana 2002), which in turn requires large-scale cultivation of the bacterial cells. An understanding of the kinetics of bacteriocin production by LAB is thus most pertinent in terms of process design and optimization (MacNeil and Harvey 1990). Information on the yield and reaction times would enable engineers to design the appropriate bioreactor size and the process design could be predicted from a model of the reaction process (Altiok et al. 2006). Modelling is therefore an essential step in predicting the requirements of the engineering system to provide information on the relationships between different culture conditions. In this respect modelling reveal the biological, chemical and physical bases of the process, provide a guide to experimental planning and facilitate result evaluation (Sinclair and Kristiansen 1987).
Kinetic models are employed to design and control microbial processes (Znad et al. 2004). By incorporating carefully designed experiments with mathematical models, the information and data generated could be used by bioengineers to evaluate the system behaviour more rapidly as compared to laboratory experiments alone. Microbial processes can be mathematically modelled by two different mechanisms—structured and unstructured models (Kar et al. 2000; Chen and Hoover 2003). Structured models account for the effects of cell structure, function and composition while in unstructured models the biological system is described solely by the intracellular reaction dynamics.
Batch is the simplest mode of bioreactor operation which could be set up and operated with limited information on the production of fermentative products (Cinar et al. 2003). A fermentation process is controlled by the physical, chemical or biochemical properties of the culture system and by manipulating the physical and chemical environmental parameters (Carrillo-Ureta et al. 2001). Several mathematical models of bacteriocin production by batch-cultivated LAB have been proposed (Guerra and Castro 2003). These models are based on the Luedeking–Piret-like equation, which take into consideration the growth-associated bacteriocin production and bacteriocin degradation or adsorption (Guerra et al. 2005). To date, models with respect to BLIS secretion by Pediococcus spp. have not been reported.
This study reports the effect of agitation speed on the growth and the secretion of bacteriocin-like inhibitory substance (BLIS) by P. acidilactici Kp10 during batch fermentation in a 2 L stirred tank bioreactor. Using biokinetic models, the experimental data were analysed for their suitability to describe the reaction process. The sensitivity and robustness of the models were evaluated by comparing the modelled determination coefficients and mean relative percentage deviations with those of analysed experimental data. Further, for commercial application as a preservative in the food industry, the stability of BLIS against pH, detergents, and temperature was also assessed.
Materials and methods
Microorganism and inoculum preparation
The BLIS-producing bacterium, P. acidilactici Kp10, was isolated and characterised as described in our previous study (Abbasiliasi et al. 2012). The bacterium was maintained as a stock culture on agar slants at 4 °C and sub-cultured twice in M17 broth (Merck, Darmstadt, Germany) prior to inoculum preparation. A single colony of P. acidilactici Kp10 was propagated in 10 mL of M17 broth and incubated at 37 °C for 24 h. This initial culture (1% v/v) was sub-cultured at 37 °C for 18–24 h in the same medium until the optical density at 650 nm (OD650) reached 0.9–1.2. This OD650 reading was used to standardise the inoculum size at 3% v/v throughout the study.
Culture medium
The standard LAB cultivation media, M17 and de Man, Rogosa and Sharpe (MRS), were initially tested for P. acidilactici Kp10 cultivation and BLIS secretion at three incubation temperatures (30, 35 and 37 °C). On both media, BLIS secretion was maximum at 30 °C and markedly reduced at 37 °C. At 30 °C, BLIS secretion was nine times higher in M17 medium (1427.66 AU mL−1) as compared to that obtained in MRS medium (159.98 AU mL−1) in spite of cell growth being similar on both media. Hence, M17 medium was selected as the growth medium in ensuing experiments. The nitrogen and carbon composition of M17 medium is listed in Table 1. Nitrogen was sourced from yeast extract, meat extract and various peptones. Lactose was used as the sole carbon source.
Bioreactor set up and fermentation
BLIS-producing P. acidilactici Kp10 was cultivated in a 2 L stirred tank bioreactor (B-DCU Model, Sartorius, Germany) with a working volume of 1 L. The bioreactor was agitated by a single six bladed Rushton turbine (impeller diameter = 0.052 m) and the temperature, pH and dissolved oxygen tension (DOT) were monitored by a control module system.
To initiate fermentation, the bioreactor containing 1 L of M17 medium was seeded with 3% v/v inoculum. During the fermentation, agitation speed was fixed at a selected impeller speed (100, 250, 400, 600 or 800 rpm). In all fermentations, the cultures were aerated by sparging sterile air at a fixed flow rate (0.5 vvm) using an integral thermal mass flow controller. The culture pH was monitored on-line by an in situ sterilisable pH electrode (Mittler Toledo, Switzerland). DOT level was measured by a polarographic DO electrode (Mittler Toledo, Switzerland) and recorded throughout the fermentation. To suppress foaming, antifoam reagent (Silicon antifoam, Sigma, USA) was added during the fermentation. Temperature within the bioreactor vessel was controlled at 28.5 °C by circulating water through the jacketed vessel. During the fermentation, 10 mL of the cultures were withdrawn from the bioreactor vessel at 2 h intervals for 24 h. The cultures were centrifuged (at 18,000×g for 25 min at 4 °C) and the supernatant was immediately stored at − 20 °C for further analysis. All experiments were carried out in technical triplicates.
Analytical procedures
Bacterial growth was measured by the attenuation (OD) at 650 nm using a spectrophotometer (Perkin Elmer, Lambda 25, USA) and converted into dry cell weight (DCW) from a standard curve using an experimentally predetermined factor of 0.37, where one OD unit was equivalent to 0.37 of DCW per volume (g L−1). The culture samples were centrifuged (at 18,000×g for 25 min at 4 °C) and the supernatant retained was used for determination of BLIS activity, lactose and organic acid concentration. Total sugars (TS) were determined using a YSI bioanalyzer (Illinois, USA). Total nitrogen content was estimated by the micro-Kjeldahl method (Havilah et al. 1977).
The concentrations of organic acids (lactic, acetic and citric) were determined using high performance liquid chromatography (HPLC; Agilent, Australia). The HPLC system was equipped with an Aminex (R)HPX-87H, 300 mm × 7.8 mm (Bio-Rad, CA) column and a UV detector read at 210 nm. The column temperature was kept at 25 °C. The elution solvent was sulphuric acid (0.4 mM) flowing at 0.6 mL min−1. All measurements were performed in triplicate.
Determination of antimicrobial activity
Antimicrobial activity of the isolates was determined by the agar well diffusion method as described in our previous study (Abbasiliasi et al. 2012). Briefly, cells produced were separated by centrifugation at 12,000×g for 20 min at 4 °C (rotor model 1189, Universal 22R centrifuge, Hettich AG, Switzerland). Aliquots of supernatant in twofold dilution (100 µL) were placed in wells (6 mm diameter) of cooled soft agar plates (25 mL) previously seeded (1% v/ v) with the actively growing test strain (Listeria monocytogenes ATCC 15313). The plates were then incubated at 37 °C for 24 h for the growth of L. monocytogenes. After 24 h the diameters of the inhibition zones were measured and the antimicrobial activity (AU mL−1) defined as the mean reciprocal of the highest dilution showing inhibition of the indicator lawn as expressed in our previous study (Abbasiliasi et al. 2016). This BLIS activity assay was a generalized assay for bacteriocin activity which includes all forms of pediocin but the forms of pediocin was not distinguishable.
Mathematical models
Growth, substrate consumption and product formation were modelled by the logistic and Luedeking–Piret equations. These unstructured models are given by:
where X and S denote the cell and lactose concentrations, respectively, P is the activity of BLIS, μ is the specific growth rate, q P is the volumetric BLIS secretion rate and q S is the volumetric lactose uptake rate.
The modified logistic equation, proposed by Zwietering et al. (1990), accounts for the lag phase, λ (h):
where X is the biomass density (g L−1), X mX is the maximum biomass density (g L−1), t is the elapsed time (h), μmX is the specific growth rate (1/h), λ is the growth lag time, determined as the t-intercept of the tangent through the inflection point (h), a, b, and c are model parameters.
In the present study, Eq. (4) was modified by an additional cell death term, \( \frac{{t - t_{{{\text{m}}X}} }}{\tau } \) to capture the decreasing BLIS secretion towards the end of fermentation. A further two parameters were included:
-
1.
(t mX ) the time of maximum biomass.
-
2.
\( \left( \tau \right) \) the time elapsed since tmx during which the biomass declines to zero, designed such that τ becomes negative once the biomass has peaked in Eq. (5), i.e., t > t mx .
Implementing the above changes, our modified logistic equation is given by:
where t mX is the time (h) at which \( \frac{dX}{dt} \approx 0 \), t is the time (h) required since t mX for biomass to decline to zero.
Product formation and substrate consumption are evolved by the Luedeking–Piret models given in Eqs. (6) and (7), respectively:
where α and β are the growth-associated and non-growth-associated BLIS production constants, respectively and m and n are the respective growth-associated and non-growth-associated lactose consumption constants.
Inserting \( \frac{\Delta X}{\Delta t} \) and \( \frac{\Delta P}{\Delta t} \) from Eqs. (1) and (2) into (5), and \( \frac{\Delta S}{\Delta t} \) and \( \frac{\Delta X}{\Delta t} \) from Eqs. (1) and (3) into (5), Eqs. (4) and (5) become simplified as Eqs. (8) and (9), respectively;
All models were fitted by the SigmaPlot program, version 10.0 (Systat Software Inc., California, USA). The coefficients of the models with p values below 0.05 were considered statistically significant. For each model, the goodness-of-fit criteria were the determination coefficient (R2), the standard error of prediction (SEP) (Lomauro et al. 1985) and the root mean square error (RMSE).
The % SEP is given by
where obs is the experimental (observed) value, pred is the calculated (predicted) value and n is the number of experimental data.
The % SEP and R2 determine the quality of the model fit. For practical purposes a good model fitting was defined by % SEP < 10% and R2 ≥ 0.90% (Lomauro et al. 1985). A slope approximating unity with low RMSE, high R2 and less than 10% SEP indicates that the model appropriately describes the data. The goodness-of-fits of the fitted models were evaluated by the mean square error (MSE) and bias factor. The bias factor quantifies the average extent to which a model over-predicts (bias factor > 1) or under-predicts (bias factor < 1) the observed data.
The RMSE is calculated using Eq. (11):
where Xi,exp is the experimental response, Xi,cal is the calculated response and n is the number of experiments.
Bias factor is defined by Eq. (12):
where n is the number of observations used in the calculation. The bias factor of a perfect model is 1 (Zhao et al. 2001).
The calculated biokinetics parameters were analysed by one-way ANOVA. Post-hoc tests for each dataset were undertaken by Tukey’s HSD method. The means of all calculated parameters were compared by the Tukey function of SPSS. All statistics were based on the 95% confidence level, and p < 0.05 was considered as statistically significant.
Effect of pH, detergents, and temperature on BLIS stability and activity
The effect of certain detergents (SDS, Tween 40, Tween 60, urea and Tween 80), NaCl, acetone, methanol and ethanol, added at different concentrations on BLIS in cell-free supernatants was determined as described by Abbasiliasi et al. (2010). Sterile M17 broth sample, each supplemented with the above-mentioned chemicals, were served as controls.
In a separate experiment, the effect of pH on BLIS activity was determined by adjusting the cell-free supernatant to pH 2–12 with sterile 1N HCl or 1N NaOH. After 2 h of incubation at 37 °C, the activity was determined as described earlier. The effect of temperature on BLIS activity was determined by heating the cell-free supernatants to various temperatures (30, 37, 45, 60, 80 and 100 °C) using water bath and 121 °C using an autoclave for 15 min. Residual BLIS activity was tested as described previously (Abbasiliasi et al. 2010). As control, sterile MRS were exposed to the same temperatures and pH for experiments, and subsequently tested against L. monocytogenes ATCC 13513.
Results and discussion
Model fitting for cell growth, BLIS secretion and lactose uptake by P. acidilactici Kp10
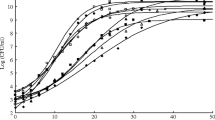
The typical time course of BLIS secretion by P. acidilactici Kp10 in the stirred tank bioreactor using M17 medium shown in Fig. 1 also shows the fitness of the proposed models to the experimental data. The exponential growth phase (2–14 h) was followed by a stationary growth phase after approximately 16 h. BLIS secretion was observed after 6–8 h of fermentation, depending on the agitation speed. BLIS secretion was increased with increased bacterial growth and ended at the stationary phase. The fitnesses of the evaluated growth models to the experimental growth data of P. acidilactici Kp10 are summarised in Table 2.
Batch fermentation of BLIS-producing P. acidilactici Kp10 in a stirred tank bioreactor agitated at different impeller speeds (100, 250, 400, 600 and 800 rpm). The growth medium was M17 medium and the air flow rate was fixed at 0.5 vvm. Solid lines and symbols represent the simulated and experimental data, respectively. G growth, Actual-G observed bacterial growth, M Logistic-G calculated growth from modified logistic model, RM Logistic-G calculated growth from Re-Modified Logistic model, P production, Actual-P observed production, Luedeking–Piret-P calculated production from Luedeking model, M Luedeking–Piret-P calculated production from Modified Luedeking model, S lactose consumption/uptake, Actual-S observed lactose uptake, Luedeking–Piret-S calculated lactose uptake from Luedeking–Piret model. a Agitation speed, 100 rpm. b Agitation speed, 250 rpm. c Agitation speed, 400 rpm. d Agitation speed 600 rpm. e Agitation speed, 800 rpm
In all fermentations, up to 90% of the total lactose supply was consumed. After 16 h of fermentation, lactose consumption was increased with increasing bacterial growth. In the final fermentation stages, lactose uptake was related linearly with bacterial growth, implying that lactose was consumed for maintenance. Lactose consumption was significantly reduced in the cell death phase but continued after cessation of overall cell growth and stabilisation of dry cell weight. Lactose consumption rate was also decreased with increasing agitation speed. Lactose consumption was maximum at 16 h, which coincided with the time of maximum cell growth and BLIS secretion (Fig. 1).
The growth curves generated by the modified and re-modified logistic functions were subjectively compared by plotting the predicted and experimental data on the same graph (Fig. 1). For further comparison, the regression curves of the experimental and modelled data were forced through the origin. The models were validated by strong correlations between the experimental and calculated data (R2 > 0.9). The R2 values of all growth models were above 0.9. At all agitation speeds, the % SEP between the experimental data and the re-modified logistic model results remained below 10%, which are comparable to the inherent analytical precision of fermentation data and the precision of other models (Table 2). The cell growth models presented in Table 2 favourably agree with the experimental values. The high R2 values (> 0.9), approximately unity slope, low RMSE and low % SEP indicate that cell growth is adequately described by these models. However, low correlations between the experimental and calculated data were obtained by the BLIS secretion model (Table 2). In this case R2 did not exceeded 0.9 and % SEP was higher than 10, indicating that Luedeking–Piret model did not describe BLIS secretion by P. acidilactici Kp10 sufficiently. In this study, high values of extracellular BLIS activity (up to 3500 AU mL−1) were recorded. This could be attributed to high discrepancy in the experimental data and calculated data in model fitting. Therefore, a suitable model capable of describing BLIS secretion more precisely needs to be developed in the future studies. Lactose consumption model was also validated by strong correlation between the experimental and calculated data. In this model, R2 exceeded 0.9 at all agitation speeds. Again, the slope and B f approximated unity, and the RMSE (< 1) and SEP (< 10%) were acceptably small (Table 2).
Bacteriocins production by LAB usually follows primary metabolite growth-associated kinetics, which concur with several reports on biomass production from the members of LAB population (De Vuyst et al. 1996; Parente et al. 1997; Leroy and De Vuyst 2002; Zhang et al. 2012). However, the relationship between bacteriocin production and cell growth is strain dependent (Parente et al. 1997). The growth associated process for bacteriocin production by LAB has been reported (Abriouel et al. 2003). Some bacteriocins were also produced during a stationary growth phase, indicating that the process is non-growth associated (Guerra et al. 2001; Leroy and De Vuyst 2002; Coetzee 2007).
In the present study, P. acidilactici Kp10 exhibited a typical sigmoid growth trend comprising of a lag phase, a logarithmic phase and a stationary phase. The experimental growth data fitted well by the modified and re-modified logistic models. Since the re-modified logistic model takes into account the stationary growth phase, this model is more suitable to describes the entire growth curve. The parameters obtained from the proposed models (positive α and negative β) indicated an important role on the growth-associated factor in BLIS secretion by P. acidilactici Kp10. Consistent with this result, Cabo et al. (2001) reported that α typically exceeds β in unstructured models of nisin production. The higher values of α and m compared to β and n support a growth-associated process for BLIS production and lactose consumption which is consistent with those of many other bacteriocins in existing literature (Savadogo et al. 2006; Owusu-Kwarteng et al. 2013).
Effect of agitation speed on DOT and culture pH
Figure 2 plots the DOT profiles and pH changes during BLIS secretion at different impeller speeds. At low and moderate impeller speeds (100, 250 and 400 rpm) the DOT level dramatically declined to 0% saturation after 5–8 h fermentation. At high impeller speeds (600 and 800 rpm) the DOT level was reduced to 50% saturation after 12 h fermentation and rose to high levels (> 75% saturation) towards the end of fermentation. It is important to note that the experimental set-up in this study did not allow separate oxygen effects from the more general shear effects created by the agitation speed. Shear effect involves the sum of physical effects which is direct effect of shear on bacterial cell and nutrient supply but not limited to the supply of oxygen to the bacterial cell in the culture. Thus, the effect of agitation speed on the behavior and performance of the fermentation process could not be explained solely attributed to the effect of the DOT level.
BLIS secreted by P. acidilactici Kp10 was stable at a very wide pH range (pH 2–9) and the activity was denatured at pH above 10 (Abbasiliasi et al. 2012). The culture pH during BLIS fermentation by P. acidilactici Kp10 at different agitation speeds ranged from 5.9 to 7.2. Thus, it can be inferred that BLIS activity during the fermentation was stable with the variation in culture pH. However, the effect of different shear rates on the stability of BLIS activity was not investigated in this study. Since BLIS activity was stable at a wide pH range, reduced extracellular BLIS activity during the fermentation was not attributable to the reduction in culture pH. At pH 6, decreased extracellular BLIS activity at the end of fermentation could obscure possible BLIS secretion during the growth slowdown period. Extracellular BLIS activity was maximum when the medium pH decreased from 7 to approximately 6 (5.95–6.6), indicating that mildly acidic cultural conditions favoured BLIS secretion and BLIS accumulated during the final phase of growth. A similar trend of maximum accumulation during the stationary growth phase has been previously reported (Pilet et al. 1995; Kumar et al. 2012). Both extracellular BLIS activity and lactic acid production were synchronised with cell growth and followed a similar biokinetic profile. The pH dependence of BLIS secretion suggested that the expression of the involved biosynthetic genes is pH-regulated, as previously reported for several classes of genes (Olson 1993). Extracellular BLIS activity could also be enhanced by more efficient energy use at low growth rates. Enzymatic reactions are sensitive to pH and are compromised, with concomitant slower growth rate under acidic conditions. This slowdown could positively affect BLIS synthesis, increasing the pools of essential metabolites including ATP (Møretrø et al. 2000).
Two important operating variables in the stirred tank bioreactor are impeller speed and air flow rate. Both variables influence the DOT level in the culture. In this study, BLIS secretion was impeded at high agitation speeds (600 and 800 rpm). High DOT levels in the culture encouraged the formation of free radicals such as superoxides and hydroxyl radicals which affected protein synthesis (Chow et al. 2011). This phenomenon is commonly recognized as oxidative stress. In bacteria, free radicals are destroyed by a mechanism involving catalase and superoxide dismutase. If the oxidizing species concentration exceeds the capacity of the cellular defence system, this mechanism breaks down and the cells experience oxidative stress (Cabiscol et al. 2000). “Foreign” proteins expressed in highly aerated systems may undergo oxidative damage such as carboxylation with consequent reduction in their activity (Voulgaris et al. 2011). Hence, the DOT level in the culture of P. acidilactici Kp10 should be optimally controlled during the fermentation process to enhance the secretion of BLIS.
The agitation provided by the impeller not only affects oxygen supply but also created the shear affect. The effect of shear on the growth morphology of P. acidilactici Kp10 and the stability of BLIS was not systematically investigated in this study. The experimental set-up which is capable of investigating the separate effects of oxygen supply and shear rate produced by the impeller agitation need to be carried in future studies to generate more information for better understanding of BLIS fermentation by P. acidilactici Kp10.
Effect of agitation speeds on growth and BLIS secretion
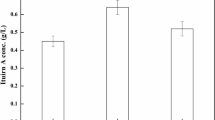
The effect of agitation speed on maximum cell concentration (XmX) obtained during the fermentation is shown in Table 3. XmX was increased with increased agitation speed up to 600 rpm. However, XmX was reduced at very high agitation speed (800 rpm). The highest XmX (3.96 g L−1) was obtained at agitation speed of 600 rpm while the highest maximum BLIS activity (PmX) (12,222.10 AU mL−1) was obtained at 400 rpm. The highest accumulation of organic acids such as lactic acid and acetic acid was observed at 400 rpm. The highest specific growth rate (μmax) (0.56 h−1) was observed at agitation speed of 600 rpm. However, the value was not significantly different to that of μmax (0.55 h−1) obtained at 800 rpm. The positive coefficient of growth-associated BLIS secretion (α) and negative coefficient of non-growth associated BLIS secretion (β) indicated that the BLIS secretion in fermentation employing P. acidilactici Kp10 was a growth associated process. Similarly, the positive values of growth associated lactose consumption coefficient (m) and negative non-growth associated of lactose consumption coefficient (n) showed that the lactose consumption was a growth associated process where lactose consumption was concomitant to the cell growth. Results from this study also showed that both BLIS secretion and acid accumulation took place simultaneously with cell growth following similar biokinetic profiles.
In the present study, BLIS secretion by P. acidilactici Kp10 was significantly affected by agitation speed while lactose and nitrogen consumption were independent of this parameter. Among the evaluated agitation speeds (100–800 rpm), BLIS secretion was maximum at 400 rpm. This peak of BLIS secretion coincided with the highest accumulation of lactic and acetic acids. Reduced extracellular BLIS activity at high agitation speeds (from 600 and 800 rpm) may not be due to increased in shear rate because growth was enhanced at high agitation speed. Reduced BLIS activity at high agitation speeds may be due to high DOT level in the culture system or the creation of non-oxygen limited culture conditions. High DOT level during the fermentation could lead to BLIS degradation due to high protease activity, low culture pH, and/or reabsorption by the producer cell surface (Cheigh et al. 2002; Ferreira et al. 2007). Increased protease activity was normally observed towards the end of the fermentation cycle and the excretion of protease during the stationary growth phase is common for dairy fermentation of several oligopeptide antibiotics (De Vuyst and Vandamme 1992; Parente and Ricciardi 1994). Production of bacterial proteases is often induced by the presence of complex organic nitrogen and suppressed by rapidly metabolizable nitrogen sources (Beg et al. 2002).
Bacteriocin production is a defensive mechanism which apparently is suppressed in nutrient-rich environments, suggesting a potential role for bacteriocin production in natural, competitive, and nutrient-depleted ecosystems. In artificial cultivation environments, bacteria could resort to self-protective behaviours. High levels of bacteriocin in the cellular environment might inhibit further bacteriocin production because the producer cells are relatively susceptible to their own bacteriocin. In other words bacteriocin production cannot exceed a critical plateau of activity beyond which bacteriocin would accumulate to toxic levels in the cellular environment (Leroy and De Vuyst 2001). The above rationale could adequately explain the decline in BLIS secretion under high agitation conditions. However, there is a possibility that the bacteriocin could be reabsorbed by the bacterium itself. Cellular re-absorption has been reported by several researchers (Cheigh et al. 2002; Messens et al. 2003). With an agitation speeds beyond 400 rpm, a marked increase in bacterial population was observed implying that nutrients uptake was enhanced in the aerated fermentation systems, which in turn, increased the substrate consumption. Since substrate consumption was consistently high throughout the growth phase, cellular re-absorption of bacteriocin could also be the rationale for reduced BLIS activity in highly agitated P. acidilactici Kp10 cultures. It is notable that high agitation speeds also increased shear rate that negatively affect the growth morphology and cell viability of P. acidilactici Kp10. Low agitation speed (200 rpm) was preferred for optimal growth of many natural isolates (Natarajan and Rajendran 2009; Hamzah et al. 2012). P. acidilactici can tolerate to the shear created by the agitation speed ranging from 200 to 1000 rpm (Beshay and Moreira 2004), suggesting that this bacterial cell is not shear-sensitive.
Effect of pH, detergents, NaCl and temperature on BLIS stability
Analysis of the effect of detergent on bacteriocin activity provided an indirect information with regards to the structure of the active molecule which is of interest in the screening of novel peptides (Abbasiliasi et al. 2010). In this case, the cell free supernatant of P. acidilactici Kp10 was prepared to include non-ionic detergents (Tween 40, 60, 80 and urea) and anionic detergents (SDS), which were added to reach a final concentration of 0.1, 1 and 5% (w/v) or (v/v) (Table 4). Anionic detergents are known to unfold proteins by complexing to the interior hydrophobic core of their native structure thus affecting their three-dimensional conformation (Ivanova et al. 2000). BLIS activity was not affected by the presence of Tween 40, Tween 60 and Tween 80, 0.05% urea, and SDS. Similar results were obtained for two bacteriocins from P. acidilactici (Albano et al. 2007) and pediocin AcH (Biswas et al. 1991). Similar results were also recorded for other bacteriocins from other LAB strains such as bozacin B14 (Ivanova et al. 2000), plantaricin C19 (Atrih et al. 2001) bacteriocin from Lb. plantarum F1 and Lb. brevis OG1 (Ogunbanwo et al. 2003) and Lc. lactis (Rajaram et al. 2010).
The anti-Listeria substance of P. acidilactici Kp10 could tolerate a wide range of pH and temperature (Table 4). At 121 °C for 15 min or pH 10, BLIS of P. acidilactici Kp10 still had the ability to inhibit L. monocytogenes. BLIS activity was not detected at pH 11 and 12 (Table 4). BLIS with thermo and pH stable characteristics are essential for commercial applications as the food product could be subjected to sterilization process and exposed to different pH conditions, as well as the application in acidic foods such as cheese and sausage (Settanni and Corsetti 2008; Albano et al. 2009). Several bacteriocins produced by LAB were reported to be stable at a pH ranging from 2 to 10 as BLIS from P. acidilactici P9 (Jia et al. 2014), BLIS from Lb. plantarum DM5 (Das and Goyal 2014), BLIS from Lb. plantarum BM-1 (Zhang et al. 2013) and BLIS from Lb. plantarum Lp6SH (Marie et al. 2012). BLIS activity was not affected by exposure to NaCl and organic solvents at a concentration of 50% (Table 4), which were similar for BLIS of P. pentosaceus K23-2 (Shin et al. 2008), pediocin of P. pentosaceus Pep1 (Osmanagaoglou et al. 2001) and BLIS of Bacillus cereus NS02 (Senbagam et al. 2013). The observations from above are in agreement with the previous reports by Kimura et al. (1997) which claimed that pediocin (bacteriocin from Pediococcus strains) is resistant to heat and some organic solvents and is active over a wide range of pH.
Conclusion
This study provided strong support that the re-modified logistic and Luedeking–Piret models are applicable to BLIS secretion by P. acidilactici Kp10 in a stirred tank bioreactor. Both models accurately replicated the experimental data at different agitation speeds. The modelling suggests that BLIS secretion by P. acidilactici Kp10 is a growth-associated process and highly sensitive to agitation conditions. BLIS secretion was enhanced under moderate agitation speeds (400 rpm). In addition, lactose was almost completely depleted, while only 5–6% of the available nitrogen was consumed. This suggests a need for balancing the composition of the culture medium or a lactose fed-batch process. BLIS from this LAB strain possessed highly sought after qualities such as heat resistance, acid and alkaline tolerance and not affected by surfactant, detergents and organic solvents. Based on these characters BLIS from this isolate could be recommended as a biopreservative in food industry.
References
Abbasiliasi S, Ramakrishnan NR, Ibrahim TAT, Mustafa S, Mohamad R, Ariff AB (2010) Partial characterization of antimicrobial compound produced by Lactobacillus paracasei LA07, a strain isolated from Budu. Minerva Biotecnol 22:75–82
Abbasiliasi S, Tan JS, Bashekouh F, Ibrahim TAT, Mustafa S, Vakhshiteh F, Ariff AB (2017) In vitro assessment of Pediococcus acidilactici Kp10 for its potential use in the food industry. BMC Microbiol 17:121–132
Abbasiliasi S, Tan JS, Ibrahim TAT, Ramanan RN, Vakhshiteh F, Mustafa S, Ling TC, Rahim RA, Ariff AB (2012) Isolation of Pediococcus acidilactici Kp10 with ability to secrete bacteriocin-like inhibitory substance from milk products for applications in food industry. BMC Microbiol 12:260
Abbasiliasi S, Tan J, Kadkhodaei S, Nelofer R, Ibrahim TAT, Mustafa S, Ariff AB (2016) Enhancement of BLIS production by Pediococcus acidilactici kp10 in optimized fermentation conditions using an artificial neural network. RSC Adv 6:6342–6349
Abriouel H, Valdivia E, Martínez-Bueno M, Maqueda M, Gálvez A (2003) A simple method for semi-preparative-scale production and recovery of enterocin AS-48 derived from Enterococcus faecalis subsp. liquefaciens A-48-32. J Microbiol Methods 55:599–605
Albano H, Oliveira M, Aroso R, Cubero N, Hogg T, Teixeira P (2007) Anti- listerial activity of lactic acid bacteria isolated from ‘‘Alheiras’’ (traditional Portuguese fermented sausages): in situ assays. Meat Sci 76:796–800
Albano H, Pinho C, Leite D, Barbosa J, Silva J, Carneiro L, Magalhães R, Hogg T, Teixeira P (2009) Evaluation of a bacteriocin-producing strain of Pediococcus acidilactici as a biopreservative for “Alheira”, a fermented meat sausage. Food Control 20:764–770
Altiok D, Tokatli F, Harsa S (2006) Kinetic modelling of lactic acid production from whey by Lactobacillus casei (NRRL B-441). J Chem Technol Biotechnol 81:1190–1197
Atrih A, Rekhif N, Moir AJG, Lebrihi A, Lefebvre G (2001) Mode of action, purification and amino acid sequence of plantaricin C19, an anti-Listeria bacteriocin produced by Lactobacillus plantarum C19. Int J Food Microbiol 68:93–109
Beg QK, Saxena RK, Gupta R (2002) De-repression and subsequent induction of protease synthesis by Bacillus mojavensis under fed-batch operations. Proccess Biochem 37:1103–1109
Beshay U, Moreira A (2004) Protease production from marine microorganism by immobilized cells. In: AIChE ann meet conf proc, pp 8717–8729
Biswas SR, Ray P, Johnson MC, Ray B (1991) Influence of growth conditions on the production of a bacteriocin, pediocin AcH, by Pediococcus acidilactici H. Appl Environ Microbiol 57:1265–1267
Cabiscol E, Piulats E, Echave P, Herrero E, Ros J (2000) Oxidative stress promotes specific protein damage in Saccharomyces cerevisiae. J Biol Chem 275:27393–27398
Cabo ML, Murado MA, Ma Gonzalez, Pastoriza L (2001) Effects of aeration and pH gradient on nisin production: a mathematical model. Enzyme Microb Technol 29:264–273
Carrillo-Ureta GE, Roberts PD, Becerra VM (2001) Genetic algorithms for optimal control of beer fermentation. In: IEEE int symp intell control conf proc, pp 391–396
Cheigh C-I, Choi H-J, Park H, Kim S-B, Kook M-C, Kim T-S, Hwang J-K, Pyun Y-R (2002) Influence of growth conditions on the production of a nisin-like bacteriocin by Lactococcus lactis subsp. lactis A164 isolated from kimchi. J Biotechnol 95:225–235
Chen H, Hoover DG (2003) Bacteriocins and their food applications. Compr Rev Food Sci Food Saf 2:82–100
Chow KL, Mak NK, Wong MH, Zhou XF, Liang Y (2011) Generation of reactive oxygen species and oxidative stress in Escherichia coli and Staphylococcus aureus by a novel semiconductor catalyst. J Nanopart Res 13:1007–1017
Cinar A, Parulekar S, Ündey C, Birol G (2003) Batch fermentation: modeling, monitoring, and control. Marcel Dekker, New York
Coetzee JCJ (2007) Increased production of bacST4SA by Enterococcus mundtii in an industrial-based medium with pH-control. University of Stellenbosch, Stellenbosch
Das D, Goyal A (2014) Characterization of a noncytotoxic bacteriocin from probiotic Lactobacillus plantarum DM5 with potential as a food preservative. Food Func 5:2453–2462
De Vuyst L, Vandamme EJ (1992) Influence of the carbon source on nisin production in Lactococcus lactis subsp. lactis batch fermentations. J Gen Microbiol 138:571–578
De Vuyst L, Callewaert R, Pot B (1996) Characterization of the antagonistic activity of Lactobacillus amylovorus DCE 471 and large scale isolation of its bacteriocin amylovorin L471. Syst Appl Microbiol 19:9–20
Ferreira AE, Canal N, Morales D, Fuentefria DB, Corção G (2007) Characterization of enterocins produced by Enterococcus mundtii isolated from humans feces. Braz Arch Biol Technol 50:249–258
Guerra NP, Castro LP (2003) Enhancement of nisin production by Lactococcus lactis in periodically re-alkalized cultures. Biotechnol Appl Biochem 38:157–167
Guerra NP, Pastrana L (2002) Dynamics of pediocin biosynthesis in batch fermentation on whey. Electron J Environ Agric Food Chem 1:96–106
Guerra NP, Rua ML, Pastrana L (2001) Nutritional factors affecting the production of two bacteriocins from lactic acid bacteria on whey. Int J Food Microbiol 70:267–281
Guerra NP, Agrasar AT, Macías CL, Pastrana L (2005) Modelling the fed-batch production of pediocin using mussel processing wastes. Proc Biochem 40:1071–1083
Hamzah A, Zarin MA, Hamid AA, Omar O, Senafi S (2012) Optimal physical and nutrient parameters for growth of Trichoderma virens UKMP-1M for heavy crude oil degradation. Sains Malays 41:71–79
Havilah EJ, Wallis DM, Morris R, Woolnough JA (1977) A microcolorimetric method for determination of ammonia in Kjeldahl digests with a manual spectrophotometer. Lab Pract 26:545–547
Ivanova I, Kabadjova P, Pantev A, Danova S, Dousset X (2000) Detection, purification and partial characterization of a novel bacteriocin substance produced by Lactococcus lactis subsp. lactis B14 isolated from boza-Bulgarian traditional cereal beverage. Biocatalysis 41:47–53
Jia N, Fengwei T, Jianxin Z, Hao Z, Wei C (2014) Partial characterization of an anti-listeria substance produced by Pediococcus acidilactici P9 isolated from pickle in china. Int Dairy J 34:275–279
Kar B, Banerjee R, Bhattacharyya BC (2000) Modeling gallic acid production rate by empirical and statistical analysis. Braz Arch Biol Technol 43:509–514
Kimura H, Nagano R, Matsusaki H, Sonomoto K, Ishizaki A (1997) A bacteriocin of strain Pediococcus sp. ISK-1 isolated from Nukadoko, bed of fermented rice bran. Biosci Biotechnol Biochem 61:1049–1051
Kumar M, Jain AK, Ghosh M, Ganguli A (2012) Statistical optimization of physical parameters for enhanced bacteriocin production by L. casei. Biotechnol Bioprocess Eng 17:606–616
Leroy F, De Vuyst L (2001) Growth of the bacteriocin-producing Lactobacillus sakei strain CTC 494 in MRS broth is strongly reduced due to nutrient exhaustion: a nutrient depletion model for the growth of lactic acid bacteria. Appl Environ Microbiol 67:4407–4413
Leroy F, De Vuyst L (2002) Bacteriocin production by Enterococcus faecium RZS C5 is cell density limited and occurs in the very early growth phase. Int J Food Microbiol 72:155–164
Line JE, Svetoch EA, Eruslanov BV et al (2008) Isolation and purification of enterocin E-760 with broad antimicrobial activity against gram-positive and gram-negative bacteria. Antimicrob Agents Chemother 52:1094–1100
Lomauro CJ, Bakshi AS, Labuza TP (1985) Evaluation of food moisture sorption isotherm equations. Part I: fruit, vegetable and meat products. Lebensm Wiss Technol 18:111–117
MacNeil B, Harvey LM (1990) Fermentation: a practical approach. IRL press, Oxford
Marie KP, François ZN, Abbasi A, Anwar F, Ali SA, Victor SD, Félicité TM (2012) Characterization of a bacteriocin produced by Lactobacillus plantarum Lp6SH isolated from “Sha’a”, a maize-based traditionally fermented beverage from Cameroon. Int J Biol 4:149–158
Messens W, Verluyten J, Leroy F, De Vuyst L (2003) Modelling growth and bacteriocin production by Lactobacillus curvatus LTH 1174 in response to temperature and pH values used for European sausage fermentation processes. Int J Food Microbiol 81:41–52
Møretrø T, Aasen IM, Storrø I, Axelsson L (2000) Production of sakacin P by Lactobacillus sakei in a completely defined medium. J Appl Microbiol 88:536–545
Natarajan K, Rajendran A (2009) Effect of fermentation parameters on extra cellular tannase production by Lactobacillus plantarum MTCC 1407. J Chem 6:979–984
Ogunbanwo ST, Sanni AI, Onilude AA (2003) Characterization of bacteriocin produced by Lactobacillus plantarum F1 and Lactobacillus brevis OG1. Afr J Biotechnol 2:219–227
Olson ER (1993) Influence of pH on bacterial gene expression. Mol Microbiol 8:5–14
Osmanagaoglou O, Beyatli Y, Gunduz U (2001) Isolation and characterization of pediocin producing Pediococcus pentosaceus Pep1 from vacuum-packed sausages. Turk J Biol 25:133–143
Owusu-Kwarteng J, Tano-Debrah K, Akabanda F, Nielsen DS, Jespersen L (2013) Partial characterization of bacteriocins produced by Lactobacillus reuteri 2-20B and Pediococcus acidilactici 0-11A isolated from Fura, a Millet-based fermented food in Ghana. J Food Res 2:50–58
Parente E, Ricciardi A (1994) Influence of pH on the production of enterocin 1146 during batch fermentation. Lett Appl Microbiol 19:12–15
Parente E, Brienza C, Ricciardi A, Addario G (1997) Growth and bacteriocin production by Enterococcus faecium DPC1146 in batch and continuous culture. J Ind Microbiol Biotechnol 18:62–67
Pilet M-F, Dousset X, Barre R, Novel G, Desmazeaud M, Piard J-C (1995) Evidence for two bacteriocins produced by Carnobacterium piscicola and Carnobacterium divergens isolated from fish and active against Listeria monocytogenes. J Food Prot 58:256–262
Rajaram G, Manivasagan P, Thilagavathi B, Saravanakumar A (2010) Purification and characterization of a bacteriocin produced by Lactobacillus lactis isolated from marine environment. Adv J Food Sci Technol 2:138–144
Ray B, Daeschel M (1992) Bacteriocins of lactic acid bacteria as food biopreservatives: an overview. In: Bibek R, Daeschel M (eds) Food biopreservatives of microbial origin. CRC Press, Boca Raton
Savadogo A, Ouattara ATC, Bassole HNI, Traore SA (2006) Bacteriocins and lactic acid bacteria-a minireview. Afr J Biotechnol 5:678–683
Senbagam D, Gurusamy R, Senthilkumar B (2013) Physical chemical and biological characterization of a new bacteriocin produced by Bacillus cereus NS02. Asian Pac J Trop Med 6:934–941
Settanni L, Corsetti A (2008) Application of bacteriocins in vegetable food biopreservation. Int J Food Microbiol 121:123–138
Shin MS, Han SK, Ryu JS, Kim KS, Lee WK (2008) Isolation and partial characterization of a bacteriocin produced by Pediococcus pentosaceus K23-2. isolated from Kimchi. J Appl Microbiol 105:331–339
Sinclair CG, Kristiansen B (1987) Fermentation kinetics and modelling. Taylor and Francis, New York
Voulgaris I, Arnold SA, Speight R, Harvey LM, McNeil B (2011) Effects of dissolved oxygen availability and culture biomass at induction upon the intracellular expression of monoamine oxidase by recombinant E. coli in fed batch bioprocesses. Proc Biochem 46:721–729
Zhang J, Zhang Y, S-n Liu, Han Y, Z-j Zhou (2012) Modelling growth and bacteriocin production by Pediococcus acidilactici PA003 as a function of temperature and pH value. Appl Biochem Biotechnol 166:1388–1400
Zhang H, Liu L, Hao Y, Zhong S, Liu H, Han T, Xie Y (2013) Isolation and partial characterization of a bacteriocin produced by Lactobacillus plantarum BM-1 isolated from a traditionally fermented Chinese meat product. Microbiol Immun 57:746–755
Zhao L, Chen Y, Schaffner DW (2001) Comparison of logistic regression and linear regression in modeling percentage data. Appl Environ Microbiol 67:2129–2135
Znad H, Blazej M, Bales V, Markos J (2004) A kinetic model for gluconic acid production by Aspergillus niger. Chem Pap 58:23–28
Zwietering MH, Jongenburger I, Rombouts FM, Van’t Riet K (1990) Modeling of the bacterial growth curve. Appl Environ Microbiol 56:1875–1881
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abbasiliasi, S., Tan, J.S., Ibrahim, T.A.T. et al. Kinetic modeling of bacteriocin-like inhibitory substance secretion by Pediococcus acidilactici Kp10 and its stability in food manufacturing conditions. J Food Sci Technol 55, 1270–1284 (2018). https://doi.org/10.1007/s13197-018-3037-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3037-x